Introduction
Lung cancer remains the most prevalent malignant
disease and leading cause of mortality worldwide (1). Treatment for the majority of patients
with lung cancer includes surgery, chemotherapy, radiation therapy,
or a combination of these treatments. Among these, chemotherapy is
an effective treatment strategy for lung cancer, which can improve
the overall survival rate of patients following surgery. However,
side-effects such as nausea, vomiting and drug resistance often
limit the use of chemotherapeutic agents (2). Among the chemotherapeutic drugs,
naturally occurring flavonoids, such as baicalin, liquiritin,
hesperetin and quercetin have garnered substantial interest due to
their potential effects on cancer cells and lower toxicity on
normal cells (3-6).
Several studies have demonstrated that reactive
oxygen species (ROS) are associated with a variety of cellular
processes, such as transcription factor activation, cell
proliferation and apoptosis (7).
The generation of intracellular ROS is an important source of
mitochondrial electron transport chains. The excessive generation
of intracellular ROS reduces mitochondrial membrane potential
(MMP), leading to apoptosis (8).
Moreover, the ROS-mediated mitogen-activated protein kinase (MAPK)
pathway is closely associated with cell proliferation,
differentiation and apoptosis. Among MAPK families, there is ample
evidence that p38/MAPK, c-Jun N-terminal kinases (JNKs) and
extracellular signal-regulated kinases (ERKs) are involved on
cancer initiation and progression (9). In addition, the ROS-mediated signal
transducer and activator of transcription 3 (STAT3) and nuclear
factor-κB (NF-κB) pathways play an important role in cancer
progression (10-13). Increasing evidence has suggested
that ROS induce cell apoptosis by activating the MAPK, STAT3 and
NF-κB signaling pathways in cancer cells (14,15).
Isoorientin
(3',4',5,7-tetrahydroxy-6-C-glucopyranosyl flavone, ISO) is a well
characterized naturally occurring flavonoid with biological
properties representative of this group of compounds. It has been
extracted from several plant species, including Patrinia,
Crataegus pentagyna and Drosophyllum lusitanicum
(16-18) and has anti-bacterial and
anti-inflammatory activities. It has been demonstrated that ISO
inhibits the rate of protein synthesis of Salmonella
typhimurium, alters the permeability of the cell membrane of
the bacteria, and eventually causes the leakage of nucleic acids
and electrolytes; it also inhibits the proliferation of bacteria,
thereby exerting antibacterial effects (19). In addition, ISO attenuates
neuroinflammation by inhibiting the ROS-related MAPK/NF-κB
signaling pathway, thereby exerting anti-inflammatory effects
(20,21). Furthermore, ISO has been shown to
induce apoptosis and cell cycle arrest of HT-29 colorectal
adenocarcinoma cells (22).
However, the effects of ISO-induced apoptosis on human lung cancer
cells remain unknown.
The present study evaluated the anticancer effects
of ISO on human lung cancer cells (A549, NCI-H23 and NCI-H460). In
addition, ISO-induced apoptosis through the ROS-mediated MAPK,
STAT3 and NF-κB signaling pathways in A549 cells was evaluated.
Materials and methods
Cell lines and cell culture
The human lung cancer cell lines A549, NCI-H23 and
NCI-H460 cells, were obtained from the American Type Culture
Collection (ATCC) and maintained in Dulbecco's modified Eagle's
medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.). Normal lung
IMR-90 and normal stomach GES-1 cells were obtained from ATCC and
Saiqi Biotech Co., Ltd., and maintained in DMEM (Gibco; Thermo
Fisher Scientific, Inc.). All cells were supplemented with
heat-inactivated fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.), 100 µg/ml streptomycin (Gibco; Thermo
Fisher Scientific, Inc.) and 100 U/ml penicillin (Gibco; Thermo
Fisher Scientific, Inc.). The cells were then cultured at 37°C in a
5% CO2 atmosphere.
Cell viability assay
The cytotoxic effects of ISO treatment on lung
cancer cells (A549, NCI-H23 and NCI-H460) and the side-effects of
ISO (purity ≥99%; Chengdu Herbpurify Co., Ltd.) on normal cells
(IMR-90 and GES-1) were assessed using the Cell Counting Kit-8
(CCK-8) assay. Briefly, cells were seeded in 96-well culture plates
at a density of 1×104 cells per well and incubated for
24 h at 37°C. 5-FU, as the one of the first anticancer drug ued,
inhibits thymidine nucleotide synthetase, blocks the conversion of
deoxypyrimidine nucleotides into thymidine nucleus and interferes
with DNA synthesis. In addition, cisplatin can bind to DNA and
causes cross-linking, thereby destroying the function of DNA and
inhibiting cell mitosis. It is a potent broad-spectrum anticancer
drug (23,24). Thus, in the present study, 5-FU and
cisplatin were selected as positive control drugs. The lung cancer
cells and normal cells were treated with 5-FU (Medchem Express),
cisplatin (Solarbio Science & Technology Co., Ltd.) and ISO at
various concentrations (20, 40, 60, 80 and 100 µM) for 24 h
or different periods of time (3, 6, 12, 24 and 36 h) at 46.81
µM (the IC50 value of A549 cells). Subsequently,
10 µl CCK-8 solution were added to each well followed by
incubation for 3 h at 37°C. The absorbance values were measured
using a microplate reader (BioTek Instruments Inc.) at 450 nm and
the experiments were performed 3 times with 16 wells per
experiment. Finally, the obtained OD values were analyzed using
GraphPad Prism software, and the corresponding IC50
values were then obtained using GraphPad Prism software.
Analysis of cell apoptosis
The effects of ISO on cell apoptosis were measured
using the Apoptosis and Necrosis Assay kit and Annexin V Detection
kit (Beyotime Institute of Biotechnology). The A549 cells were
seeded in 6-well culture plates at a density of 1×105
per well and treated with 46.81 µM ISO for different periods
of time (3, 6, 12 and 24 h). After the cells were collected, they
were re-suspended in 100 μl cell staining buffer, followed by the
addition of 5 µl Hoechst 33342 staining solution and 1.5
µl propidium iodide (PI) staining solution, incubation for
10 min at 37°C, and observation with a fluorescence microscope
(Thermo Fisher Scientific, Inc.). The collected A549 cells were
then cultured in 195 µl Annexin V staining buffer, followed
by the addition of 3 µl Annexin V-FITC and 2 µl PI
for 10 min. The percentages of apoptotic cells were analyzed using
a flow cytometry (Beckman Coulter, Inc.).
Detection of MMP
The MMP of A549 cells was detected using the MMP
Detection kit (JC-1; Beyotime Institute of Biotechnology). After
the A549 cells were grown in a 6-well culture plate at a density of
1×105 cells per well, they were treated with 46.81
µM ISO for different periods of time (3, 6, 12 and 24 h).
The A549 cells were incubated with JC-1 working solution at 37°C
for 20 min. The cells were then washed twice with 1X JC-1 staining
buffer solution. The data were analyzed by flow cytometry.
Analysis of cell cycle arrest
The cell cycle arrest of ISO-treated A549 cells was
assessed by a DNA Content Quantitation assay (Solarbio Science
&Technology Co., Ltd.). Briefly, the A549 cells were treated
with 46.81 µM ISO for different periods of time (3, 6, 12
and 24 h). After pre-cooling with 70% ethanol overnight, they were
washed twice with phosphate-buffered saline (PBS), followed by the
addition of 100 µl RNase A solution and the re-suspension of
cells at 37°C for 30 min. Finally, 400 µl PI staining
solution were added, and the cells were incubated for 30 min at
4°C. The data were then analyzed by flow cytometry.
Preparation of nuclear extracts
The Nuclear Protein Extraction kit was used to
prepare the nuclear extract. The A549 cells were treated with 46.81
µM ISO for different periods of time (3, 6, 12 and 24 h),
and then washed with PBS once; the cells were then centrifuged at
500 × g for 3 min in room temperature. After the cells were
resuspended with 80 µl plasma protein extraction reagent,
they were incubated on ice for 10 min. The cells were then
centrifuged at 12,000 × g for 10 min at 4°C and the supernatants
were used as the cytosolic extract. The precipitate was then
resuspended in 50 µl nuclear protein extraction reagent and
incubated on ice for 10 min. The cells were then centrifuged at
12,000 × g for 10 min at 4°C. The supernatant was used as the
nuclear protein.
Western blot analysis
The expression levels of relevant proteins were
measured by western blot analysis. After the A549 cells were
treated with 46.81 µM ISO for different periods of times (3,
6, 12 and 24 h), the cells were collected, and protein was
extracted using cell lysis buffer. For the inhibitor-treated cell
samples, the A549 cells were pre-treated 30 min with 10 µM
MAPK inhibitors (the pharmacological inhibitor of p38, SB203580;
the pharmacological inhibitor of JNK, SP600125; and the
pharmacological inhibitor of ERK, FR180204; all from MedChem
Express) at 37°C and were then treated with 46.81 µM ISO for
24 h. For N-acetylcysteine (NAC)-treated cell samples, the
A549 cells were pre-treated 30 min with 10 µM NAC
(Sigma-Aldrich; Merck KGaA) at 37°C and then treated with 46.81
µM ISO for 24 h. Briefly, the cells were centrifuged at
12,000 × g for 30 min at 4°C. An equal amount of protein (30
µg) was loaded onto 10-12% SDS-PAGE gels and
electro-transferred onto nitrocellulose membranes (EMD Millipore).
The membranes were then blocked in 5% skim milk in Tris-buffered
saline Tween-20 (TBST) for 2 h, followed by overnight incubation at
4°C with specific primary antibodies (from Santa Cruz
Biotechnology, Inc.), against mouse monoclonal β-actin (1:2,500;
cat. no. sc-47778), Lamin B1 (1:2,500; cat. no. sc-374015), Bax
(1:1,500; cat. no. sc-493), Bcl-2 (1:1,500; cat. no. sc-7382),
cleaved-caspase-3 (cle-cas-3; 1:1,500; cat. no. sc-373730),
poly(ADP) ribose polymerase (PARP)-1 (1:1,500; cat. no. sc-8007),
p-p38 (1:1,500; cat. no. sc-7973), p-JNK (1:1,500; cat. no.
sc-6254), JNK (1:1,500; cat. no. sc-7345), p-ERK (1:1,500; cat. no.
sc-7383), p-STAT3 (1:1,500; cat. no. sc-8059), STAT3 (1:1,500; cat.
no. sc-8019), NF-κB (1:1,500; cat. no. sc-8008), p-NF-κB (1:1,500;
cat. no. sc-166748), inhibitor of IκB-α (IκB-α; 1:1,500; cat. no.
sc-1643), p-IκB-α (1:1,500; cat. no. sc-8404), cyclin B1 (1:1,500;
cat. no. sc-245), CDK1/2 (1:1,500; cat. no. sc-53219), against
rabbit monoclonal p38α/β (1:1,500; cat. no. sc-7149), p27 (1:1,500;
cat. no. sc-528), p21 (1:1,500; cat. no. sc-397). The membranes
were then incubated with horseradish peroxidase (HRP)-conjugated
secondary antibodies (i.e., HRP-conjugated AffiniPure goat
anti-mouse IgG and HRP-conjugated AffiniPure goat anti-rabbit IgG)
for 1 h at room temperature. Proteins were measured using enhanced
chemiluminescence kits (Bio-Rad Laboratories, Inc.). Band intensity
was assessed using ImagesJ software version 1.42q.
Detection of intracellular ROS
levels
Intracellular ROS levels were measured using the
2',7'-dichlorofluorescein diacetate (DCFH-DA; Beyotime Institute of
Biotechnology) fluorescent probe. The cells were treated with 46.81
µM ISO for different periods of time (3, 6, 12 and 24 h).
The collected cells were washed twice with PBS. The cells were then
incubated with DCFH-DA for 30 min at 37°C, and were again washed
twice with PBS. The fluorescence intensity of DCF, which represents
intracellular ROS levels, was analyzed using a flow cytometer
(Beckman Coulter, Inc.) in the cell samples.
Statistical analysis
All data are presented as the means ± standard
deviation from 3 experiments. Continuous data were analyzed by
one-way analysis of variance followed by Tukey's post-hoc test
using SPSS software version 21.0. P-values <0.05 were considered
to indicate statistically significant differences.
Results
ISO exerts cytotoxic effects on human
lung cancer cells
To determine the effects of ISO on lung cancer cell
viability, the A549, NCI-H23 and NCI-H460 cells were treated with
5-FU and ISO, and after 24 h, cell viability was measured by CCK-8
assay. As shown in Fig. 1A,
5-FU, cisplatin and ISO exerted
significant cytotoxic effects on lung cancer cells (A549, NCI-H23
and NCI-H460) in a dose-dependent manner. As human lung fibroblasts
(IMR-90) are extracted from embryonic cells, their primary
properties have not transformed. This can directly reflect the
toxic effects to the human lungs in the toxicity experiment of the
compounds. Furthermore, GES-1 cells are human gastric mucosal
epithelial cells. The drug used in this experiment is a compound
extracted from Chinese herbal medicine, which is mainly digested
and metabolized in the stomach. Therefore, the IMR-90 and GES-1
cells were selected for the determination of ISO cytotoxicity.
Importantly, compared with 5-FU and cisplatin, following treatment
with ISO, the survival rate of the human normal cells (IMR-90 and
GES-1) did not evidently decrease (Fig. 1B). Among the lung cancer cells, the
A549 cells were more sensitive to ISO than the NCI-H23 and NCI-H460
cells, and the half-maximal inhibitory values (IC50) of
ISO for the lung cancer cells are presented in Table I. In addition, the results of CCK-8
assay revealed that 5-FU, cisplatin and ISO exerted evident
growth-inhibitory effects on the lung cancer cells and the
IC50 value was reached in the A549 cells at 24 h
(Fig. 1C). It was found that the
concentration of ISO did not reach its IC50 value when
it exceeded 100 µM; thus, it was considered that its
IC50 value exceeds 100 µM. In addition, in order
to ensure that normal cells and cancer cells were compared at the
same level, the IC50 value of ISO for the experiments
was selected based on the toxicity of normal cell time gradients.
As shown in Fig. 1D, compared with
5-FU and cisplatin, treatment with 46.81 µM ISO for 24 h
exerted no obvious effects on normal cells (Fig. 1D). Based on the results shown in
Fig. 1C and D, it was found that
the cytotoxic effects of ISO on lung cancer cells were similar to
those of 5-FU and cisplatin; however, ISO exerted less side-effects
than 5-FU and cisplatin on normal cells. In addition, when the
cells were treated with 46.81 µM ISO for different periods
of time (3, 6, 12, 24 and 36 h), the cells begun to undergo
apoptosis and gradually die. In particular, when the treatment time
reached 36 h, a large number of cells were apoptotic and exfoliated
from the plate, rendering subsequent experiments impossible. Thus,
subsequent experiments were conducted at different time points (3,
6, 12 and 24 h).
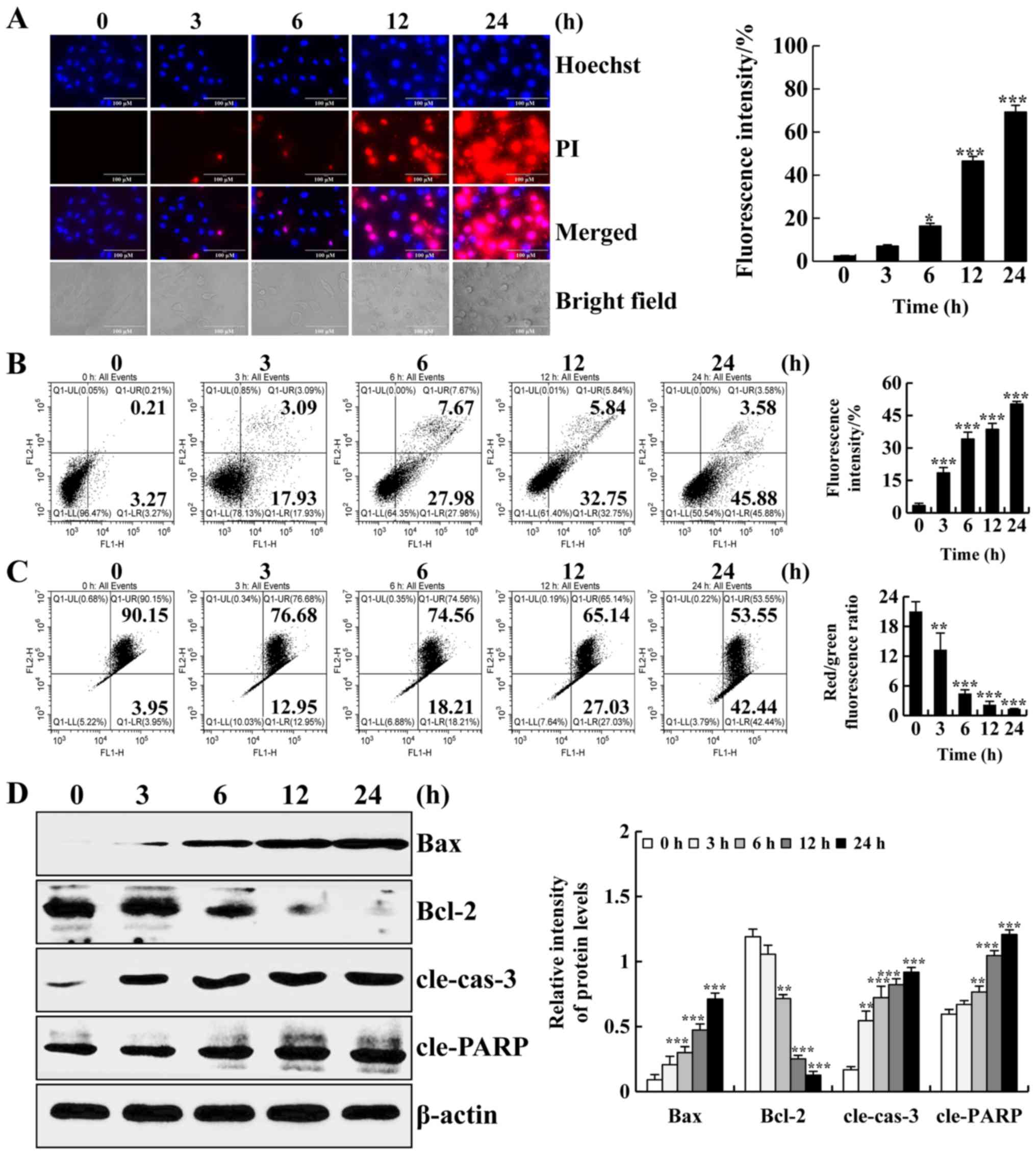
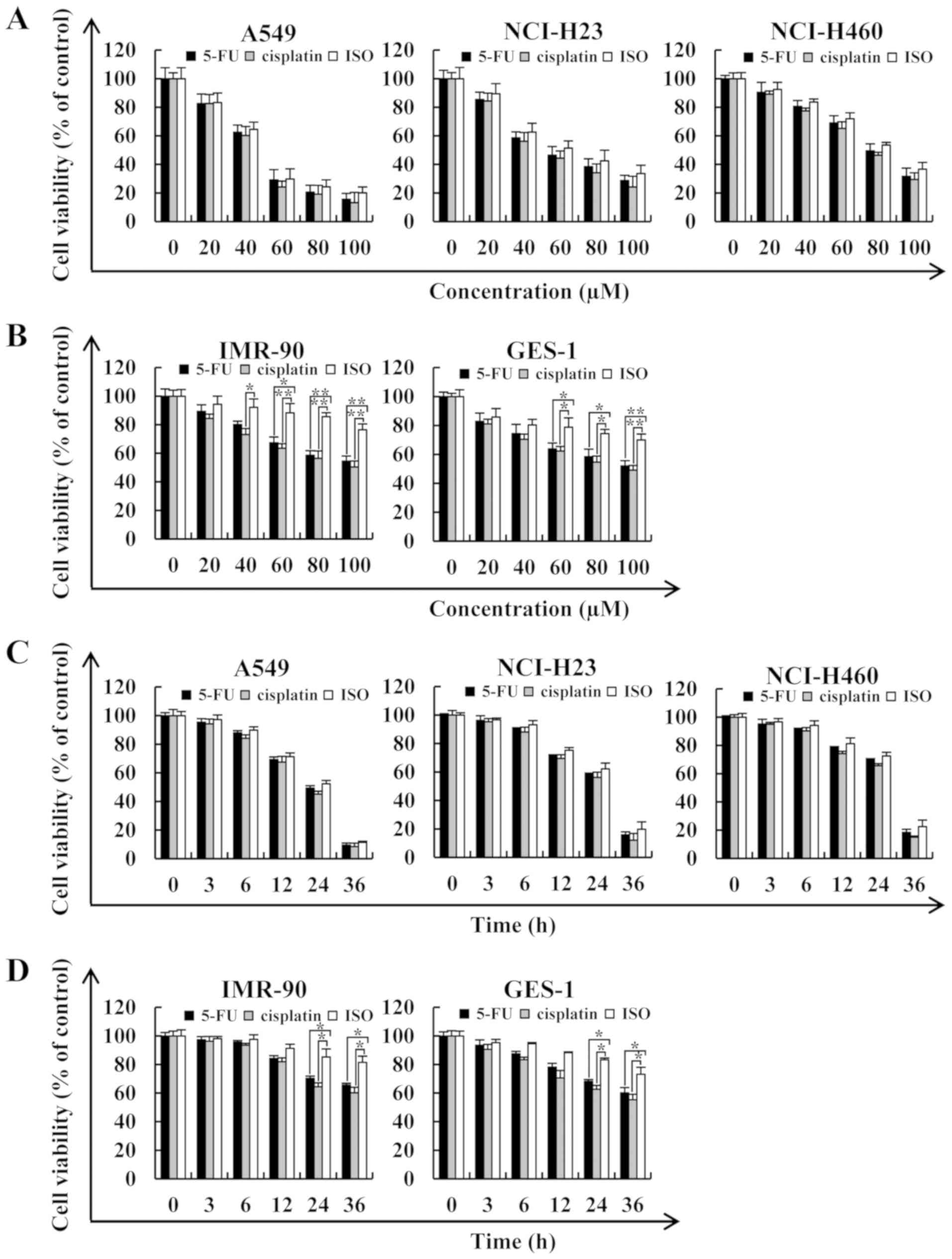 | Figure 1Cytotoxic effects of ISO on human
lung cancer cells. (A) A549, NCI-H23 and NCI-H460 lung cancer cells
were treated with various concentrations of 5-FU, cisplatin and ISO
(20, 40, 60, 80 and 100 µM) for 24 h, after which their cell
viabilities were determined by CCK-8 assay. (B) IMR-90 and GES-1
normal cells were treated with various concentrations of 5-FU,
cisplatin and ISO (20, 40, 60, 80 and 100 µM) for 24 h,
after which their cell viabilities were determined by CCK-8 assay.
(C) A549, NCI-H23 and NCI-H460 cells were treated for different
periods of time (3, 6, 12, 24 and 36 h) with the IC50
value of ISO, after which their cell viabilities were determined by
CCK-8 assay. (D) IMR-90 and GES-1 cells were treated for different
periods of time (3, 6, 12, 24 and 36 h) with the IC50
value of ISO, after which their cell viabilities were determined by
CCK-8 assay. Data are expressed as the means ± SD.
*P<0.05 and **P<0.01 vs. control. ISO,
isoorientin. |
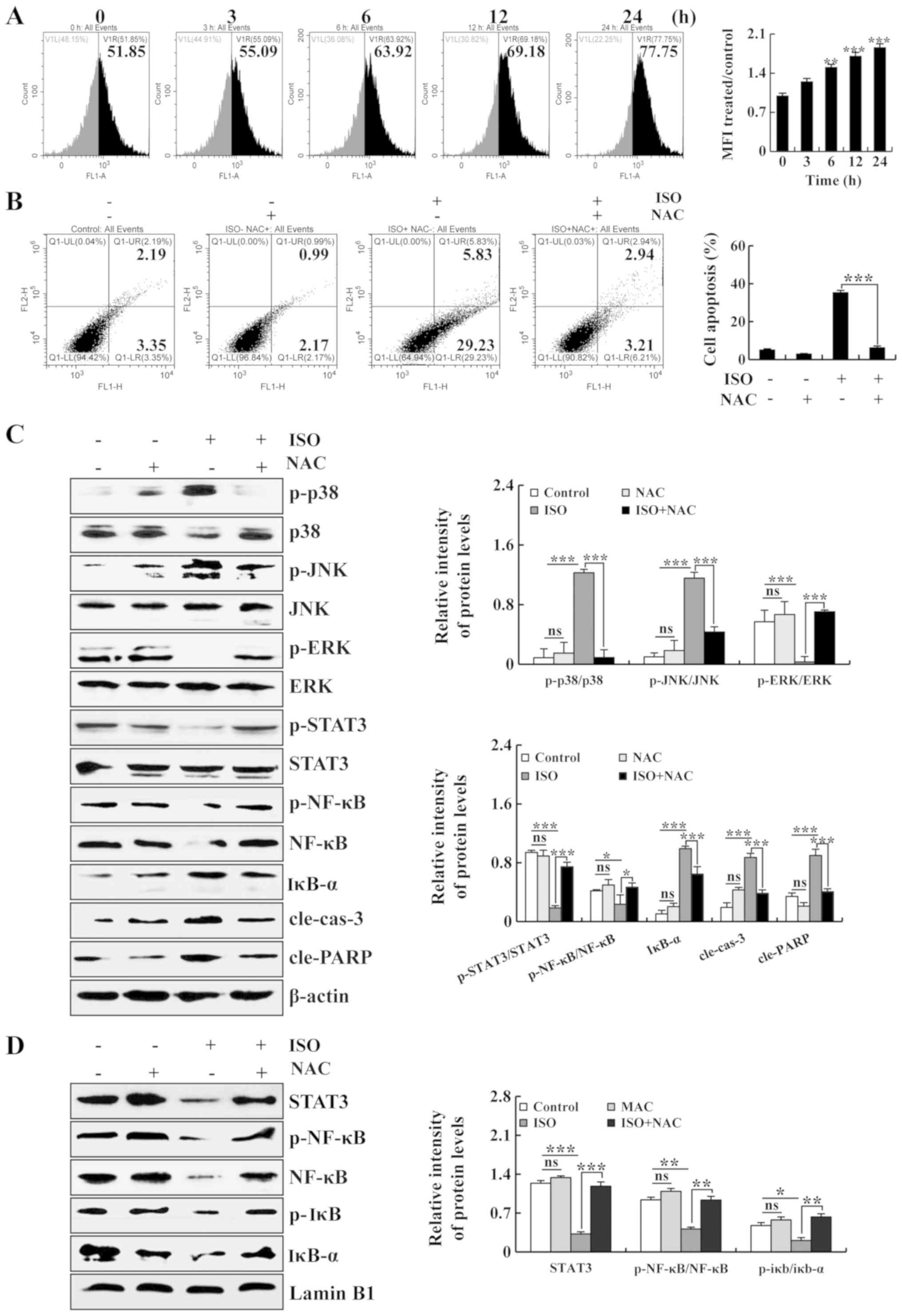 | Figure 5Promoting effects of ISO on ROS
generation and the induction of apoptosis of A549 cells. (A) A549
cells were treated with ISO, and the intracellular ROS levels were
measured by flow cytometry. (B) A549 cells were treated with NAC
and ISO. The percentages of apoptotic cells were then measured by
flow cytometry. (C) A549 cells were treated with ISO and NAC, after
which the expression levels of MAPKs, STAT3, NF-κB, cle-cas-3 and
cle-PARP were detected by western blot analysis. (D) A549 cells
were treated with ISO and NAC, after which the expression levels of
STAT3, p-NF-κB, NF-κB, p-iκb and iκb-α in the nucleus were detected
by western blot analysis. The phosphorylated proteins were
quantified with corresponding total proteins. β-actin and Lamin B1
was used as the loading controls. *P<0.05,
**P<0.01 and ***P<0.001 vs. the NAC +
ISO group. ns, not significant; ISO, isoorientin. |
 | Table IIC50 values of ISO and
5-FU in lung cancer cells. |
Table I
IC50 values of ISO and
5-FU in lung cancer cells.
| Cell line | 5-FU
(µM) | ISO
(µM) |
|---|
| A549 | 43.52±1.83 | 46.81±2.37 |
| NCI-H23 | 57.15±2.14 | 63.88±1.49 |
| NCI-H460 | 79.82±1.54 | 81.69±1.56 |
ISO induces the apoptosis of A549
cells
To verify the effects of ISO on lung cancer cell
apoptosis, the A549 cells were processed with ISO for different
periods of time (3, 6, 12 and 24 h), and the fluorescence intensity
was detected with a fluorescence microscope. As shown in Fig. 2A, the fluorescence intensity of
Hoechst 33342 and PI and the degree of cell shrinkage were
increased. Next, we detected the apoptotic effects of ISO in A549
cells by flow cytometry. As shown in Fig. 2B, the ratio of apoptotic cells was
increased. In addition, early apoptotic cells were accompanied by
changes in MMP. As shown in Fig.
2C, the ratio of red to green fluorescence was significantly
decreased, indicating that ISO reduced MMP in a time-dependent
manner. Consistently, to further investigate the molecular
mechanisms through which ISO induced the apoptosis of A549 cells,
the expression levels of apoptosis-related proteins were examined
by western blot analysis. As shown in Fig. 2D, the protein expression levels of
Bax, cleaved caspase-3 (cle-cas-3) and cleaved PARP (cle-PARP) were
increased. Furthermore, the protein expression levels of Bcl-2 were
decreased. Thus, these results demonstrated that ISO induced
apoptosis via a mitochondrial-dependent pathway in A549 cells.
ISO induces apoptosis through the MAPK,
STAT3 and NF-κB signaling pathways in A549 cells
To further determine the molecular mechanisms
responsible for the ISO-induced apoptosis of A549 cells, the
related protein expression levels of MAPK, STAT3 and NF-κB were
measured by western blot analysis. As shown in Fig. 3A, the protein expression levels of
p-p38, p-JNK and IκB-α were increased, whereas the protein
expression levels of p-ERK, p-STAT3, p-NF-κB and NF-κB were
decreased. As a nuclear transcription factor, the nuclear
translocation of proteins (STAT3, p-NF-κB, NF-κB, p-IκB and IκB-α)
is required for their function. As shown in Fig. 3B, the nuclear protein expression
levels of STAT3, p-NF-κB, NF-κB, p-IκB and IκB-α were decreased. In
addition, to verify the effects of MAPK and STAT3 signaling
pathways on the ISO-induced apoptosis of human lung cancer cells,
the A549 lung cancer cells were pre-treated with 10 µM
SB203580 (a pharmacological inhibitor of p38), 10 µM
SP600125 (a pharmacological inhibitor of JNK) and 10 µM
FR180204 (a pharmacological inhibitor of ERK), and the protein
expression levels of the MAPKs and STAT3 were measured by western
blot analysis. As shown in Fig. 4A and
B, following pre-treatment with SB203580 or SP600125 alone, no
significant differences were observed between the SB203580 group or
SP600125 group and the control group. Compared with the control
group, the protein expression levels of p-STAT3 and Bcl-2 were
decreased, while the protein expression levels of p-p38, p-JNK,
cle-cas-3 and cle-PARP were increased in the ISO group. Moreover,
following pre-treatment with SB203580 or SP600125, compared with
the ISO group, the protein expression levels of p-STAT3 and Bcl-2
were increased, while the protein expression levels of p-p38,
p-JNK, cle-cas-3 and cle-PARP were decreased in the ISO + SB203580
group or ISO + SP600125 group. As shown in Fig. 4C, following pre-treatment with
FR180204 alone, the expression level of p-ERK was decreased and
that of the other 4 proteins exhibit no significant difference
between the FR180204 group and the control group. Briefly, compared
with the control group, the protein expression levels of p-ERK,
p-STAT3 and Bcl-2 were decreased, while the protein expression
levels of cle-cas-3 and cle-PARP were increased in the ISO group.
Furthermore, following pre-treatment with FR180204, compared with
the ISO group, the protein expression levels of p-ERK, p-STAT3 and
Bcl-2 were decreased, while the protein expression levels of
cle-cas-3 and cle-PARP were increased in the ISO + FR180204 group.
Taken together, these results indicated that the MAPK/STAT3/NF-κB
signaling pathways were associated with the ISO-induced apoptosis
of A549 cells.
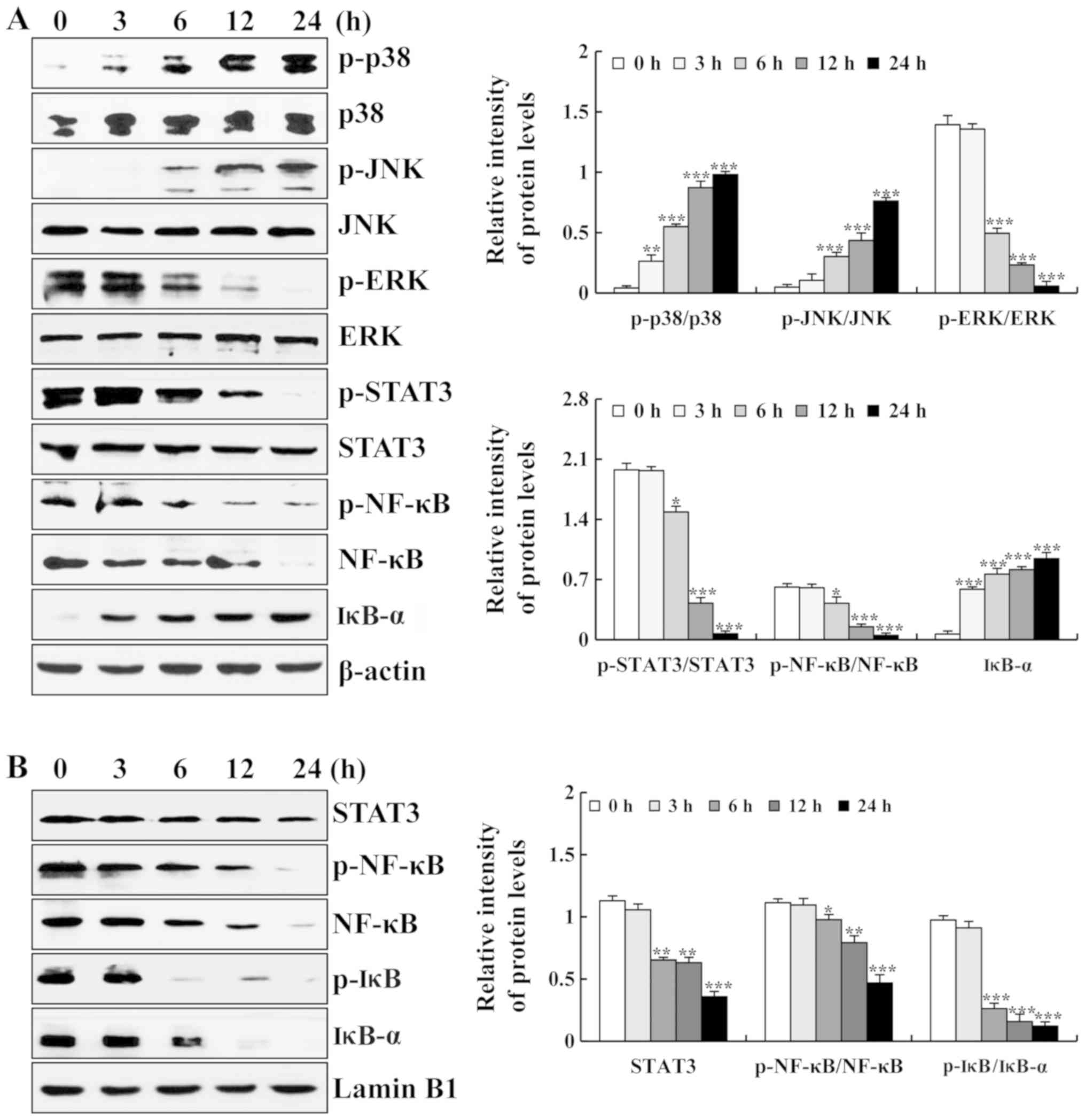 | Figure 3Effects of ISO on MAPK/STAT3/NF-κB
signaling pathway in A549 cells. (A) Expression levels of p-p38,
p-JNK, p-ERK, p-STAT3, p-NF-κB, NF-κB and IκB-α were measured by
western blot analysis. (B) Expression levels of STAT3, p-NF-κB,
NF-κB, p-IκB and IκB-α in the nucleus were measured by western blot
analysis. The phosphorylated proteins were quantified with
corresponding total proteins. β-actin and Lamin B1 were used as the
loading controls. *P<0.05, **P<0.01 and
***P<0.001 vs. 0 h. ISO, isoorientin. |
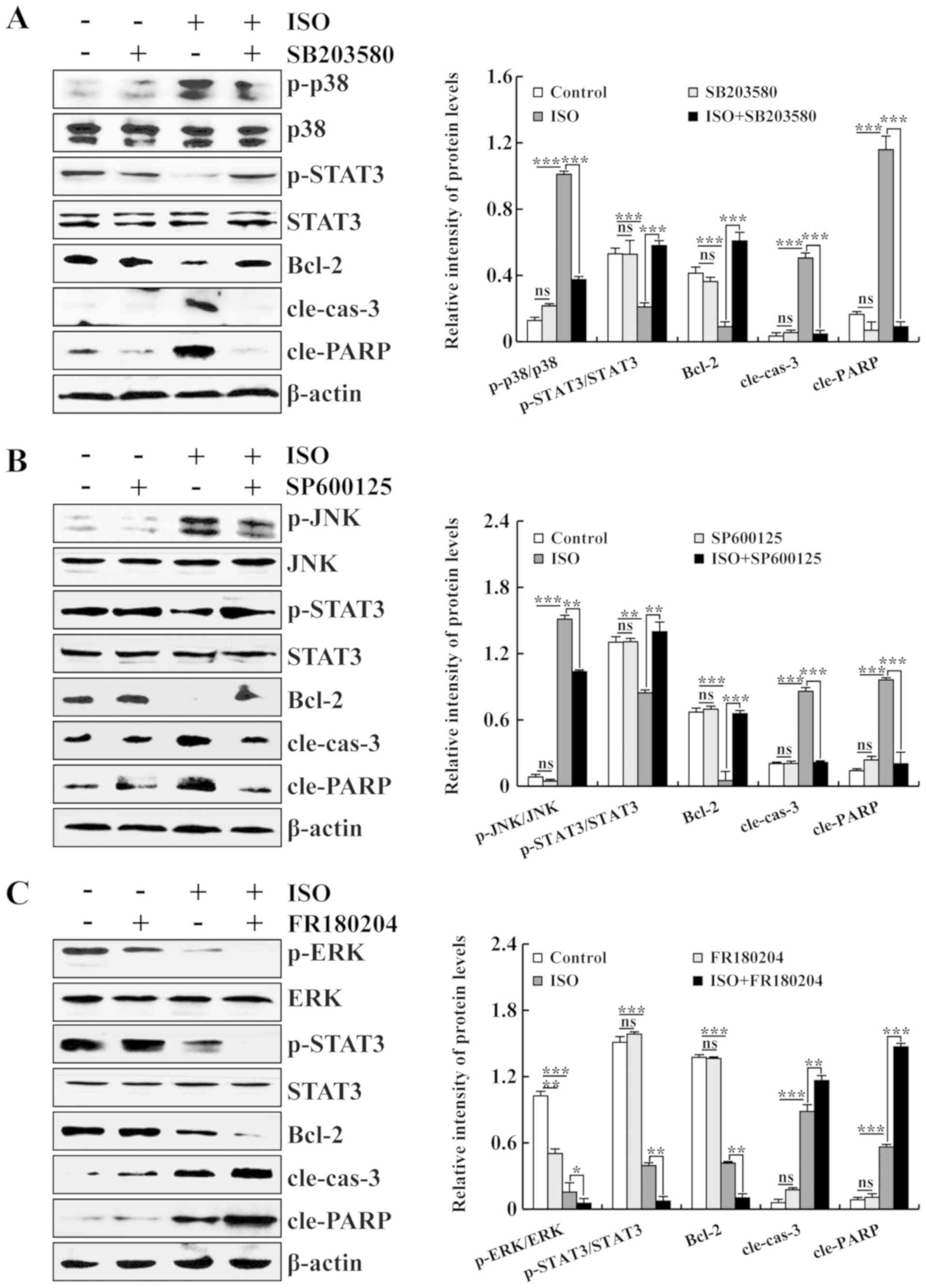 | Figure 4Effects of ISO on the MAPK and STAT3
signaling pathways in A549 cells. (A) Expression levels of p-p38,
p-STAT3, Bcl-2, cle-cas-3 and cle-PARP proteins in ISO-treated and
p38 inhibitor-treated A549 cells. (B) Expression levels of p-JNK,
p-STAT3, Bcl-2, cle-cas-3 and cle-PARP proteins in ISO-treated and
JNK inhibitor-treated A549 cells. (C) Expression levels of p-ERK,
p-STAT3, Bcl-2, cle-cas-3 and cle-PARP proteins in ISO-treated and
ERK inhibitor-treated A549 cells. The phosphorylated proteins were
quantified with corresponding total proteins. β-actin was used as
the loading control. *P<0.05, **P<0.01
and ***P<0.001 vs. ISO + MAPK inhibition. ns, not
significant; ISO, isoorientin. |
ISO-induced cell apoptosis is dependent
on the ROS-mediated MAPK/STAT3/NF-κB signaling pathway in A549
cells
To investigate whether ROS are associated with the
ISO-induced apoptosis of A549 cells, intracellular ROS levels were
detected by flow cytometry. As shown in Fig. 5A, following treatment with ISO, the
intracellular ROS levels were significantly increased. In addition,
the apoptosis of A549 cells was significantly decreased following
pre-treatment with NAC (Fig. 5B).
To further investigate whether ISO induces cell apoptosis via the
ROS-mediated MAPK, STAT3 and NF-κB signaling pathways, the A549
cells were pre-treated with NAC, and the protein expression levels
of MAPK, STAT3, p-NF-κB and NF-κB, as well as the nuclear protein
expression levels of STAT3, p-NF-κB, NF-κB, p-IκB and IκB-α were
then detected. As shown in Fig.
5C, following pre-treatment with NAC, no significant
differences were observed between the NAC group and the control
group. Compared with the control group, the expression levels of
p-p38, p-JNK, IκB-α, cle-cas-3 and cle-PARP were significantly
upregulated, while the expression levels of p-ERK, p-STAT3, p-NF-κB
and NF-κB were downregulated in the ISO group. Furthermore,
following pre-treatment with NAC, compared with the ISO group, the
expression levels of p-p38, p-JNK, IκB-α, cle-cas-3 and cle-PARP
were significantly downregulated, while the expression levels of
p-ERK, p-STAT3, p-NF-κB and NF-κB were upregulated in the ISO + NAC
group. In addition, as shown in Fig.
5D, following pre-treatment with NAC, no significant
differences were observed between the NAC group and the control
group. Compared with the control group, the nuclear protein
expression levels of STAT3, p-NF-κB and NF-κB were significantly
decreased in the ISO group. In brief, following pre-treatment with
NAC, compared with the ISO group, the nuclear protein expression
levels of STAT3, p-NF-κB, NF-κB, p-IκB and IκB-α were increased in
the ISO + NAC group.
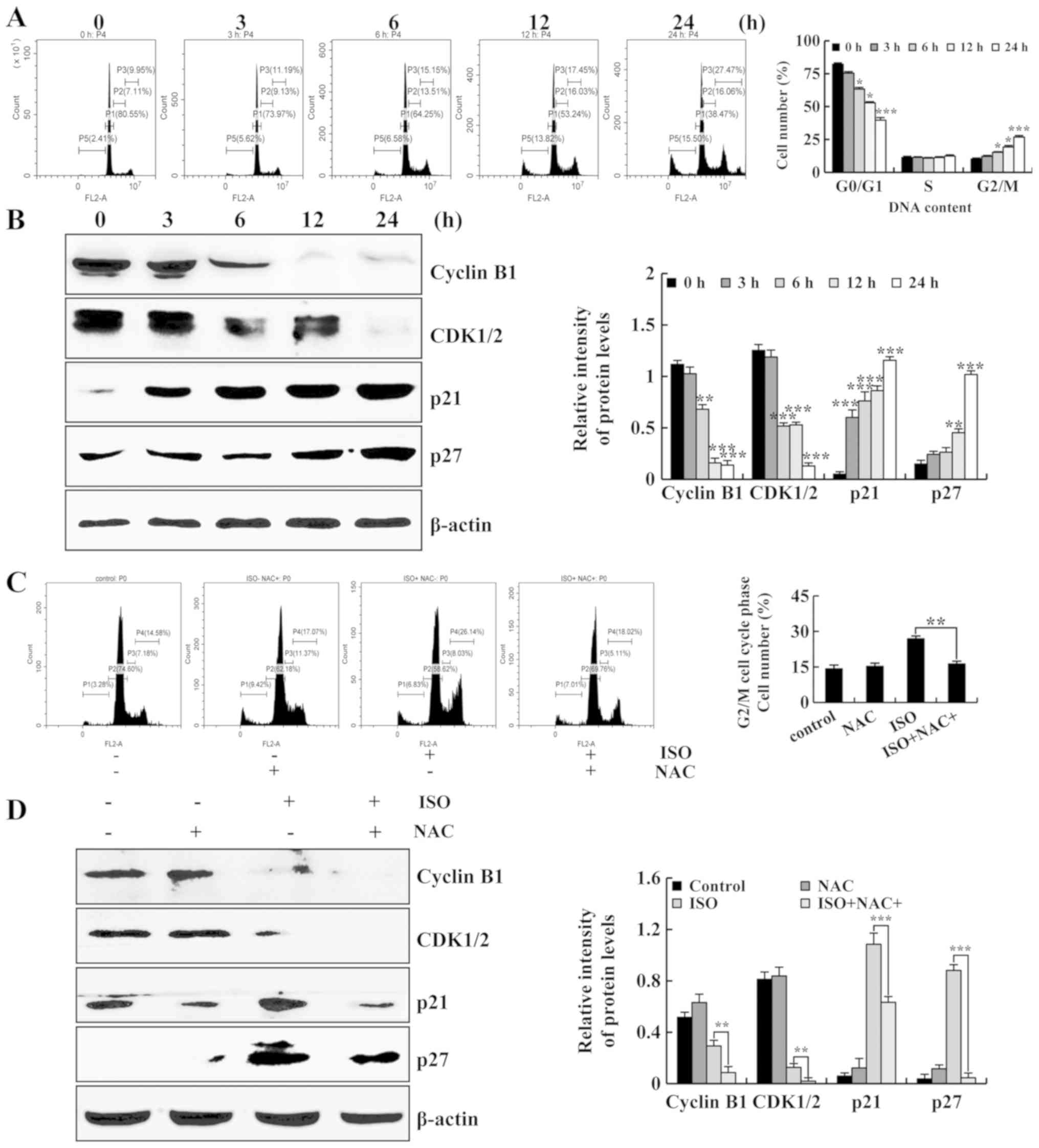
ISO leads to the G2/M cell cycle arrest
of the A549 cells
To determine the effects of ISO on lung cancer cell
cycle arrest, the A549 cells were treated with ISO for different
periods of time (3, 6, 12 and 24 h) followed by detection with flow
cytometry. As shown in Fig. 6A,
the population of A549 cells in the G2/M phase was markedly
increased. Subsequently, the expression of G2/M cell cycle-related
proteins was detected by western blot analysis. It was found that
the protein expression levels of cyclin B1 and CDK1/2 were
decreased. Moreover, the protein expression levels of p21 and p27
were increased. These data indicated that ISO caused the G2/M cell
cycle arrest of the A549 cells. Furthermore, the cells were
pre-treated with NAC, and the population of A549 cells in the G2/M
phase was measured by flow cytometry. As shown in Fig. 6C, compared with the ISO group, the
population of G2/M phase cells was significantly decreased in the
ISO + NAC group. Moreover, NAC significantly downregulated the
expression levels of cyclin B1 and CDK1/2, and upregulated the
expression levels of p21 and p27 compared to ISO treatment alone
(Fig. 6D). Taken together, these
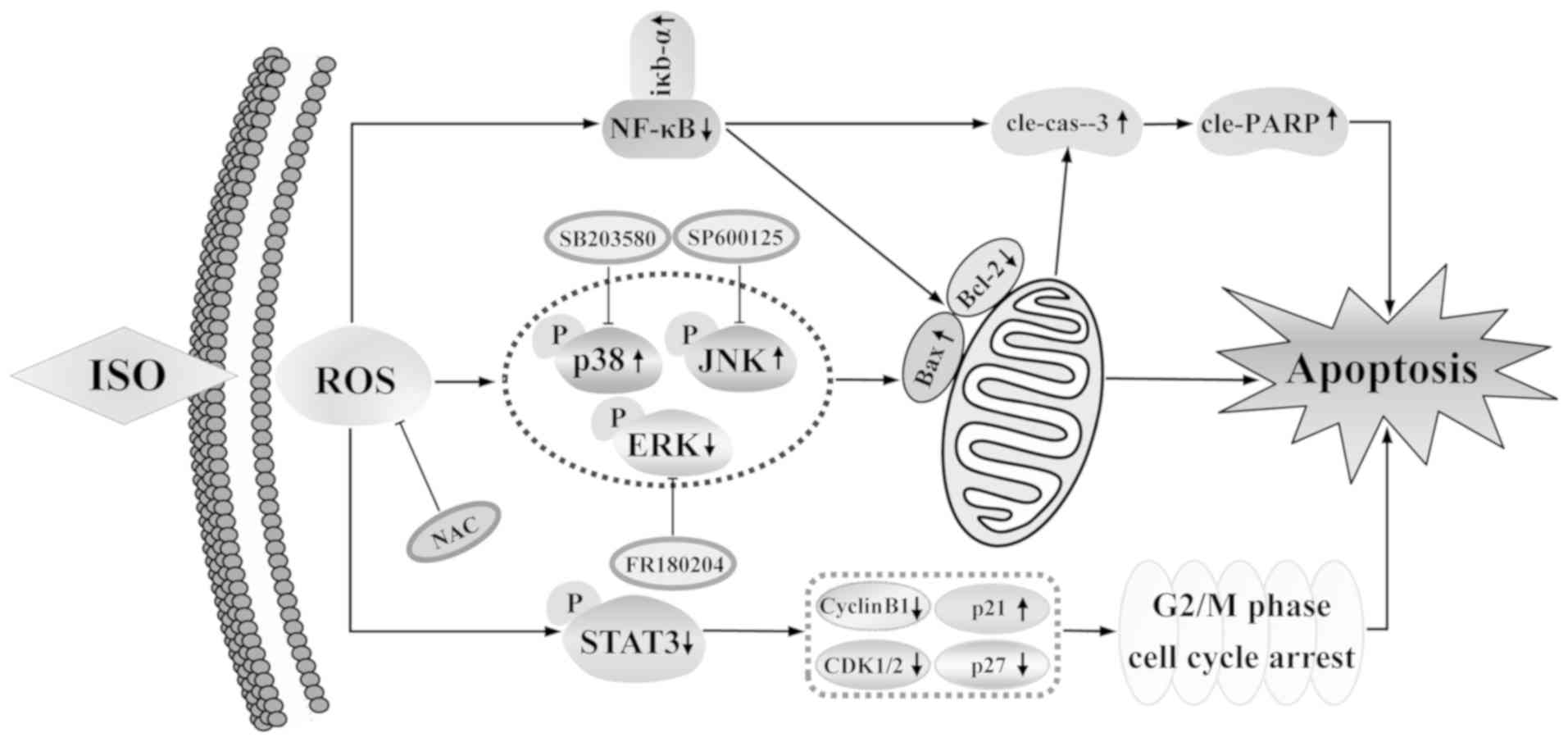
results suggested that ISO induced apoptosis and G2/M cell cycle
arrest via the ROS-mediated MAPKs/STAT3/NF-κB signaling pathways in
A549 human lung cancer cells (Fig.
7).
Discussion
Traditional Chinese herbal medicine is one of the
topics in the antitumor drug development arena. Certain studies
have noted that herbs with anticancer effects can be separated into
several compounds including flavonoids (25,26).
ISO, a naturally C-glycosyl flavone, has been shown to inhibit the
proliferation of HepG2 liver cancer cells, with no obvious
cytotoxicity on HL-7702 normal human liver cells (27). Several studies have demonstrated
that the anticancer mechanisms of ISO involve the inhibition of
cancer cell proliferation, the promotion of oxidative stress, the
induction of cell cycle arrest, and ultimately, in the induction of
apoptosis (22,27-34).
However, the experimental data of the present study revealed that
ISO induced ROS accumulation, apoptosis and G2/M cycle arrest in
the lung cancer cells, and the present study also investigated the
relevant molecular mechanisms. It was found that ROS accumulation
induced by ISO activated the MAPK signaling pathway and further
inhibited the STAT3 and NF-κB signaling pathways. ISO also exerted
effects on nuclear transcription factors, such as the nuclear
translocation of proteins (STAT3, p-NF-κB, NF-κB and p-IκB),
finally causing the apoptosis of A549 lung cancer cells. On the
other hand, ROS accumulation regulated the expression of CDK and
cyclin, causing the G2/M phase arrest of the A549 lung cancer
cells. Finally, a theoretical basis was provide for the drug design
and anticancer drug development by ISO. It has previously found
that the IC50 value of the HT-29 cells was 125
µM, the IC50 of HepG2 cells was 80 µM, the
IC50 of PATU-8988 cells was 300 µM, and the
IC50 of PANC-1 cells was 120 µM (22,27,29,31).
The present study demonstrated that ISO decreased A549 cell
viability in dose-dependent and time-dependent manner; moreover, no
evident side-effects were observed on the normal cells in the 5-FU
and cisplatin group; the IC50 value of the A549 cells
was 46.81 μM, the IC50 of the NCI-H23 cells was 63.88
µM and the IC50 of the NCI-H460 cells was 81.69
µM (Fig. 1 and Table I). In addition, following treatment
with ISO for 24 h, the A549 cells exfoliated from the plate and
exhibited morphology similar to apoptosis (Fig. 2A) (35). To further define the underlying
mechanisms of ISO, the effects of ISO on the induction of apoptosis
of the A549 cells were then investigated.
Apoptosis plays a crucial role in the process of
cell proliferation, differentiation and death.
Mitochondrial-dependent apoptosis is an intrinsic apoptosis,
causing the release of cytochrome c, which results in caspase-3
cleavage and ultimately leads to apoptosis (36). Bcl-2 family members are major
regulators of the mitochondrial release of cytochrome c and can
alter their conformation and form mitochondrial permeability
transition pores in the mitochondrial outer membrane, releasing
cytochrome c from the mitochondria into the cytosol (37,38).
In addition, previous studies have demonstrated that Bcl-2 and Bax
control programmed cell apoptosis (39,40).
The results of the present study revealed that ISO significantly
inhibited the expression levels of Bcl-2 and increased the
expression of Bax, cle-cas-3 and cle-PARP (Fig. 2D). Therefore, ISO-induced apoptosis
is associated with mitochondrial-dependent pathways.
The activation of the MAPK signaling pathway plays a
key role in the apoptosis of cancer cells induced by natural
compounds (41,42). Accumulating evidence has identified
that the MAPK, STAT3 and NF-κB signaling pathways play important
roles in cell apoptosis (43,44).
The results of the present study demonstrated that ISO activated
the p38 and JNK pathways, inhibiting the ERK, STAT3 and NF-κB
signaling pathways. In addition, the expression levels of p-STAT3
were decreased following the addition of p38 and JNK inhibitors (10
µM) and were increased following the addition of an ERK
inhibitor (10 µM), indicating that MAPK was involved in the
regulation of the STAT3 signaling pathway in A549 lung cancer cells
(Fig. 4) (32,45-48).
A number of studies have demonstrated that some
natural flavonoids appear as pro-oxidants or antioxidants depending
upon the target cell (49,50). The previous studies have
demonstrated that ISO can upregulate ROS levels in HepG2
hepatocellular carcinoma cells to induce cell apoptosis (20). The results revealed that ISO
upregulated the levels of ROS. In addition, following treatment of
A549 cells with NAC the number of apoptotic cells was significantly
reduced (Fig. 5B). It has been
reported that ROS can induce apoptosis as a second messenger of
MAPK and NF-κB transcription factors in cancer cells. NF-κB factor
is localized in the cytoplasm and is sequestered by IκB molecules.
Under some stimulating conditions, IκB undergoes ubiquitination and
degradation, resulting in the translocation of NF-κB dimers into
the nucleus (51-53). In the present study, it was found
that ROS was involved in ISO-induced MAPK/STAT3/NF-κB signaling
pathways as an upstream signal. Furthermore, NAC regulated the
activation of the MAPK, STAT3 and NF-κB signaling pathways
(Fig. 5C). The results also
revealed that ISO decreased the expression levels of STAT3,
p-NF-κB, NF-κB, p-IκB and IκB-α in the nucleus (Fig. 5D). These results indicated that ROS
activated the MAPK, STAT3 and NF-κB signaling pathways on the
ISO-induced apoptosis of A549 cells.
The balance of the cell cycle is critical for
maintaining intracellular stability; however, when cells are
damaged, the cell cycle is arrested by various mechanisms, such as
the expression of CDKs and the inhibition of cyclins (54-56).
In the present study, the results of flow cytometry revealed that
the number of cells in the G2/M phase increased following ISO
treatment and the number of cells in the G0/G1 phase decreased.
Western blot analysis revealed that G2/M phase-associated protein
expression levels of CDK1/2 and cyclin B1 decreased following ISO
treatment. In addition, the expression levels of p21 and p27
proteins were increased. To summarize, ISO induced the cell cycle
arrest of A549 lung cancer cells in the G2/M phase.
In conclusion, the present study demonstrated that,
ISO upregulated intracellular ROS levels, caused G2/M cell cycle
arrest, and induced apoptosis by regulating the MAPK/STAT3/NF-κB
signaling pathway in A549 human lung cancer cells. These results
demonstrate the possibility of ISO as a potential treatment agent
for lung cancer.
Funding
The present study was funded by the Heilongjiang
Farms and Land Reclamation Administration Support Project for Key
Scientific Research (grant no. HKKYZD190705), the Heilongjiang Bayi
Agricultural University Support Program for 'San Zong' (grant no.
TDJH201905), Heilongjiang Touyan Innovation Team Program
(2019HTY078), the Natural Science Foundation of Heilongjiang
Province of China (QC2015121) and the Scientific Research
Innovation Program for College Graduates of Heilongjiang Bayi
Agricultural University (YJSCX2019-Y69).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CHJ and DJZ conceived and designed the experiments.
WTX and GNS wrote the manuscript and participated in the
experiments. TZL and YZ assessed the cytotoxic effects of the
drugs. TZ and HX performed the cell cycle and apoptotic analyses.
WBZ performed the western blot analysis. YNL performed the
signaling analysis. All authors read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reinmuth N, Gröschel A, Schumann C,
Sebastian M, Wiewrodt R and Reck M: Updated recommendation for
treatment of metastatic non-small cell lung cancer. Pneumologie.
72:138–154. 2018.In German.
|
|
3
|
Xu Z, Mei J and Tan Y: Baicalin attenuates
DDP (cisplatin) resistance in lung cancer by downregulating MARK2
and p-Akt. Int J Oncol. 50:93–100. 2017. View Article : Google Scholar
|
|
4
|
Zhou Y and Ho WS: Combination of
liquiritin, isoliquiritin and isoliquirigenin induce apoptotic cell
death through upregulating p53 and p21 in the A549 non-small cell
lung cancer cells. Oncol Rep. 31:298–304. 2014. View Article : Google Scholar
|
|
5
|
Elango R1, Athinarayanan J, Subbarayan VP,
Lei DKY and Alshatwi AA: Hesperetin induces an apoptosis-triggered
extrinsic pathway and a p53-independent pathway in human lung
cancer H522 cells. J Asian Nat Prod Res. 6:559–569. 2018.
View Article : Google Scholar
|
|
6
|
Chang JH, Lai SL, Chen WS, Hung WY, Chow
JM, Hsiao M, Lee WJ and Chien MH: Quercetin suppresses the
metastatic ability of lung cancer through inhibiting
Snail-dependent Akt activation and Snail-independent ADAM9
expression pathways. Biochim Biophys Acta Mol Cell Res.
1864:1746–1758. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang DX and Gutterman DD: Mitochondrial
reactive oxygen species-mediated signaling in endothelial cells. Am
J Physiol Heart Circ Physiol. 292:H2023–H2031. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang W and Liu HT: MAPK signal pathways
in the regulation of cell proliferation in mammalian cells. Cell
Res. 12:9–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sheng W, Chen C, Dong M, Wang G, Zhou J,
Song H, Li Y, Zhang J and Ding S: Calreticulin promotes EGF-induced
EMT in pancreatic cancer cells via Integrin/EGFR-ERK/MAPK signaling
pathway. Cell Death Dis. 8:pp. e31472017, View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu H, Lee H, Herrmann A, Buettner R and
Jove R: Revisiting STAT3 signalling in cancer: New and unexpected
biological functions. Nat Rev Cancer. 14:736–746. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nguyen TT, Ung TT, Li S, Lian S, Xia Y,
Park SY and Do Jung Y: Metformin inhibits lithocholic acid-induced
interleukin 8 upregulation in colorectal cancer cells by
suppressing ROS production and NF-kB activity. Sci Rep. 9:20032019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu D, Zhou WY, Lin XT, Fang L and Xie CM:
Bufalin induces apoptosis via mitochondrial ROS-mediated caspase-3
activation in HCT-116 and SW620 human colon cancer cells. Drug Chem
Toxicol. 42:444–450. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu WT, Shen GN, Luo YH, Piao XJ, Wang JR,
Wang H, Zhang Y, Li JQ, Feng YC, Zhang Y, et al: New naphthalene
derivatives induce human lung cancer A549 cell apoptosis via
ROS-mediated MAPKs, Akt, and STAT3 signaling pathways. Chem Biol
Interact. 304:148–157. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang JR, Shen GN, Luo YH, Piao XJ, Shen M,
Liu C, Wang Y, Meng LQ, Zhang Y, Wang H, et al: The compound
2-(naphthalene-2-thio)-5,8-dimethoxy-1,4-naphthoquinone induces
apoptosis via reactive oxygen species-regulated mitogen-activated
protein kinase, protein kinase B, and signal transducer and
activator of transcription 3 signaling in human gastric cancer
cells. Drug Dev Res. 79:295–306. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiang Y, Ye W, Huang C, Lou B, Zhang J, Yu
D, Huang X, Chen B and Zhou M: Brusatol inhibits growth and induces
apoptosis in pancreatic cancer cells via JNK/p38
MAPK/NF-κb/Stat3/Bcl-2 signaling pathway. Biochem Biophys Res
Commun. 487:820–826. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Peng J, Fan G, Hong Z, Chai Y and Wu Y:
Preparative separation of isovitexin and isoorientin from Patrinia
villosa Juss by high-speed counter-current chromatography. J
Chromatogr A. 1074:111–115. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Prinz S, Ringl A, Huefner A, Pemp E and
Kopp B: 4'''-Acetylvitexin-2''-O-rhamnoside, isoorientin, orientin,
and 8-methoxykaempferol-3-O-glucoside as markers for the
differentiation of Crataegus monogyna and Crataegus pentagyna from
Crataegus laevigata (Rosaceae). Chem Biodivers. 4:2920–2931. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Budzianowski J, Budzianowska A and Kromer
K: Naphthalene glucoside and other phenolics from the shoot and
callus cultures of Drosophyllum lusitanicum. Phytochemistry.
61:421–425. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yuan L, Li X, He S, Gao C, Wang C and Shao
Y: Effects of natural flavonoid isoorientin on growth performance
and gut microbiota of mice. J Agric Food Chem. 66:9777–9784. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yuan L, Wu Y, Ren X, Liu Q, Wang J and Liu
X: Isoorientin attenuates lipopolysaccharide-induced
pro-inflammatory responses through down-regulation of ROS-related
MAPK/NF-κB signaling pathway in BV-2 microglia. Mol Cell Biochem.
386:153–165. 2014. View Article : Google Scholar
|
|
21
|
Lee W, Ku SK and Bae JS: Vascular barrier
protective effects of orientin and isoorientin in LPS-induced
inflammation in vitro and in vivo. Vascul Pharmacol. 62:3–14. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gundogdu G, Dodurga Y, Elmas L, Tasci SY
and Karaoglan ES: Investigation of the anticancer mechanism of
isoorientin isolated from Eremurus spectabilis leaves via cell
cycle pathways in HT-29 human colorectal adenocarcinoma cells.
Eurasian J Med. 50:168–172. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Daniel B, Longley, Harkin D Paul and
Johnston Patrick G: 5-fluorouracil: Mechanisms of Action and
Clinical Strategies. Nat Rev Cancer. 5:330–338. 2003.
|
|
24
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rashed KN, Ćirić A, Glamočlija J, Calhelha
RC, Ferreira IC and Soković M: Antimicrobial activity, growth
inhibition of human tumour cell lines, and phytochemical
characterization of the hydromethanolic extract obtained from
Sapindus saponaria L. aerial parts BioMed Res Int.
2013:6591832013.
|
|
26
|
Li L, Henry GE and Seeram NP:
Identification and bioactivities of resveratrol oligomers and
flavonoids from Carex folliculata seeds. J Agric Food Chem.
57:7282–7287. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yuan L, Wang J, Xiao H, Xiao C, Wang Y and
Liu X: Isoorientin induces apoptosis through mitochondrial
dysfunction and inhibition of PI3K/Akt signaling pathway in HepG2
cancer cells. Toxicol Appl Pharmacol. 265:83–92. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Czemplik M, Mierziak J, Szopa J and Kulma
A: Flavonoid C-glucosides derived from flax straw extracts reduce
human breast cancer cell growth in vitro and induce apoptosis.
Front Pharmacol. 7:282–295. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin X, Wei J, Chen Y, He P, Lin J, Tan S,
Nie J, Lu S, He M, Lu Z, et al: Isoorientin from Gypsophila elegans
induces apoptosis in liver cancer cells via mitochondrial-mediated
pathway. J Ethnopharmacol. 187:187–194. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vadde R, Radhakrishnan S, Reddivari L and
Vanamala JK: Triphala extract suppresses proliferation and induces
apoptosis in human colon cancer stem cells via suppressing
c-Myc/Cyclin D1 and elevation of Bax/Bcl-2 ratio. BioMed Res Int.
2015:6492632015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ye T, Su J, Huang C, Yu D, Dai S, Huang X,
Chen B and Zhou M: Isoorientin induces apoptosis, decreases
invasiveness, and downregulates VEGF secretion by activating AMPK
signaling in pancreatic cancer cells. OncoTargets Ther.
9:7481–7492. 2016. View Article : Google Scholar
|
|
32
|
Yuan L, Wang J, Xiao H, Wu W, Wang Y and
Liu X: MAPK signaling pathways regulate mitochondrial-mediated
apoptosis induced by isoorientin in human hepatoblastoma cancer
cells. Food Chem Toxicol. 53:62–68. 2013. View Article : Google Scholar
|
|
33
|
Yuan L, Wang Y, Wang J, Xiao H and Liu X:
Additive effect of zinc oxide nanoparticles and isoorientin on
apoptosis in human hepatoma cell line. Toxicol Lett. 225:294–304.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yuan L, Wei S, Wang J and Liu X:
Isoorientin induces apoptosis and autophagy simultaneously by
reactive oxygen species (ROS)-related p53, PI3K/Akt, JNK, and p38
signaling pathways in HepG2 cancer cells. J Agric Food Chem.
62:5390–5400. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Na D, Song Y, Jiang CG, Sun Z, Xu YY, Wang
ZN, Zhao ZZ and Xu HM: Induction of apoptosis in human peritoneal
mesothelial cells by gastric cancer cell supernatant promotes
peritoneal carcinomatosis. Tumour Biol. 35:8301–8307. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Morales-Cruz M, Figueroa CM,
González-Robles T, Delgado Y, Molina A, Méndez J, Morales M and
Griebenow K: Activation of caspase-dependent apoptosis by
intracellular delivery of Cytochrome c-based nanoparticles. J
Nanobiotechnology. 12:332014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang M, Zheng J, Nussinov R and Ma B:
Release of cytochrome c from bax pores at the mitochondrial
membrane. Sci Rep. 7:26352017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shimizu S, Narita M and Tsujimoto Y and
Tsujimoto Y: Bcl-2 family proteins regulate the release of
apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature.
399:483–487. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Oltvai ZN, Milliman CL and Korsmeyer SJ:
Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that
accelerates programmed cell death. Cell. 74:609–619. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Korsmeyer SJ: BCL-2 gene family and the
regulation of programmed cell death. Cancer Res. 59(Suppl):
1693s–1700s. 1999.PubMed/NCBI
|
|
41
|
Park SG, Kim SH, Kim KY, Yu SN, Choi HD,
Kim YW, Nam HW, Seo YK and Ahn SC: Toyocamycin induces apoptosis
via the crosstalk between reactive oxygen species and p38/ERK MAPKs
signaling pathway in human prostate cancer PC-3 cells. Pharmacol
Rep. 69:90–96. 2017. View Article : Google Scholar
|
|
42
|
Chen X and Chen J: miR-3188 Regulates Cell
Proliferation, Apoptosis, and Migration in Breast Cancer by
Targeting TUSC5 and Regulating the p38 MAPK Signaling Pathway.
Oncol Res. 26:363–372. 2018. View Article : Google Scholar
|
|
43
|
Zou F, Mao R, Yang L, Lin S, Lei K, Zheng
Y, Ding Y, Zhang P, Cai G, Liang X, et al: Targeted deletion of
miR-139-5p activates MAPK, NF-κB and STAT3 signaling and promotes
intestinal inflammation and colorectal cancer. FEBS J.
283:1438–1452. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Meng LQ, Liu C, Luo YH, Piao XJ, Wang Y,
Zhang Y, Wang JR, Wang H, Xu WT, Liu Y, et al: Quinalizarin exerts
an anti-tumour effect on lung cancer A549 cells by modulating the
Akt, MAPK, STAT3 and p53 signalling pathways. Mol Med Rep.
17:2626–2634. 2018.
|
|
45
|
Hwang HS, Park SJ, Lee MH and Kim HA:
MicroRNA-365 regulates IL-1β-induced catabolic factor expression by
targeting HIF-2α in primary chondrocytes. Sci Rep. 7:178892017.
View Article : Google Scholar
|
|
46
|
Fu Y, O'Connor LM, Shepherd TG and
Nachtigal MW: The p38 MAPK inhibitor, PD169316, inhibits
transforming growth factor beta-induced Smad signaling in human
ovarian cancer cells. Biochem Biophys Res Commun. 310:391–397.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hour MJ, Lee KT, Wu YC, Wu CY, You BJ,
Chen TL and Lee HZ: A novel antitubulin agent, DPQZ, induces cell
apoptosis in human oral cancer cells through Ras/Raf inhibition and
MAP kinases activation. Arch Toxicol. 87:835–846. 2013. View Article : Google Scholar
|
|
48
|
Ito K, Matsuzaki M, Sasahara T, Shin M and
Yayama K: Orthovanadate-Induced Vasoconstriction of Rat Mesenteric
Arteries Is Mediated by Rho Kinase-Dependent Inhibition of Myosin
Light Chain Phosphatase. Biol Pharm Bull. 38:1809–1816. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yang S, Zhang H, Yang X, Zhu Y and Zhang
M: Evaluation of antioxidative and antitumor activities of
extracted flavonoids from Pink Lady apples in human colon and
breast cancer cell lines. Food Funct. 6:3789–3798. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang Y, Qian J, Cao J, Wang D, Liu C, Yang
R, Li X and Sun C: Antioxidant capacity, anticancer ability and
flavonoids composition of 35 citrus (Citrus reticulata Blanco)
varieties. Molecules. 22:E11142017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zuo D, Zhou Z, Wang H, Zhang T, Zang J,
Yin F, Sun W, Chen J, Duan L, Xu J, et al: Alternol, a natural
compound, exerts an anti-tumour effect on osteosarcoma by
modulating of STAT3 and ROS/MAPK signalling pathways. J Cell Mol
Med. 21:208–221. 2017. View Article : Google Scholar
|
|
52
|
Meng LQ, Wang Y, Luo YH, Piao XJ, Liu C,
Wang Y, Zhang Y, Wang JR, Wang H, Xu WT, et al: Quinalizarin
induces apoptosis through reactive oxygen species (ROS)-mediated
mitogen-activated protein kinase (MAPK) and signal transducer and
activator of transcription 3 (STAT3) signaling pathways in
colorectal cancer cells. Med Sci Monit. 24:3710–3719. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liu C, Shen GN, Luo YH, Piao XJ, Jiang XY,
Meng LQ, Wang Y, Zhang Y, Wang JR, Wang H, et al: Novel
1,4-naphthoquinone derivatives induce apoptosis via ROS-mediated
p38/MAPK, Akt and STAT3 signaling in human hepatoma Hep3B cells.
Int J Biochem Cell Biol. 96:9–19. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Cheng CW, Leong KW, Ng YM, Kwong YL and
Tse E: The peptidyl-prolyl isomerase PIN1 relieves cyclin-dependent
kinase 2 (CDK2) inhibition by the CDK inhibitor p27. J Biol Chem.
292:21431–21441. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bonelli P, Tuccillo FM, Borrelli A,
Schiattarella A and Buonaguro FM: CDK/CCN and CDKI alterations for
cancer prognosis and therapeutic predictivity. BioMed Res Int.
2014:3610202014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sarita Rajender P, Ramasree D, Bhargavi K,
Vasavi M and Uma V: Selective inhibition of proteins regulating
CDK/cyclin complexes: Strategy against cancer - a review. J Recept
Signal Transduct Res. 30:206–213. 2010. View Article : Google Scholar : PubMed/NCBI
|