Introduction
Head and neck cancers (HNCs), in general, have a
poor prognosis with a worldwide 5-year survival rate of <50%
(1-3). In part, this prognosis is due to the
fact that numerous HNC patients with locoregionally advanced,
difficult-to-treat, inoperable, recurrent and drug-resistant tumors
may be ineligible for the standard of care surgery or may not
tolerate chemo-therapy and radiation (3-6). As
a standard of care therapy, cisplatin is a primary treatment for
HNC, breast, cervical, bladder, brain and other cancers. However,
some patients do not tolerate the current standard dose regimen and
exhibit increased side-effects or drug resistance (5-8).
Cisplatin, an antineoplastic or anticancer drug,
binds to DNA purine bases and interferes with the cellular repair
mechanisms, irreparably damaging the DNA and subsequently inducing
apoptosis in the cell (8,9). This approach does not limit cell
death to the locoregional area of the tumor. Consequently, normal
tissue and healthy cells throughout the body are also affected,
resulting in one of the biggest patient-centered challenges with
cisplatin: side effects. Not all patients will experience all of
the known or listed side-effects, but some of the most common
side-effects include nausea, vomiting, nephrotoxicity and
ototoxicity, with the latter two being the most severe (10-12).
The severity of some of these side-effects prevents
the more widespread use of cisplatin (11-13).
Additional complications with this form of treatment are the
possibility of drug resistance, aggressive recurrence and
metastasis (3,11,14,15).
Following the onset of drug resistance, these patients usually do
not survive past one year (6,16,17).
Poor survival rates and outcomes are among the reasons that the
scientific community is beginning to explore strategies to increase
the efficacy of cisplatin at lower doses by combining it with other
treatment modalities and manipulating the dosing schedule (6,10,18-21).
Dosing typically depends on the patient's height, weight, general
health and any specific health condition. The standard cisplatin
dose and schedule are usually 3 cycles of 100 mg/m2
every 3 to 4 weeks when used individually as a monotreatment for
HNC patients (22-24). Recent studies on manipulating the
cisplatin dosage have compared high-dose cisplatin with a
cisplatin-based combination therapy (radiation, chemotherapy, or
other intervention) to decrease side-effects, increase treatment
efficacy and improve patient outcomes and overall survival rates
(8,10,11,21,24-27).
Nanoparticles and nanomaterials have been used in
the treatment of cancer to enhance targeted drug delivery and tumor
specificity to minimize side-effects (28-32).
The present study focuses on a particular class of laser-activated
nanoparticles, specifically, a thermal ablation platform therapy
using near-infrared excitation of gold nanorods (AuNRs),
laser-activated nanotherapy (LANT). This LANT platform is not
designed to enhance targeting, but specifically to induce cell
death at the site of laser-activation for the sole purpose of its
therapeutic effect. LANT has demonstrated almost 100% cell death
in vitro and approximately 100% tumor regression in
vivo (33). However, to the
best of our knowledge, no such platform has been approved by the U.
S. Food and Drug Administration (FDA) for humans to date. LANT
presents an opportunity to override some of the biological
obstacles encountered within the tumor microenvironment and with
cisplatin specificity, efficacy, and treatment time. The present
study investigates the mechanisms through which LANT, as part of an
adjuvant therapy regimen, can enhance the therapeutic efficacy of
lower doses of cisplatin for the treatment of 3 head and neck
squamous cell carcinoma (HNSCC) cell lines, Detroit 562, FaDu and
CAL 27.
Materials and methods
Materials
Gold (III) chloride trihydrate (HAuCl4),
cetyltri-methylammonium bromide (CTAB), sodium borohydride
(NaBH4), silver nitrate (AgNO3), L-ascorbic
acid, potassium carbonate (K2CO3) and
dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich; Merck
KGaA. Thiol-terminated methoxy poly-(ethylene glycol) (mPEG-SH, MW
5,000K) and cisplatin were purchased from Creative PEGWorks and
Selleck Chemicals, respectively. UltraPure water (18 MΩ) was used
for gold nanorod preparation.
Cell lines
In total, 3 human HNSCC cell lines were used in this
study: a human pharyngeal carcinoma cell line, Detroit 562, and 2
human squamous cell carcinoma cell lines, FaDu and CAL 27. The cell
lines were purchased from the American Type Culture Collection
(ATCC). Upon receiving the cell lines from ATCC, the passage number
was set at one and cells at passage 3-7 were used. The cells tested
negative for mycoplasma. The HNSCC cell lines were cultured in
Dulbecco's modified Eagle medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc.) containing 10% v/v heat- inactivated fetal bovine
serum (Corning, Inc.), supplemented with 4.5 g/l glucose,
L-glutamine and penicillin-streptomycin (Corning, Inc.) and
incubated at 37°C in a 5% CO2 humidified atmosphere.
Cell death calculation from cell
viability
Cell viability was determined using PrestoBlue™ Cell
Viability Reagent (Thermo Fisher Scientific, Inc.). Briefly, the
culture medium containing AuNRs or the drug was removed and
replaced with culture medium containing PrestoBlue™ Cell Viability
Reagent (10% v/v) and the cells were incubated at 37°C for 30 min.
The plate was read at a 560/590 nm excitation/emission wavelength
using the SpectraMax® M5 Microplate Reader (Molecular
Devices, LLC). The fluorescence reading of the blank was subtracted
from all samples. The fluorescence readings from the test samples
were divided by those of the control and multiplied by 100 to yield
the percentage of cell viability. The percentage of cell death was
then calculated by subtracting the percentage of cell viability
from 100% (see formula below). The results are expressed as the
means ± standard deviation of sextuplet (n=6) in each treatment
group.
Preparation of AuNRs
The seed-mediated growth of AuNRs was performed at
25°C using a freshly prepared aqueous solution according to our
previously described methods, Green et al (34). Briefly, the PEGylated AuNRs
solution was centrifuged at 7,600 × g for 20 min at 25°C and
re-dispersed in deionized water to remove excess CTAB and
non-specifically bound mPEG-SH molecules. The PEGylated AuNRs were
characterized by UV/VIS Spectrophotometer UV5Nano (Mettler Toledo,
LLC) to determine the absorption and by Transmission Electron
Microscope to verify consistency in shape and size. The
zeta-potential of the AuNRs in PBS were evaluated using a Zetasizer
Nano-ZS (Malvern Panalytical, Ltd.).
LANT in vitro
LANT was performed according to our previously
described methods, Green et al (34). A total of 6×104
cells/well were seeded in 96-well culture plates and treated at
approximately 100% confluence. The concentration of the AuNRs were
calculated by the Beer-Lambert Law based on the previously
determined molar absorptivity, ε=5 ×109 l/mol/cm for 808
nm and aspect ratio, R=4 (34).
Serially diluted AuNRs (25 µl) were added to each well and
exposed to a diode near-infrared (NIR) laser (Information
Unlimited) with an 808 nm wavelength at 1.875 W/cm2
(spot size around 4 mm) for 4 min. Immediately, within 1-5 min
after the laser excitation of the AuNRs, the percentage of cell
death was determined by the PrestoBlue Assay, as described
above.
Cell death induced by cisplatin
The HNSCC cell lines, Detroit 562, FaDu and CAL 27,
were seeded in 96-wells plates at 1×104 cells/well and
allowed to adhere overnight. The culture medium was then replaced
with fresh medium containing cisplatin at various concentrations,
0.05-40 µM, and the cells were incubated at 37°C for 48 h.
The percentage of cell death was determined by the PrestoBlue
Assay, as described above.
Combination of cisplatin and LANT in
vitro
The HNSCC cell lines were seeded in 96-wells plates
at 1×104 cells/well and allowed to adhere overnight. The
culture medium was then replaced with fresh medium containing
cisplatin at 2 concentrations (1 or 2 µM), and the cells
were incubated with cisplatin at 37°C for 48 h. Immediately after
the 48-h incubation, the medium containing cisplatin was removed,
and the cells were washed with PBS once. Subsequently, 25 µl
of AuNRs in PBS at the concentration of 2.5 or 5 nM were added to
the cisplatin-treated cells and exposed to 4 min of 808 nm
wavelength NIR irradiation at 1.875 W/cm2. As described
above, the final percentage of cell death induced by the Cis + LANT
combination treatment was evaluated using the PrestoBlue Assay
immediately following LANT treatment. Each treatment combination
was performed in quadruplicate (n=4), and the results are expressed
as the means ± standard deviation.
Calculations for EC50 and Cisplatin dose
reduction
The half-effective concentrations (EC50) of
cisplatin and LANT for the 3 HNSCC cell lines were calculated with
the IC50 calculator provided by AAT Bioquest® according
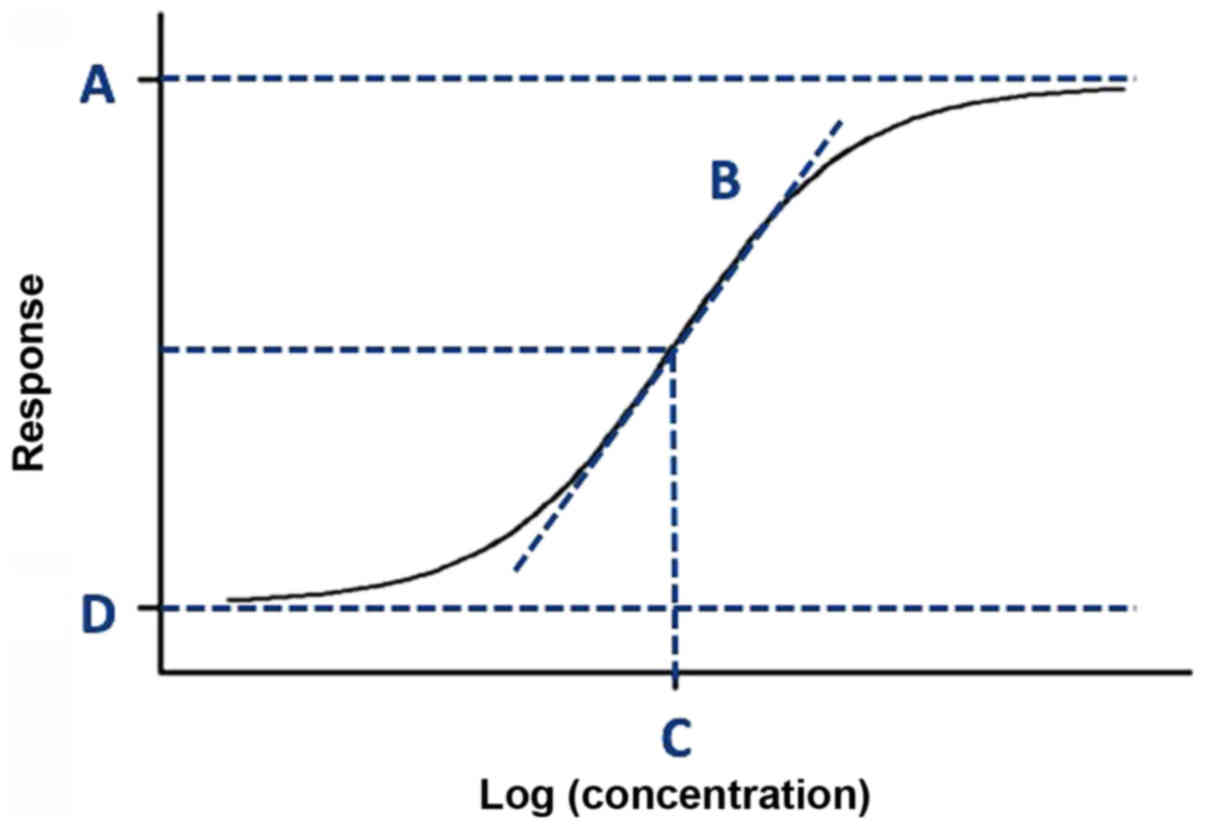
to the Four-Parameter Logistic (4PL) model equation (Equation 1) describing the sigmoid-shaped
response pattern as shown in Fig.
1 (35):
where y (x) is the percentage of cell death induced by the
treatment that corresponds to each 'x'; 'x' is the concentration of
the treatment used to establish the dose-response curve in
logarithmic form; 'A' is the highest percentage of cell death on
the dose-response curve (ymax); 'B' is the hill slope of
the dose-response curve; 'C' is the x-value (concentration)
corresponding to the midway between 'A' and 'D' on the
dose-response curve (i.e., the EC50 value); and 'D' is the lowest
percentage of cell death on the dose-response curve
(ymin) (
Fig. 2).
To describe the synergistic therapeutic efficacy,
the 4PL model equation was used to estimate the decrease in the
cisplatin dose, comparing the combination treatment to the
monotreatment. As LANT is not a drug, the combination index (CI)
and other traditional methods for calculating the synergistic
effects of a combination therapy did not apply to this study. Thus,
calculating the dose reduction required an evaluation of the
difference in doses at the same percentage of cell death: the
percentage of cell death induced by the Cis + LANT combination
treatment (y′) was evaluated at the same percentage of cell death
caused by the cisplatin monotreatment (y) (i.e., y=y′). Using the
4PL equation, the concentration of cisplatin monotreatment (x) that
would be required to induce the percentage of cell death (y)
equivalent to y′ (the percentage of cell death induced by the Cis +
LANT combination treatment) was determined. The 4PL equation was
also used to determine the dose reduction LANT introduced to
cisplatin.
Cisplatin monotreatment dose reduction
calculation
Equation 1 above
was used to calculate the percentage of cell death induced by each
Cis monotreatment concentration corresponding to each concentration
of Cis monotreatment used to establish the dose-response curve for
the 3 HNSCC cell lines. The measured and calculated values for A,
B, C and D were inserted for the Detroit 562, FaDu and CAL27 cells,
according to Equations 1a,
1b and 1c, respectively:
For Detroit 562 cells,
For FaDu cells,
For CAL 27 cells,
Cis + LANT combination treatment dose
comparison
Equation 2 was
used to calculate the percentage of cell death induced by the
combination, Cis + LANT treatment, that corresponds to each
concentration of Cis used to establish the dose-response curve.
For Cis + PNT combination treatment,
where y′ (x′) is the percentage of cell death induced by the
combination Cis + LANT treatment that corresponds to each 'x'; 'x'
is the concentration of the combination Cis + LANT treatment used
to establish the dose-response curve in logarithmic form; 'A' is
the highest percentage of cell death on the dose-response curve
(y′max); 'B' is the hill slope of the dose-response
curve; 'C' is the x′-value (concentration) corresponding to the
midway between 'A' and 'D' on the dose-response curve (i.e., the
EC50 value); and 'D' is the lowest percentage of cell death on the
dose-response curve (y′min).
Considering that y′ (x′) in Equation 2 was derived from real data, y′
(x′)=y (x) from Equation 1, and
the present study were only interested in the percentage of cell
death that is in common with both Cis monotreatment and Cis + LANT
combination treatment, the y′ (x′) from Equation 2 was substituted for y(x) in
Equation 1 and solved for 'x' to
calculate the Cis monotreatment dose.
Therefore, the dose reduction realized by combining
Cis with PNT was calculated according to Equation 3:
Statistical analysis
To assess differences in cell death percent-ages
across the 6 treatment conditions, a one-way Analysis of Variance
(ANOVA) was performed with post hoc tests using the Bonferroni
correction. The statistical significance was set at P<0.05.
Prior to the ANOVA estimation, statistical tests were performed for
the assumptions of homogeneity of variance and normality using
Bartlett's and Shapiro-Wilk tests, respectively. For these tests,
P>0.05 indicated that these assumptions were met. These
procedures were repeated for each cell line, resulting in a total
of 3 sets of analyses. The comparisons of interest for the present
study are those between cisplatin alone treatments (i.e., 1
µM Cis and 2 µM Cis) and the treatments involving a
combination of the cisplatin and LANT (i.e., 1 µM Cis + 2.5
nM LANT; 1 µM Cis + 5 nM LANT; 2 µM Cis + 2.5 nM
LANT; and 2 µM Cis + 5 nM LANT). All analyses were performed
using R statistical software (R Core Team, 2019).
Results
Effects of LANT monotreatment
The therapeutic efficacy, dose-response curves and
half effective concentration (EC50) were established in
vitro for LANT as a monotherapy for HNSCC. To determine the
percentage of cell death induced by AuNRs alone (Laser OFF)
compared to LANT (Laser ON), an 808 nm NIR laser, for 4 min at
1.875 W/cm2, was used to excite the AuNRs at 8 or 9
concentrations: 0, 2.5, 5, 7.5, 10, 15, 20, 25 and 30 nM. Fig. 2 illustrates the
concentration-dependent cell death induced by LANT monotherapy for
3 HNSCC cell lines, Detroit 562 (Fig.
2A), FaDu (Fig. 2B) and CAL 27
(Fig. 2C). Under the conditions in
this study, LANT induced substantial death in all cell lines
compared to the AuNRs alone (Laser OFF). Increasing the AuNR
concentration in LANT directly increased the percentage of cell
death. Furthermore, the CAL 27 cells were the least sensitive to
LANT at the lower AuNR concentrations (2.5 and 5 nM) compared to
the Detroit 562 and FaDu cells under the same conditions. However,
the CAL 27 cells were the most sensitive to LANT at the higher AuNR
concentrations used for LANT (7.5, 10, 15, 20 and 25 nM) compared
to the Detroit 562 and FaDu cells under the same conditions.
The FaDu cells required a higher EC50 value of LANT
than the Detroit 562 and CAL 27 cells: the EC50 values of LANT for
treating the Detroit 562, FaDu and CAL 27 cells were 8.08, 11.03
and 6.68 nM, respectively (Table
I). Furthermore, the FaDu cells required an additional
treatment condition, at 30 nM, to achieve the approximately 100%
cell death obtained in the other cell lines at 25 nM (Fig. 2). Consistent with previous findings
(34), LANT induced ~100% cell
death in all 3 HNSCC cell lines at 25 nM and higher doses.
 | Table IEC50 values for LANT and cisplatin
mono-treatments. |
Table I
EC50 values for LANT and cisplatin
mono-treatments.
| EC50 | Cell line
|
|---|
| Detroit 562 | FaDu | CAL 27 |
|---|
| LANT (nM) | 8.08 | 11.03 | 6.68 |
| Cisplatin
(µM) | 9.33 | 5.05 | 4.05 |
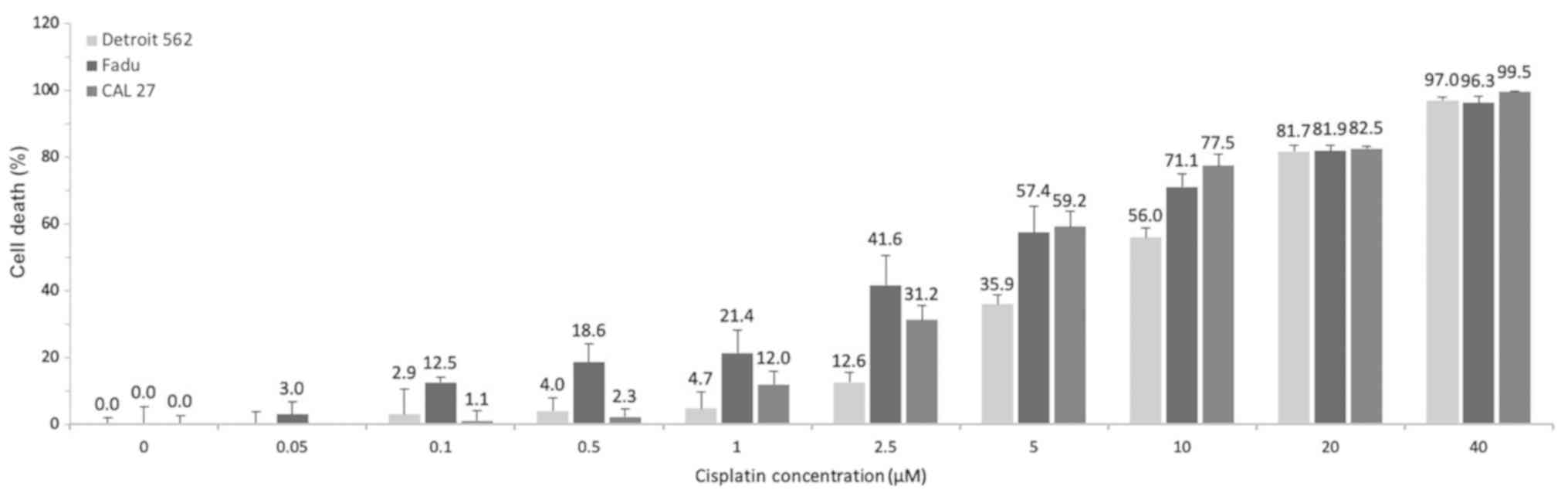
Effects of cisplatin monotreatment
To establish the dose-response curves and EC50 for
cisplatin as a monotherapy for the treatment of the HNSCC cell
lines, Detroit 562, FaDu and CAL 27, the percentage of cell death
induced was determined after incubating the cells with cisplatin
for 48 h at 9 different concentrations ranging from 0.05-40
µM. The concentration-dependent cell death induced by
cisplatin monotherapy is illustrated in Fig. 3.
Increasing the cisplatin concentration was directly
proportional to the increase in the percentage of cell death.
However, administering the high cisplatin doses in humans necessary
to achieve a complete therapeutic response after 48 h would result
in patient intolerance due to increased severe side-effects and
toxicity. The FaDu cells were more sensitive to cisplatin at doses
≤2.5 µM than the Detroit 562 and CAL 27 cells, whereas all 3
cell lines were equally responsive to cisplatin at doses ≥20
µM. The EC50 values of cisplatin for treating the Detroit
562, FaDu, and CAL 27 cells were 9.33, 5.05 and 4.05 µM,
respectively (Table I). In the
present study, 40 µM of cisplatin resulted in approximately
100% cell death in all 3 cell lines during the 48-h treatment
window.
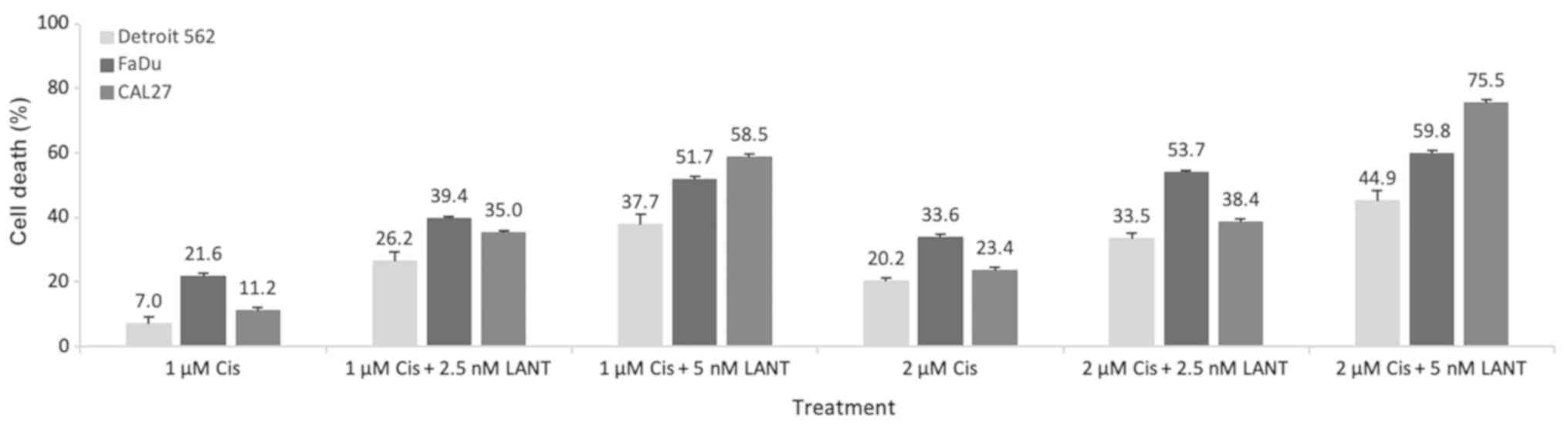
Combination of cisplatin and LANT
treatments
The monotreatment EC50 values that induced 50% cell
death (Table I) informed the dose
selection for the combination experiments to specifically narrow
the focus to low doses for both cisplatin and LANT. To delineate
and emphasize the efficacy of the Cis + LANT combination treatment,
1 and 2 µM of cisplatin were used in the combination
treatment as they were less than half of the concentration of the
lowest cisplatin monotreatment EC50 values for all cell lines (4.05
µM). Likewise, 2.5 and 5 nM of AuNRs for LANT were selected
as they were also less than half of the lowest EC50 values from the
LANT monotreatment (6.68 nM). The percentage of cell death due to
the 4 Cis + LANT combination treatments, (cisplatin at 1 or 2
µM) + (LANT at 2.5 or 5 nM), was significantly higher than
that due to the 2 cisplatin monotreatments (1 or 2 µM) for
all 3 cell lines (Fig. 4).
Descriptive statistics, ANOVA and post
hoc tests
Based on the cell death percentage data shown in
Fig. 4, the descriptive
statistics, mean percentage (Mean), and standard deviation (SD)
were summarized for the 6 treatment groups and 3 cell lines in
Table II. The ANOVA test compared
the means of the 6 treatment groups for 3 cell lines. There was a
statistically significant difference in the means of most groups
for all 3 cell lines. ANOVA and post hoc test outcomes were similar
across all 3 cell types and the results are summarized in Table III. The post hoc analyses results
for all 3 cell lines indicated statistically significant
differences (P<0.05) in the majority of comparisons of interest
between the 6 treatment groups.
 | Table IIDescriptive statistics for cisplatin
monotreatment and Cis + LANT combination treatment outcomes. |
Table II
Descriptive statistics for cisplatin
monotreatment and Cis + LANT combination treatment outcomes.
| Cell line | Statistic | Treatment
|
|---|
| 1 µM
Cis | 1 µM Cis +
2.5 nM LANT | 1 µM Cis + 5
nM LANT | 2 µM
Cis | 2 µM Cis +
2.5 nM LANT | 2 µM Cis + 5
nM LANT |
|---|
| Detroit 562 | Mean | 7.04 | 26.22 | 37.73 | 20.15 | 33.48 | 44.86 |
| SD | 3.64 | 1.74 | 2.68 | 2.09 | 2.59 | 2.03 |
| Obs | 4 | 4 | 4 | 4 | 4 | 4 |
| FaDu | Mean | 21.64 | 39.44 | 51.73 | 33.62 | 53.67 | 59.77 |
| SD | 2.40 | 2.93 | 3.84 | 1.90 | 0.99 | 1.87 |
| Obs | 4 | 4 | 4 | 4 | 4 | 4 |
| CAL27 | Mean | 11.18 | 34.99 | 58.48 | 23.40 | 38.39 | 75.46 |
| SD | 2.15 | 3.58 | 5.50 | 2.22 | 0.97 | 3.91 |
| Obs | 4 | 4 | 4 | 4 | 4 | 4 |
 | Table IIITreatment group comparison. |
Table III
Treatment group comparison.
| First column vs.
second column | Detroit 562
| FaDu
| CAL 27
|
|---|
| Mean Diff | P-value | Mean Diff | P-value | Mean Diff | P-value |
|---|
| 1 µM Cis +
2.5 nM LANT | 1 µM
Cis | 19.18 | <0.0001a | 17.79 | <0.0001a | 23.81 | <0.0001a |
| 1 µM Cis + 5
nM LANT | | 30.69 | <0.0001a | 30.08 | <0.0001a | 47.30 | <0.0001a |
| 2 µM Cis +
2.5 nM LANT | | 26.44 | <0.0001a | 32.02 | <0.0001a | 27.21 | <0.0001a |
| 2 µM Cis + 5
nM LANT | | 37.82 | <0.0001a | 38.13 | <0.0001a | 64.28 | <0.0001a |
| 1 µM Cis +
2.5 nM LANT | 2 µM
Cis | 6.07 | 0.050b | 5.82 | 0.059b | 11.59 | 0.002a |
| 1 µM Cis + 5
nM LANT | | 17.58 | <0.0001a | 18.11 | <0.0001a | 35.08 | <0.0001a |
| 2 µM Cis +
2.5 nM LANT | | 13.33 | <0.0001a | 20.05 | <0.0001a | 14.99 | <0.0001a |
| 2 µM Cis + 5
nM LANT | | 24.71 | <0.0001a | 26.16 | <0.0001a | 52.06 | <0.0001a |
| 2 µM
Cis | 1 µM
Cis | 13.11 | <0.0001a | 11.97 | <0.0001a | 12.22 | 0.001a |
| 1 µM Cis + 5
nM LANT | 1 µM Cis +
2.5 nM LANT | 11.51 | <0.0001a | 12.29 | <0.0001a | 23.49 | <0.0001a |
| 2 µM Cis +
2.5 nM LANT | | 7.26 | 0.012a | 14.23 | <0.0001a | 3.40 | >0.999 |
| 2 µM Cis + 5
nM LANT | | 18.64 | <0.0001a | 20.34 | <0.0001a | 40.47 | <0.0001a |
| 1 µM Cis + 5
nM LANT | 2 µM Cis +
2.5 nM LANT | 4.25 | 0.440 | -1.94 | >0.999 | 20.09 | <0.0001a |
| 2 µM Cis + 5
nM LANT | | 11.38 | <0.0001a | 6.11 | 0.041a | 37.07 | <0.0001a |
| 2 µM Cis + 5
nM LANT | 1 µM Cis + 5
nM LANT | 7.13 | 0.014a | 8.05 | 0.004a | 16.98 | <0.0001a |
Overall, the combination of treatments was
significantly more effective than the corresponding cisplatin
monotreatment. Specifically, the combinations (1 µM Cis +
2.5 nM LANT; 1 µM Cis + 5 nM LANT; 2 µM Cis + 2.5 nM
LANT; and 2 µM Cis + 5 nM LANT) were more effective at
inducing death in all 3 cell lines than the corresponding cisplatin
monotreatment (1 µM Cis or 2 µM Cis). There were 2
(of 15) comparisons that did not exhibit statistically significant
differences in their efficacy: 1 µM Cis + 5 nM LANT vs. 2
µM Cis + 2.5 nM LANT for Detroit 562 and FaDu cell lines;
and 2 µM Cis + 2.5 nM LANT vs. 1 µM Cis + 2.5 nM LANT
for CAL 27 cells, implying that these treatments were
equivalent.
The most effective combination with the most notable
increase in cell death over its corresponding cisplatin
monotreatment was 1 µM Cis + 5 nM LANT, with approximately
2- to 5-fold greater cell death than 1 µM cisplatin
monotreatment. The lowest therapeutic efficacy improvement was
observed with the 2 µM Cis + 2.5 nM LANT combination, with
<2-fold more cell death than the 2 µM Cis
monotreatment.
The 4PL model equation was used to determine the
synergistic therapeutic efficacy of the combination treatment and
the percentage of cisplatin dose reduction (35). The cell death percentages induced
by the 4 combinations of Cis + LANT (1 or 2 µM Cis + 2.5 or
5 nM LANT) were evaluated. The dose of cisplatin necessary to
achieve the same cell death percentage as the corresponding
cisplatin used in the combination treatments was determined. The
reduction in dose was derived using cell death percentage as the
commonality (Table IV).
 | Table IVReducing effect on cisplatin dose by
Cisplatin + LANT combination treatments. |
Table IV
Reducing effect on cisplatin dose by
Cisplatin + LANT combination treatments.
| Cell line | Outcome | Treatment
combination
|
|---|
| 1 µM Cis +
2.5 nM LANT | 1 µM Cis + 5
nM LANT | 2 µM Cis +
2.5 nM LANT | 2 µM Cis + 5
nM LANT |
|---|
| Detroit 562 | Cell death (%) in
combo | 26.2 | 37.7 | 33.5 | 44.9 |
| Est. conc.
(µM) of Cis mono to obtain the same % cell death | 4.0 | 5.9 | 5.2 | 7.1 |
| Cis dose reduction
(%) | 75.2 | 82.9 | 61.2 | 72.0 |
| FaDu | Cell death (%) in
combo | 39.4 | 51.7 | 53.7 | 59.8 |
| Est. conc.
(µM) of Cis mono to obtain the same % cell death | 2.3 | 4.2 | 4.6 | 6.0 |
| Cis dose reduction
(%) | 56.6 | 76.0 | 56.1 | 66.7 |
| CAL 27 | Cell death (%) in
combo | 35.0 | 58.5 | 38.4 | 75.5 |
| Est. conc.
(µM) of Cis mono to obtain the same % cell death | 2.7 | 5.3 | 3.0 | 9.4 |
| Cis dose reduction
(%) | 62.7 | 81.2 | 32.8 | 78.7 |
The 1 µM Cis + 5 nM LANT combination
treatment resulted in the highest percentage of cisplatin dose
reduction: 82.9, 76.0 and 81.2% for the Detroit 562, FaDu and CAL
27 cell lines, respectively. For example, the 82.9% dose reduction
for the Detroit 562 cells elucidates that 5.9 µM Cis as a
monotreatment is required to achieve the same 37.7% cell death as
the 1 µM Cis used in the 1 µM Cis + 5 nM LANT
combination treatment.
Discussion
Adjuvant, neoadjuvant and combination therapies are
an emerging and viable approach to overcome the current challenges
experienced by patients who cannot receive or tolerate the standard
of care chemotherapeutic treatment regimens. This patient-centered
solution reduces the standard drug dosage administered, thereby
reducing toxicity, side-effects and poor prognosis. Cisplatin, a
standard chemotherapeutic therapy for HNSCC, has shown promise to
decrease toxicity and side-effects at lower doses when combined
with other therapeutic interventions. Several emerging clinical
studies have combined cisplatin with other interventions and
demonstrated dose reduction while maintaining efficacy. A previous
study compared cisplatin combined with paclitaxel to high-dose
cisplatin in patients with locally advanced HNSCC receiving
concurrent radiation. That study demonstrated less acute and
chronic toxicities at one-fifth of the cisplatin dose with
comparable overall survival rates and efficacy (10). In another study that followed
patients with locally advanced HNSCC, induction chemotherapy
combining docetaxel, cisplatin, and fluorouracil (TPF), in
comparison to cisplatin and fluorouracil (PF), demonstrated
significant improvement in overall-, median- and progression-free
survival without increasing treatment-related toxicity, as measured
by tracheostomies and dependence on gastric feeding tubes (18).
Pre-clinical studies are beginning to emerge and
show promise for cisplatin dose reduction and enhanced drug
delivery by combining cisplatin with various unconventional
interventions, such as nanomedicines and therapeutic
nanotechnologies (28). One such
example is a nano-enabled version of cisplatin combined with a
nano-enabled version of rapamycin. Rapamycin, which inhibits
angiogenesis and proliferation through the mTOR pathway, has been
shown to enhance human melanoma cell sensitivity to cisplatin,
induce significant apoptosis in vitro, inhibit the growth of
a xenografted tumor and permit the enhanced tumor penetration of
NPs in vivo (36). Another
example is a theranostic nanomedicine study of gold nanoclusters
conjugated to folic acid and cisplatin that significantly improved
the efficacy of cisplatin by accelerating the cellular uptake and
increasing cytotoxicity in breast cancer cells. These conjugates
also inhibited growth and lung metastasis of orthotopically
implanted breast tumors (37).
There is a class of nanoparticle drug delivery
systems (DDSs) used to facilitate the delivery of cisplatin,
relying on the enhanced permeability and retention (EPR) effect
(28). These include organic
(polymeric NPs, polymeric micelles, polymeric conjugates,
dendrimers, liposomes, polymer-coated liposomes, and nanocapsules),
inorganic (carbon nanotubes, iron oxide NPs, gold NPs, and
mesoporous silica NPs) and hybrid NPs (nanoscale coordination
polymers and polysilsesquioxane NPs) (28).
Another class of nanotechnologies that may enhance
drug performance, aside from the class of previously mentioned
nanomedicines, are the less explored, therapeutic metallic
nanoparticles. A previous study demonstrated that zinc oxide
nanoparticles (ZnO-NPs) induced tumor-selective cell death in HNSCC
in vitro and enhanced cytotoxic effects when irradiated with
UVA-1 in combination with cisplatin and paclitaxel. Although UVA-1
activated ZnO-NPs alone produced a signifi-cant decrease in viable
cells, this effect was further enhanced when combined with
cisplatin and paclitaxel, indicating a synergistic association
between the photocatalytic nanoparticles and the chemotherapeutic
drug combination (38,39).
Previous LANT research by the authors, also in this
class of therapeutic metallic nanoparticles, used AuNRs to
demonstrate approximately 100% cell death in vitro and
complete xeno-grafted tumor regression in vivo in HNSCC when
exposed to a specific excitation wavelength of near-infrared laser
light (785 nm) (34). In the
present study, LANT, combined with cisplatin as an adjuvant
therapy, improved the therapeutic efficacy of cisplatin by
>5-fold that of cisplatin monotreatment and reduced the
effective cisplatin dose in 3 HNSCC cell lines. This cisplatin +
LANT combination therapy is designed to lower the effective dose,
decrease treatment times and minimize the side-effects of cisplatin
monotreatment. This nanodrug adjuvant therapy approach may also
circumvent systemic delivery and the need for conjugation by
overriding the tumor microenvironment and avoiding the delivery
obstacles encountered during uptake by the reticuloendothelial
system. This strategy is based on effective intratumoral LANT
delivery (34) and may hold
promise of becoming an additional option for patients who cannot
tolerate the full dose of the standard cisplatin regimen.
In conclusion, the present study demonstrates the
potential of cisplatin and LANT co-therapy as a possible addition
to the adjuvant therapy options for the treatment of HNSCC. The
combination of cisplatin + LANT demonstrates up to 5.4-fold greater
therapeutic efficacy than cisplatin monotreatment. The most
effective treatment combination, 1 µM Cis + 5 nM LANT,
demonstrates an 82.9% dose reduction in Detroit 562 cells, compared
to the 5.9 µM of Cis monotreatment required to achieve the
same 37.7% cell death in 48 h. This observation suggests that a
lower cisplatin dose may be used in combination with LANT to
achieve the same therapeutic efficacy as higher doses of cisplatin
monotreatment. Directly translating this in vitro
concentration to an animal or human dose is not a process clearly
outlined in the literature. However, if the same 82.9% dose
reduction was applied to the standard human cisplatin dose
schedule, LANT could reduce the standard clinical dose of cisplatin
from 2.54 mg/kg (100 mg/m2) every 3 to 4 weeks to 0.43
mg/kg (17.1 mg/m2) in 48 h. The combination of LANT and
cisplatin suggests that LANT may boost the therapeutic effect of
low doses of cisplatin, and may result in fewer side-effects for
cancer patients and improved patient outcomes. It also suggests
that adding LANT to the current standard cisplatin dose schedule
may provide a more aggressive treatment option if desired; however,
this requires additional study.
It is suggested that these findings may be extended
to a variety of other cancer types. It is also suggested that these
findings may extend to the development of novel adjuvant therapy
formulations, incorporating other metallic-based nanoparticle
technologies, such as other gold, silver, platinum and iron
nanoparticles. Other therapeutic nanotechnologies, such as
dendrimers, polymers and liposomes may also serve as adjuvant,
multi-step interventions that may be less expensive and more
effective than the conjugated, hybrid versions of the same
components. Consequently, future studies should also consider the
improvement cisplatin may have to the other treatment component in
the adjuvant therapy, including, but not limited to, LANT and other
nanotechnologies. Future studies are also required to address the
impact of the combination treatment on oral keratinocytes,
fibroblasts, the mechanism of cell death and decreased cellular
proliferation.
Funding
The study was supported by Award no. I01BX007080
from the Biomedical Laboratory Research and Development Service of
the VA Office of Research and Development.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
All authors listed made substantial, direct, and
intellectual contributions to the work discussed in this
manuscript. HNG developed the LANT technology and protocols. HNG,
GYL and JAM designed, drafted and revised the manuscript. GYL
performed the experiments. JAM, KPS and SMP performed the
statistical analysis. All authors analyzed the data, read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
AuNRs
|
gold nanorods
|
|
AuNPs
|
gold nanoparticles
|
|
NPs
|
nanoparticles
|
|
LANT
|
laser-activated nanotherapy
|
|
Cis
|
cisplatin
|
|
HNC
|
head and neck cancer
|
|
HNSCC
|
head and neck squamous cell
carcinoma
|
Acknowledgments
The authors would like to thank Dr Adejare Adeboye,
University of the Sciences; Dr William E. Grizzle, University of
Alabama at Birmingham; Dr James Lillard, Morehouse School of
Medicine; Mr. Eric Spears, Ora Lee Smith Cancer Research
Foundation; Miss Maya Cothran, Ora Lee Smith Cancer Research
Foundation; and Dr Ed Childs, Morehouse School of Medicine for
their support and encouragement during the preparation of this
manuscript. The authors would also like to thank the supporters and
volunteers at the Ora Lee Smith Cancer Research Foundation for
their endeavors to translate LANT from bench to bedside, while
making it affordable and accessible.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Blanchard P, Baujat B, Holostenco V,
Bourredjem A, Baey C, Bourhis J and Pignon JP; MACH-CH
Collaborative group: Meta-analysis of chemotherapy in head and neck
cancer (MACH-NC): A comprehensive analysis by tumour site.
Radiother Oncol. 100:33–40. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marur S and Forastiere AA: Head and neck
squamous cell carcinoma: Update on epidemiology, diagnosis, and
treatment. Mayo Clin Proc. 91:386–396. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dauzier E, Lacas B, Blanchard P, Le QT,
Simon C, Wolf G, Janot F, Horiuchi M, Tobias JS, Moon J, et al:
Role of chemo-therapy in 5000 patients with head and neck cancer
treated by curative surgery: A subgroup analysis of the
meta-analysis of chemotherapy in head and neck cancer. Oral Oncol.
95:106–114. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alsahafi E, Begg K, Amelio I, Raulf N,
Lucarelli P, Sauter T and Tavassoli M: Clinical update on head and
neck cancer: Molecular biology and ongoing challenges. Cell Death
Dis. 10:5402019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pendleton KP and Grandis JR:
Cisplatin-based chemotherapy options for recurrent and/or
metastatic squamous cell cancer of the head and neck. Clin Med
Insights Ther. 2013: View Article : Google Scholar : 2013.PubMed/NCBI
|
|
8
|
Ghosh S: Cisplatin: The first metal based
anticancer drug. Bioorg Chem. 88:1029252019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Furqan M, Snyders TP, Saqlain MU, Mott SL,
Laux D, Snow A, Anderson CM, Watkins JM and Clamon GH: Comparing
high-dose cisplatin with cisplatin-based combination chemo-therapy
in definitive concurrent chemoradiation setting for locally
advanced head and neck squamous cell carcinoma (LAHNSCC). Cancer
Med. 8:2730–2739. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Florea AM and Büsselberg D: Cisplatin as
an anti-tumor drug: Cellular mechanisms of activity, drug
resistance and induced side effects. Cancers (Basel). 3:1351–1371.
2011. View Article : Google Scholar
|
|
12
|
Towne TG and Murray A: Encyclopedia of
Toxicology. 3rd edition. Academic Press; 2014
|
|
13
|
Pratt WB and Ruddon RW: The anticancer
drugs. Oxford University Press; 1979
|
|
14
|
Makovec T: Cisplatin and beyond: Molecular
mechanisms of action and drug resistance development in cancer
chemotherapy. Radiol Oncol. 53:148–158. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamano Y, Uzawa K, Saito K, Nakashima D,
Kasamatsu A, Koike H, Kouzu Y, Shinozuka K, Nakatani K, Negoro K,
et al: Identification of cisplatin-resistance related genes in head
and neck squamous cell carcinoma. Int J Cancer. 126:437–449. 2010.
View Article : Google Scholar
|
|
16
|
Marur S and Forastiere AA: Head and neck
cancer: Changing epidemiology, diagnosis, and treatment. Mayo Clin
Proc. 83:489–501. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
American Cancer Society: Global cancer
facts and figures 2018. Atlanta; American Cancer Society; 2018
|
|
18
|
Cho H, Nishiike S, Yamamoto Y, Takenaka Y,
Nakahara S, Yasui T, Hanamoto A and Inohara H: Docetaxel,
Cisplatin, and fluorouracil for patients with inoperable recurrent
or metastatic head and neck squamous cell carcinoma. Auris Nasus
Larynx. 42:396–400. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lorch J, Goloubeva O, Haddad R, Cullen K,
Sarlis N, Tishler R, Tan M, Fasciano J and Sammartino DE: Induction
chemotherapy with Cisplatin and fluorouracil alone or in
combination with docetaxel in locally advanced squamous-cell cancer
of the head and neck: Long-term results of the TAX 324 randomized
phase 3 trial. Lancet Oncol. 12:153–159. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Porceddu SV, Scotté F, Aapro M, Salmio S,
Castro A, Launay-Vacher V and Licitra L: treating patients with
locally advanced squamous cell carcinoma of the head and neck
unsuitable to receive cisplatin-based therapy. Front Oncol.
9:15222020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Szturz P, Cristina V, Herrera Gómez RG,
Bourhis J, Simon C and Vermorken JB: Cisplatin eligibility issues
and alternative regimens in locoregionally advanced head and neck
cancer: Recommendations for clinical practice. Front Oncol.
9:4642019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peng H, Chen L, Li WF, Guo R, Mao YP,
Zhang Y, Zhang F, Liu LZ, Tian L, Lin AH, et al: The cumulative
cisplatin dose affects the long-term survival outcomes of patients
with nasopharyngeal carcinoma receiving concurrent
chemoradiotherapy. Sci Rep. 6:243322016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Le X and Hanna EY: Optimal regimen of
cisplatin in squamous cell carcinoma of head and neck yet to be
determined. Ann Transl Med. 6:2292018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Strojan P, Vermorken JB, Beitler JJ, Saba
NF, Haigentz M Jr, Bossi P, Worden FP, Langendijk JA, Eisbruch A,
Mendenhall WM, et al: Cumulative cisplatin dose in concurrent
chemoradiotherapy for head and neck cancer: A systematic review.
Head Neck. 38(Suppl 1): E2151–E2158. 2016. View Article : Google Scholar
|
|
25
|
Szturz P, Wouters K, Kiyota N, Tahara M,
Prabhash K, Noronha V, Adelstein D, Van Gestel D and Vermorken JB:
Low-dose vs. High-dose cisplatin: Lessons learned from 59
chemoradiotherapy trials in head and neck cancer. Front Oncol.
9:862019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mashhour K and Hashem W: Cisplatin weekly
versus every 3 weeks concurrently with radiotherapy in the
treatment of locally advanced head and neck squamous cell
carcinomas: What is the best dosing and schedule? Asian Pac J
Cancer Prev. 21:799–807. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nouman M, Haider G, Bukhari N, Yousuf A,
Nouman R, Shaikh MR, Hussain S, Pavan B, Rahool R, Memon P, et al:
Response rate of cisplatin plus docetaxel as primary treatment in
locally advanced head and neck carcinoma (squamous cell types).
Asian Pac J Cancer Prev. 21:825–830. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Duan X, He C, Kron SJ and Lin W:
Nanoparticle formulations of cisplatin for cancer therapy. Wiley
Interdiscip Rev Nanomed Nanobiotechnol. 8:776–791. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kayyali MN, Ramsey AJ, Higbee-Dempsey EM,
Yan L, O'Malley BW Jr, Tsourkas A and Li D: The development of a
nano-based approach to alleviate cisplatin-induced ototoxicity. J
Assoc Res Otolaryngol. 19:123–132. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mehtala JG, Torregrosa-Allen S, Elzey BD,
Jeon M, Kim C and Wei A: Synergistic effects of cisplatin
chemotherapy and gold nanorod-mediated hyperthermia on ovarian
cancer cells and tumors. Nanomedicine (Lond). 9:1939–1955. 2014.
View Article : Google Scholar
|
|
31
|
Miao L, Guo S, Zhang J, Kim WY and Huang
L: Nanoparticles with precise ratiometric co-loading and
co-delivery of gemcitabine monophosphate and cisplatin for
treatment of bladder cancer. Adv Funct Mater. 24:6601–6611. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Miao L, Wang Y, Lin CM, Xiong Y, Chen N,
Zhang L, Kim WY and Huang L: Nanoparticle modulation of the tumor
microen-vironment enhances therapeutic efficacy of cisplatin. J
Control Release. 217:27–41. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Green HN, Martyshkin DV, Rosenthal EL and
Mirov SB: A minimally invasive multifunctional nanoscale system for
selective targeting, imaging, and NIR photothermal therapy of
malignant tumors. In: Proceedings of the Reporters, Markers, Dyes,
Nanoparticles, and Molecular Probes for Biomedical Applications
III; 7910. SPIE BiOS; San Francisco, CA. 2011
|
|
34
|
Green HN, Crockett SD, Martyshkin DV,
Singh KP, Grizzle WE, Rosenthal EL and Mirov SB: A histological
evaluation and in vivo assessment of intratumoral near infrared
photothermal nanotherapy-induced tumor regression. Int J
Nanomedicine. 9:5093–5102. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sebaugh JL: Guidelines for accurate
EC50/IC50 estimation. Pharm Stat. 10:128–134. 2011. View Article : Google Scholar
|
|
36
|
Guo S, Lin CM, Xu Z, Miao L, Wang Y and
Huang L: Co-delivery of cisplatin and rapamycin for enhanced
anticancer therapy through synergistic effects and microenvironment
modulation. ACS Nano. 8:4996–5009. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhou F, Feng B, Yu H, Wang D, Wang T, Liu
J, Meng Q, Wang S, Zhang P, Zhang Z and Li Y: Cisplatin
prodrug-conjugated gold nanocluster for fluorescence imaging and
targeted therapy of the breast cancer. Theranostics. 6:679–687.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hackenberg S, Scherzed A, Harnisch W,
Froelich K, Ginzkey C, Koehler C, Hagen R and Kleinsasser N:
Antitumor activity of photo-stimulated zinc oxide nanoparticles
combined with paclitaxel or cisplatin in HNSCC cell lines. J
Photochem Photobiol B. 114:87–93. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hackenberg S, Scherzed A, Kessler M,
Froelich K, Ginzkey C, Koehler C, Burghartz M, Hagen R and
Kleinsasser N: Zinc oxide nanoparticles induce photocatalytic cell
death in human head and neck squamous cell carcinoma cell lines in
vitro. Int J Oncol. 37:1583–1590. 2010.PubMed/NCBI
|