Introduction
Endometrial cancer ranks as the most common
gynecological cancer and the fourth most common cancer among women
worldwide (1). Estimates for the
United States predicted that there would be ~62,000 new cases of
endometrial cancer with >12,000 deaths in 2019 (2). Mortality from endometrial cancer in
the United States will be almost equal to that from ovarian cancer
at ~14,000 deaths per year and, at any one time, an excess of
700,000 women are living with endometrial cancer in the United
States alone (2). Endometrial
cancer incidence as well as recurrence risk is on the rise, with a
growing disparity in outcomes among ethnic groups (3,4). For
example, the 5-year survival rate is only 64% for non-Hispanic
Black women in the United States compared with 86% for non-Hispanic
white women, a 22% difference (3).
Thus, it is important to improve our understanding of these trends
and seek ways to address them clinically. One avenue that should be
explored is new therapeutic strategies involving combinations of
agents, which must begin with in vitro studies using
endometrial cancer cell lines.
Beginning with the HeLa cell line in the 1950s
(5), cultured cancer cells have
been the workhorse of cancer research. While these cells have been
the source of numerous breakthroughs in our understanding of cancer
biology and have served as the front lines of cancer treatment
discovery, there have been contamination with other cell lines,
misidentification of cultured cells and reversals in reports of
contamination with other cell lines that have caused some to
question their utility as cancer models, particularly their
clinical relevance (6,7). However, with the development of next
generation genetic and genomic technologies, it has become possible
to fully characterize cancer cell lines in order to objectively
evaluate how representative they are. For example, four studies of
ovarian cancer cell lines have provided a wealth of information for
a total of 108 different cell lines (8-11).
There are numerous ovarian cancer cell lines and only six of them
were represented in all four studies and only eight more were found
in three of the four. Similarly, while there are >100 uterine
cancer cell lines, only a few are routinely used and none have been
sufficiently characterized to assess their status as
representatives of patients diagnosed with endometrial cancer.
To assess how well individual endometrial cancer
cell lines represent larger groups of patient tumors, the present
study selected the five of the most commonly used endometrial
cancer cell lines: Ishikawa, ECC-1, KLE, RL95-2 And Hec50co.
Specific analyses included histology, mutation screening, MutL
homolog (MLH)1 promoter methylation, homologous recombination
repair (HRR), copy number variation (CNV) and microsatellite
instability (MSI) (12-16). The issue of what ECC-1 cells really
are, as several studies have suggested that they are actually
Ishikawa cells and that ECC-1 cells no longer exist (8,17,18),
was also investigated. The current data support this view but also
suggested that there are enough differences between Ishikawa and
ECC-1 cells to regard ECC-1 as a useful cell line. Finally, the
present study assembled a database listing a total of 127
endometrial cancer cell lines, some of which are well known but
several are not. If novel and more representative pre-clinical
endometrial cancer models are identified, then these and other
lines should be examined to the extent that Ishikawa, ECC-1,
Hec50co, KLE and RL95-2 cells are in the current study.
A brief history of the five cell
lines
Ishikawa
Dr Masato Nishida first established the classic
Ishikawa cell line in 1980 (16).
Dr Nishida recognized that no one cell line could possess all of
the relevant characteristics of a cancer and that there were few
endometrial cancer cell lines available. Ishikawa cells were
derived from a 39-year-old Japanese patient who presented with a
well differentiated stage 2 endometrial adenocarcinoma. The cell
line was readily established and propagated and maintained both
estrogen and progesterone receptors in culture. Since their
original establishment, Ishikawa cells have been used as a model
for type I endometrial cancer (11) in labs around the world and have
appeared in >500 publications based on a PubMed (pubmed.
ncbi.nlm.nih.gov/) search using the search term
'Ishikawa.' In 2002, Dr Nishida reviewed the history of the cell
line and noted that the original parent cells were distributed to
other labs but that sub-clones were developed in 1993 (19-21).
These were distributed after 1993, with sub-clone 3-H-4 being the
most commonly used line between 1993 and 1996; sub-clone 3-H-12 was
the most used line after 1996 (19-21).
ECC-1
This cell line was developed by the late P.G.
Satyaswaroop by passaging cells from a well differentiated
endometrial adenocarcinoma from a 68-year-old patient into the
infrascapular region of ovarectomized Balb/c (nu/nu) mice (22). The cells were subsequently cultured
in vitro and maintained using both estrogen and progesterone
receptors in culture. Moreover, ECC-1 cells express proteins
characteristic of luminal epithelium upon stimulation with EGF
(23). In general, ECC-1 cells
were regarded as most similar to Ishikawa cells, though there were
useful unique features, including the luminal epithelium
characteristics, that differentiate ECC-1 cells from the more
glandular epithelial characteristics of other endometrial cancer
cell lines, such as Ishikawa (23). This cell line was deposited in the
American Type Culture Collection (ATCC) but is no longer available
as the ATCC no longer recognizes it as a unique cell line based
upon work by Korch (8). ATCC
(www.atcc.org) and Cellosaurus (web.expasy.
org/cellosaurus/) both note that ECC-1 cells are actually Ishikawa
contaminants. This assertion has been repeated by another study
(8), but has never fully
documented by anything beyond COmbined DNA Index System (CODIS)
genotypes.
HEC50co
The 'co' sub-line was clonally derived from HEC50
cells in the laboratory of Dr Kimberly Leslie in the late 1990s
(24). The parent cell line was
established in 1975 from ascites obtained from a Japanese patient
with a grade 3 endometrial adenocarcinoma (25). Transplants of HEC50 cells into nude
mice result in poorly differentiated adenocarcinomas with papillary
serous features (25). The HEC50co
sub-line produces the same phenotype in mice (26). A thorough characterization of the
sub-line has shown that HEC50co cells are consistent with type II
endometrial cancer, which is aggressive and has a poor prognosis
(12). This assignment was
supported by another study that also confirmed p53 status as a null
phenotype (27).
KLE
The KLE cell line was derived from a 68-year-old
Caucasian patient at the Vincent Memorial Hospital in Boston,
Massachusetts. The patient had previously been surgically treated
for a grade II/IV endometrial adenocarcinoma but upon readmission
was found to have a large, poorly differentiated adenocarcinoma
extending into the parametrium (28). A portion of this tumor was the
parent tissue of the cell line. KLE cells do not maintain either
estrogen or progesterone receptors. The cell line was deposited in
the ATCC soon after it was fully characterized and is still
available (CRL-1622).
RL95-2
A 65-year-old, obese Caucasian patient presented at
the University of Arizona Medical Center in 1980 with a grade 2
moderately differentiated adenosquamous carcinoma of the
endometrium. Tumor epithelial cells were successfully isolated from
the stromal and fibroblastic cells and cultured as the RL95-2 cell
line (29). This cell line, which
is positive for the estrogen receptor in both the cytosol and
nucleus, is consistent with glandular epithelium on microscopic and
biochemical criteria (29). The
RL95-2 cell line is available from the ATCC (CRL-1671).
Materials and methods
Database of endometrial cancer cell
lines
A list of endome-trial cancer cell lines was
compiled by mining the International Agency for Research on Cancer
(IARC) TP53 database (www.iarc.fr)
using the search term 'corpus uteri.'
Cell culture
The ECC-1, KLE and RL95-2 cells were purchased from
the American Type Culture Collection, while Ishikawa cells and the
HEC50 cells from which Hec50co originated were gifts from Dr Erlio
Gurpide (New York University) (12). Ishikawa and Hec50co cells were
cultured in DMEM supplemented with 10% fetal bovine serum (FBS) and
1% antibiotic [penicillin (p)/streptomycin (s)] (all from Gibco;
Thermo Fisher Scientific, Inc.). KLE cells were cultured in RPMI
1640 (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10%
FBS and 1% p/s. ECC-1 cells were cultured RPMI 1640 supplemented
with 5% FBS and 1% p/s. RL95-2 cells were cultured in DMEM/F12
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS
and 1% p/s.
Nucleic acid purification and quality
control (QC)
Whole cell RNAs were purified using the mirVana RNA
isolation kit according to manufacturer's recommendations (Thermo
Fisher Scientific, Inc.). Yield and purity were assessed in the
Genomics Division of the University of Iowa Institute for Human
Genetics (IIHG) using an Agilent Model 2100 DNA Analyzer and a
Trinean DropSense 16 spectrophotometer. Genomic DNA (gDNA) was
purified using the DNeasy kit according to manufacturer's
recommendations (Qiagen, Inc.). Yield and purity were determined
using a NanoDrop 2000 spectrophotometer and horizontal gel
electrophoresis [1% agarose in 1X Tris/Borate/EDTA (TBE) buffer
(0.13 M Tris, pH 7.6; 45 mM boric acid; 2.5 mM EDTA/Borate/EDTA)].
Gels were visualized on a uV transilluminator at 302 nm following
staining in a 2% (v/v) solution of ethidium bromide (Sigma).
Short tandem repeat (STR) genotyping
Quality control tested gDNA samples (100 ng per cell
line) were submitted to Bio-Synthesis, Inc. (Cat. No. CL1003,
www.biosyn.com), for 15 loci STR plus amelogenin
genotyping. The STR panel is the core technology in the CODIS
system genotyping system (30).
RNA sequencing
Cellular RNAs of sufficient quality, as determined
by an RNA Integrity Number (31)
>9.0 from the Agilent DNA Analyzer, were selected for RNA
sequencing. Sequencing was carried out by the Genomics Division of
the University of Iowa Institute for Human Genetics (IIHG).
Transcription profiling was performed starting with 500 ng total
cellular RNA, which was fragmented, converted to cDNA and ligated
to sequencing adaptors containing indexes; all steps were performed
using the Illumina TruSeq stranded total RNA library preparation
kit (Illumina, Inc.) per the manufacturer's protocol. Molar
concentrations of the indexed libraries were measured using the
Model 2100 Agilent Bioanalyzer and combined pooled in equimolar
concentrations for sequencing. The concentration of the pools was
measured using the Illumina Library Quantification kit (Kapa
Biosystems; Roche Diagnostics) per the manufacturer's protocol and
sequenced using the Illumina HiSeq 4000 genome sequencer and 150 bp
paired-end SBS chemistry per the manufacturer's protocol. RNA-seq
reads were mapped and aligned to the human reference genome
(version hg38) using STAR, a paired-end enabled algorithm (32). BAM files were produced after
alignment.
MLH1 methylation status
Methylation of the MLH1 locus was shown in TCGA
(https://portal.gdc.cancer.gov/)
characterization of endometrial cancer to be nearly diagnostic of
the MSI hypermutated type of tumor (13). With the same high quality gDNA that
was submitted for STR genotyping, bisulfite conversions on all five
cell lines were performed using the EZ DNA Methylation kit
according to manufacturer's recommendation (Zymo Research Corp.).
Converted bisDNA was then PCR amplified in the presence of 2X
ZYMOTaq pre-mix (Zymo Research Corp.) with the following
bisulfite-specific PCR primers in the promoter of the human (h)MLH1
gene per the manufacturer's protocol: hMLH1, Forward: 5′-GGAGTG AAG
GAG GTT ACG GGT AAG T-3′ and reverse: 5′-AAA AAC GAT AAA ACC CTA
TAC CTA ATC TAT C-3′. These sequences, along with bisPCR conditions
and both methylated and bis-converted control DNAs, are available
from ZYMO Research (www.zymoresearch.com). Cycling conditions were 95°C
for 10 min followed by 40 cycles of 95°C for 30 sec, 59°C for 30
sec and 72°C for one minute and a final extension step at 72°C for
seven minutes. PCR amplicons were visualized on a 1.3% horizontal
agarose gel following electrophoresis. The gel was visualized on a
uV transilluminator at 302 nm following staining in a 2% (v/v)
solution of ethidium bromide (Sigma Chemical). Controls consist of
bisulfite-converted human genomic DNA where C1 is completely
methylated and C2 is a methylation control.
MSI
High molecular weight gDNA was purified from each
cell line and QC assessed as aforementioned. Equal mass aliquots of
gDNA (500 ng) were delivered to the Clinical Microbiology
Laboratory of the University of Iowa Hospitals and Clinics
whereupon MSI testing was carried out by multiplex PCR followed by
fluorescence capillary electrophoresis, as is routinely performed
for clinical testing by the Clinical Microbiology Laboratory at the
University of Iowa Hospitals and Clinics. Following the 1997
National Cancer Institute consensus, each cell line was genotyped
for the mononucleotide and dinucleotide repeat markers BAT25,
BAT26, D2S123, D5S346 and D17S250 (33).
Variant calling
The reference genome used was hg38 with a 20-kb bin
size, as previously recommended (32). BAM files for each sample were used
for mutation discovery and base calling against the human genome
reference hg38 utilizing SAMtools and BCFtools for sorting and
indexing (34). Results were
annotated using ANNOVAR and formatted to display the number of
mutations per gene and sample (32). Only non-synonymous somatic
mutations were included. Data were cross-referenced to hg38
chromosomal coordinate in addition to base change.
CNV
Copy number was determined across the genome for
each cell line from the RNA sequencing output. CNV was determined
using SAMtools (version 1.7) and CopywriteR (version 2.20.0) using
BAM files as the input (34,35).
SAMTools was used to sort and index BAM files to be used by
CopywriteR. The reference genome used was hg38 with a 20-kb bin
size, as previously recommended (32). CopywriteR extracts copy number
information from targeted sequencing by utilizing off-target reads,
and can be used without reference and applied to sequencing data
obtained from various techniques (35). To determine the copy number
characteristics for each cell line, variation was measured as the
standard deviation of log2 copy number both for specific genes and
for chromosomal segments. The significance cut-off was 3× mean
standard deviation for the entire genome and the cell lines were
comparatively assessed based on this.
Homologous recombination repair
(HRR)
To assess the HRR status, RNA-sequencing data were
analyzed for mutations in the following 12 genes (36): Breast cancer susceptibility protein
(BRCA) 1, BRCA2, ataxia telangiectasia mutated (ATM), BRCA1
interacting protein C-terminal helicase 1 (BRIP1), checkpoint
kinase 2 (CHEK2), Fanconia anemia complementation (FANC) group A
(FANCA), FANC group I (FANCI), FANCM group M (FANCM), nibrin (NBN),
RAD51 Paralog C (RAD51C), RAD51 Paralog D (RAD51D) and RAD51
Paralog L (RAD51L). The ClinVar database (www.ncbi.nlm.nih.gov/clinvar/), dbSNP (www.ncbi.nlm.nih.gov/snp/) and ARUP Laboratories
(www.aruplab.com/) were assessed for prior reports
of each detected mutation in these 12 loci.
Results
Comprehensive list of endometrial cancer
cell lines
In 1999, Satyaswaroop compiled a characterization of
24 endometrial cancer cell lines which, at the time, represented
virtually all of the cell lines in use in the world (37). Using the IARC TP53 database
(www.iarc.fr) as a starting point, we have now
assembled information on 127 putative endometrial cancer cell
lines. Table SI presents basic
information on these cell lines to the extent possible based on the
original literature. Notably, these cell lines represent numerous
ethnic groups as well as a wide range of histological types. The
availability of each cell line is also noted where possible.
STR genotyping
The STR typing data are presented in Table I, comparing STR typing of the
present (in-house) cells with those reported by Korch et al
(8), the only published STR typing
of endometrial cancer cell lines. Based on the STR data, it was
confirmed that the cell models in our laboratory are correctly
identified compared with the results of Korch et al
(8). Ishikawa cells were
consistent with the 3-H-12 sub-cell line documented by Nishida
(21) as the sub-clone distributed
after 1996. Based on the STR genotypes of Ishikawa and ECC-1 cells,
these are likely the same cell line, as has been previously argued
(8). It also appears that the
ancestor of the current ECC-1 cell line is the 3-H-12 sub-cell line
distributed after 1996. However, genomic profiling data shown below
suggest that while Ishikawa and ECC-1 cells are very similar, they
are not identical.
 | Table ICombined DNA Index system
short-tandem repeat genotyping of the endometrial cancer cell lines
in the present study. Data are presented consistent with the STR
convention, whereby values reflect the number of repeats between
primers at each locus on each chromosome, including the X
chromosome ('X'). |
Table I
Combined DNA Index system
short-tandem repeat genotyping of the endometrial cancer cell lines
in the present study. Data are presented consistent with the STR
convention, whereby values reflect the number of repeats between
primers at each locus on each chromosome, including the X
chromosome ('X').
| Marker |
Cell line
|
|---|
Ishikawa
| ECC-1
| Hec50co
| KLE
| RL95-2
|
|---|
| Korch | In-house | Korch | In-house | Korch | In-house | Korch | In-house | Korch | In-house |
|---|
| CSF1PO | 11,12 | 11,12 | 11,12 | 11,12 | 9,12 | 9,12 | 13,14 | 13,14 | 10,11 | 10,11 |
| D3S1358 | 17,18 | 16,16 | 16,17 | 16,17 | 15,16 | 15,16,17 | 17,17 | 17,17 | 14,16 | 14,16 |
| D5S818 | 10,11 | 9,10 | 10,11 | 10,11 | 8,8 | 8,8 | 9,12 | 9,12 | 10,11 | 10,11 |
| D7S820 | 9,10 | 9,10 | 9,10 | 9,10 | 12,12 | 12,12 | 11,12 | 11,12 | 10,10 | 10,10 |
| D8S1179 | 12,16 | 12,16 | 13,16 | 13,16 | 10,15 | 10,15 | 8,14 | 8,14 | 10,14 | 10,14 |
| D13S317 | 9,12 | 9,13 | 9,12 | 9,12 | 9,9 | 9,9 | 12,12 | 12,12 | 8,12 | 8,12 |
| D16S539 | 9,9 | 9,9 | 9,9 | 9,9 | 12,12 | 12,12 | 11,12 | 11,12 | 11,13 | 11,13 |
| D18S51 | 12,13,22 | 14,20,21 | 12,19 | 12,19 | 14,14 | 14,14 | 13,17 | 13,17 | 10,14 | 10,14 |
| D21S11 | 28,28 | 28,28 | 28,28 | 28,28 | 30,30 | 30,30 | 28,30 | 28,30 | 28,29 | 28,29 |
| FGA | 21,21 | 21,21 | 21,21 | 21,21 | 20,20 | 20,20 | 23,25 | 23,25 | 20,22 | 20,22 |
| THO1 | 9,10 | 9,10 | 9,10 | 9,10 | 9,9 | 9,9 | 6,7 | 6,7 | 9,9.3 | 9,9.3 |
| TPOX | 8,8 | 8,8 | 8,8 | 8,8 | 9,9 | 9,9 | 8,11 | 8,11 | 8,8 | 8,8 |
| vWA | 14,17 | 14,18 | 14,17 | 14,17 | 14,14 | 14,14 | 16,16 | 16,16 | 16,20 | 16,20 |
| Amelogenin | X | X | X | X | X | X | X | X | X | X |
RNA sequencing
RNA sequencing output BAM files were aligned against
Build 38 (GRCh38) of the human genome (hg38). DNA base calling
differences between each cell line and the reference genome was
then tabulated for each of the cell lines. In all, Ishikawa
displayed 2,711 base calling differences compared with hg38, ECC-1
displayed 2,882, KLE displayed 1,488 base, RL95-2 displayed 2,756
and Hec50co displayed 1,508 (data not shown). As each base calling
difference between the cell lines and the reference genome was
identified both by base change and chromosome coordinate, all base
calling differences shared by two or more of the cell lines were
identified. It was observed that 274 base calling differences were
shared by all five cell lines. Given the historical origins of the
cell lines, it was assumed that it is unlikely that these base
calling differences are anything more than variations in the hg38
reference genome as opposed to systematic mutations in endometrial
cancer. The likelihood that cell lines from five unique individuals
from different ethnicities and times would share consistent
mutations compared to a sixth unique individual representing the
human reference genome is small. Thus, accepting the possibility
that potential shared mutations may be missed, base calling
differences that were unique or shared by only two cell lines were
further investigated.
TCGA-based endometrial cancer report listed a dozen
genes as frequently mutated (13).
The status of the cell lines for these loci is shown in Table II. Both Ishikawa and ECC-1 cells
had a DNA polymerase ε catalytic subunit A (POLE) mutation,
but P102S is not in the proof-reading exonuclease domain at
residues 268 to 471 (38) and
therefore not an ultra-mutating event. All five cell lines harbored
a TP53 mutation, and both Ishikawa and ECC-1 cells had an
identical TP53 mutation, M246V. The precise phenotypic
consequences of this variant are unknown, though the location of
the mutant near the end of the L3 Loop of p53 that forms part of
the crucial Zn2+ binding site (39) suggests that it is not neutral.
Similarly, RL95-2 cells had an in-frame deletion of a single amino
acid in TP53 (V218), the consequences of which are also
unknown. On the other hand, Hec50co cells showed a large deletion
in which the entirety of exon 6, G187 through E224, is absent,
rendering these cells phenotypically p53 null. Lastly, KLE cells
possessed one of the classic gain-of-function TP53 mutants,
R175H, which subverts the tumor suppressor function of p53 into an
oncomorphic one (40-42).
 | Table IIMutation profile of the five cell
lines for twelve commonly mutated loci in endometrial cancer. |
Table II
Mutation profile of the five cell
lines for twelve commonly mutated loci in endometrial cancer.
| Gene | Chr | Mutation frequency,
% | Cell line
|
|---|
| Ishikawa | ECC-1 | Hec50co | KLE | RL95-2 |
|---|
| POLE | 12 | 11.21 | P102Sa | P102Sa | Ndb | Nd | Nd |
| MLH1 | 3 | 2.59 | Nd | Nd
Promoter-Mec | Nd | Nd | Nd
Promoter-Mec |
| TP53 | 17 | 28.88 | M246V
(rs483352695)d | M246V
(rs483352695)d | del G187-E224 | R175H
(rs28934578)d | del V218 |
| PTEN | 10 | 63.73 | E91fs-ter | E91fs-ter | Nd | Nd | T321fs-ter |
| PIK3CA | 3 | 53.02 | Nd | Nd | Nd | Nd | Nd |
| PIK3R1 | 5 | 32.76 | L570P | L570P | E469G
Y470D | Nd | R386
R639ter |
| ARID1A | 1 | 33.62 | Nd | Nd | Y148ter | Nd | L649fs
R693ter |
| KRAS | 12 | 20.69 | Nd | Nd | G12D
(rs121913529)d | Nd | Nd |
| CTNNB1 | 3 | 29.74 | Nd | Nd | Nd | Nd | Nd |
| FBXW7 | 4 | 16.81 | Nd | Nd | Nd | R479Q
(rs866987936)d | Nd |
| PPP2R1A | 19 | 10.78 | Nd | Nd | R183W
(rs1057519946)d | Nd | Nd |
Three of the five cell lines are likely PTEN null.
RL95-2 cells have a late frame-shift mutant, T321fs-ter, resulting
in a premature termination in PTEN. A different PTEN
frame-shift termination mutant, E91fs-ter, was observed in both
Ishikawa and ECC-1 cells. Hec50co and RL95-2 cells had different
mutations in phosphatidylinositol 3-kinase regulatory subunit α
(PIK3R1) (E469G and Y470D in Hec50co; R386fs/R639ter in
RL95-2) and AT-rich interactive domain-containing protein 1A
(ARID1A) (Y148ter in Hec50co; L649fs/R693ter in RL95-2).
Hec50co cells also possessed mutations in KRAS at G12D,
serine/threonine-protein phosphatase 2A 65 kDa regulatory subunit A
α isoform (PPP21A) at R183W and F-box/WD repeat-containing
protein 7 (FBXW7) at R479Q.
Ishikawa and ECC-1 cells also had an identical
PIK3R1 mutant, L570P, in addition to their identical
POLE, TP53 and PTEN mutants. Comparing the
entire base calling profile of the two cell lines (censored for
base calling differences shared by three or more cell lines),
Ishikawa and ECC-1 cells share 1,017 identical base calling
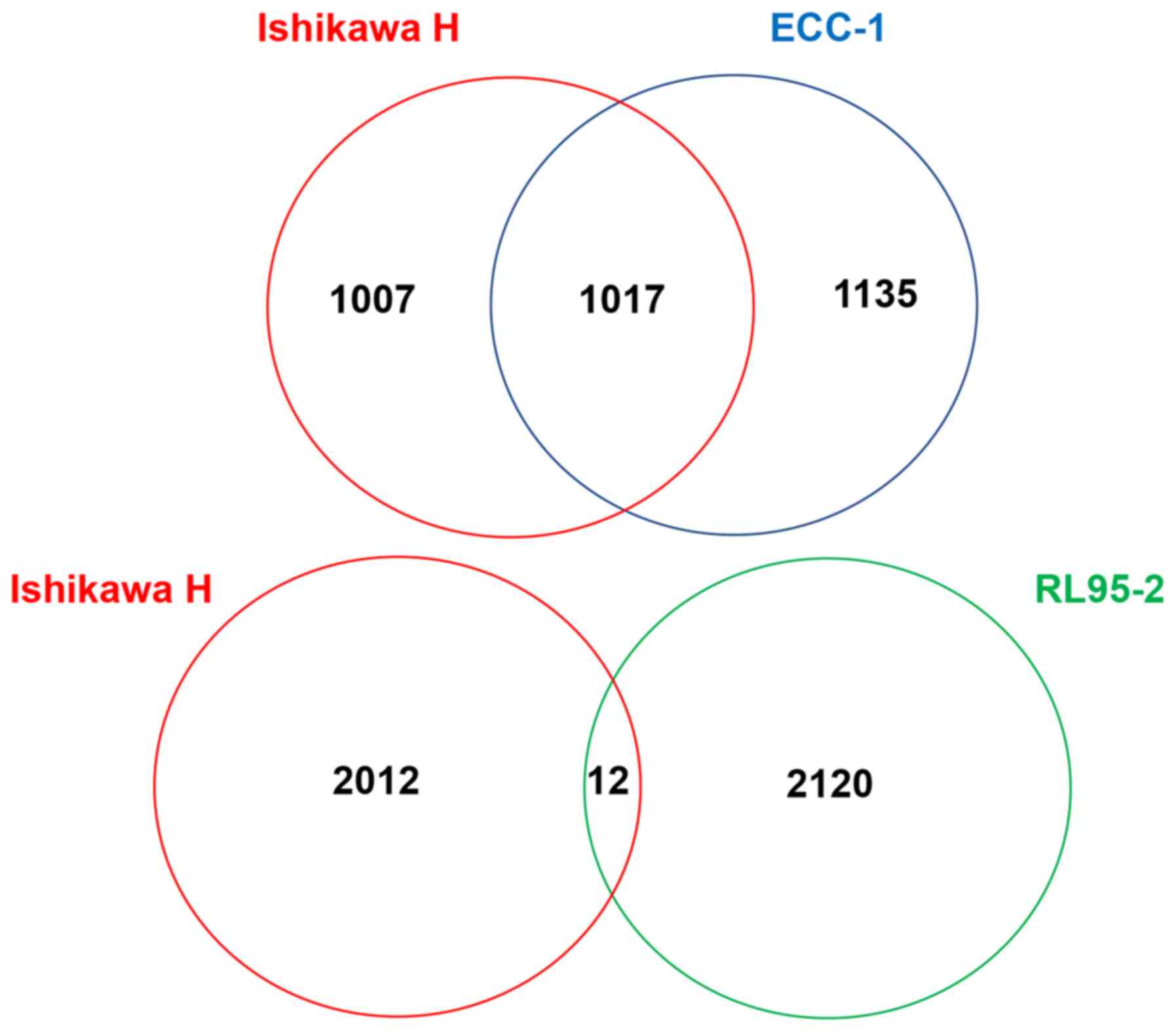
differences with the hg38 reference (Fig. 1). This accounted for approximately
one-half of all censored base calling differences. Meanwhile,
RL95-2 cells, which have a similar number of base calling
differences as Ishikawa and ECC-1 cells as aforementioned, shared
only 12 identical base calling differences with Ishikawa cells
(Fig. 1). These data substantiate
the STR genotyping data that Ishikawa and ECC-1 cells are likely
the same cell line.
MLH1 methylation status
Methylation-specific PCR of bisulfite- converted
gDNA from each of the five cell lines plus two controls are shown
in Fig. 2. Both the fully
bisulfite- converted control (C1) and the methylation control (C2)
demonstrated the expected 182-bp amplicon, indicating that the
human MLH1 promoter was methylated in these DNAs. Of the five cell
lines, ECC-1 and RL95-2 produced an amplicon, indicating that their
MLH1 promoter was methylated and thus inactive.
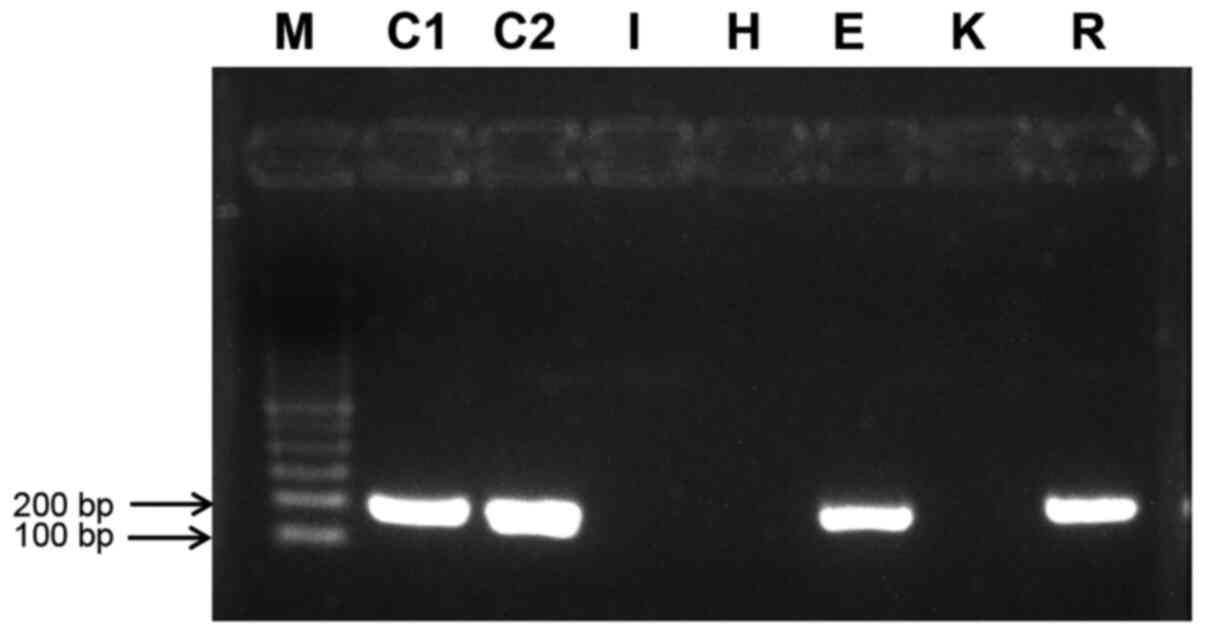 | Figure 2Methylation-specific PCR of the human
MutL homolog promoter. The 182 amplicon is produced only when the
promoter is methylated and, thus, inactive. C1 is a control
composed of bisDNA, C2 is a bisulfate conversion experimental
control. bisDNA, fully bisulfate-converted DNA; I, Ishikawa bisDNA;
H, Hec50co bisDNA; E, ECC-1 bisDNA; K, KLE bisDNA; R, RL95-2
bisDNA; M, marker. |
MSI
Results of MSI analysis are presented in Table III. Both Ishikawa and ECC-1 cells
were classified as MSI-High, RL95-2 cells as MSI-Low and both
Hec50co and KLE cells as MSI stable. The assignment of MSI-High
status to Ishikawa and ECC-1 cells agrees with the conclusion
offered by Korch et al (8)
that the cell lines are of the same origin. It should be noted that
Ishikawa but not ECC1 cells harbor a previously unreported MSH6
mutation, L398R, though the significance of this mutation to MSI
status is not known.
 | Table IIIMSI in the five endometrial cancer
cell lines. |
Table III
MSI in the five endometrial cancer
cell lines.
| Cell line | Marker
| Assignment |
|---|
| BAT26 | BAT25 | D2S123 | D5S346 | D17S250 |
|---|
| Ishikawa | 103,104 | 114,115,120 | 94,115 | 82,90 | 128,136 | MSI-High |
| ECC-1 | 105 | 114,115,118 | 97,119 | 82,88 | 126,134 | MSI-High |
| Hec50c0 | 117 | 123 | 101,109 | 82 | 132 | MSI-Stable |
| KLE | 117,118 | 122,123 | 104,106 | 86,94 | 132 | MSI-Stable |
| RL95-2 | 107 | 117,120 | 99,177 | 86 | 151,155 | MSI-Low |
HRR
The ability of cells to carry out HRR has been
linked to breast cancer susceptibility protein (BRCA) mutation
status (43). Other loci known to
influence this ability include ataxia telangiectasia mutated (ATM),
genes associated with Fanconi anemia (for example FANCD2) and the
RAD51 family of recombinases (36). RNA sequencing data were searched
for mutations in 12 relevant loci. In total, 18 mutations were
identified across the five cell lines (Table IV). Using assessments of the
likely effect of each mutation available in the ClinVar database,
dbSNP and ARUP Laboratories, it is likely that all five cell lines
are HRR proficient, although the splice site BRCA1 mutant observed
in ECC-1 cells suggested that ECC-1 may be HRR deficient; however,
additional studies are necessary to directly assess HR
proficiency.
 | Table IVMutation profile of HRR genes in the
five endometrial cancer cell lines. |
Table IV
Mutation profile of HRR genes in the
five endometrial cancer cell lines.
| Marker | Cell line
|
|---|
| Ishikawa | ECC-1 | Hec50co | KLE | RL95-2 |
|---|
| BRCA1 | P871L
S1634G | P871L
S1634G 17:4307705 splice | Nd | Nd | Nd |
| BRCA2 | N289H | N289H | Nd | A2852fs | K2551E |
| ATM | Nd | R1312fs | Nd | Nd | Nd |
| BRIP1 | Nd | Nd | Nd | Nd | L195P |
| CHEK2 | Nd | Nd | Nd | Nd | Nd |
| FANCA | Nd | Nd | Nd | Nd | Nd |
| FANCI | L781R | Nd | S371G | Nd | Nd |
| FANCM | R798G | H742Y | I1460V
P1812A | Nd | Nd |
| NBN | Nd | Nd | Nd | Nd | Nd |
| RAD51C | Nd | Nd | Nd | Nd | Nd |
| RAD51D | Nd | Nd | Nd | Nd | Nd |
| RAD51L | Nd | Nd | R154Q | Nd | Nd |
CNV
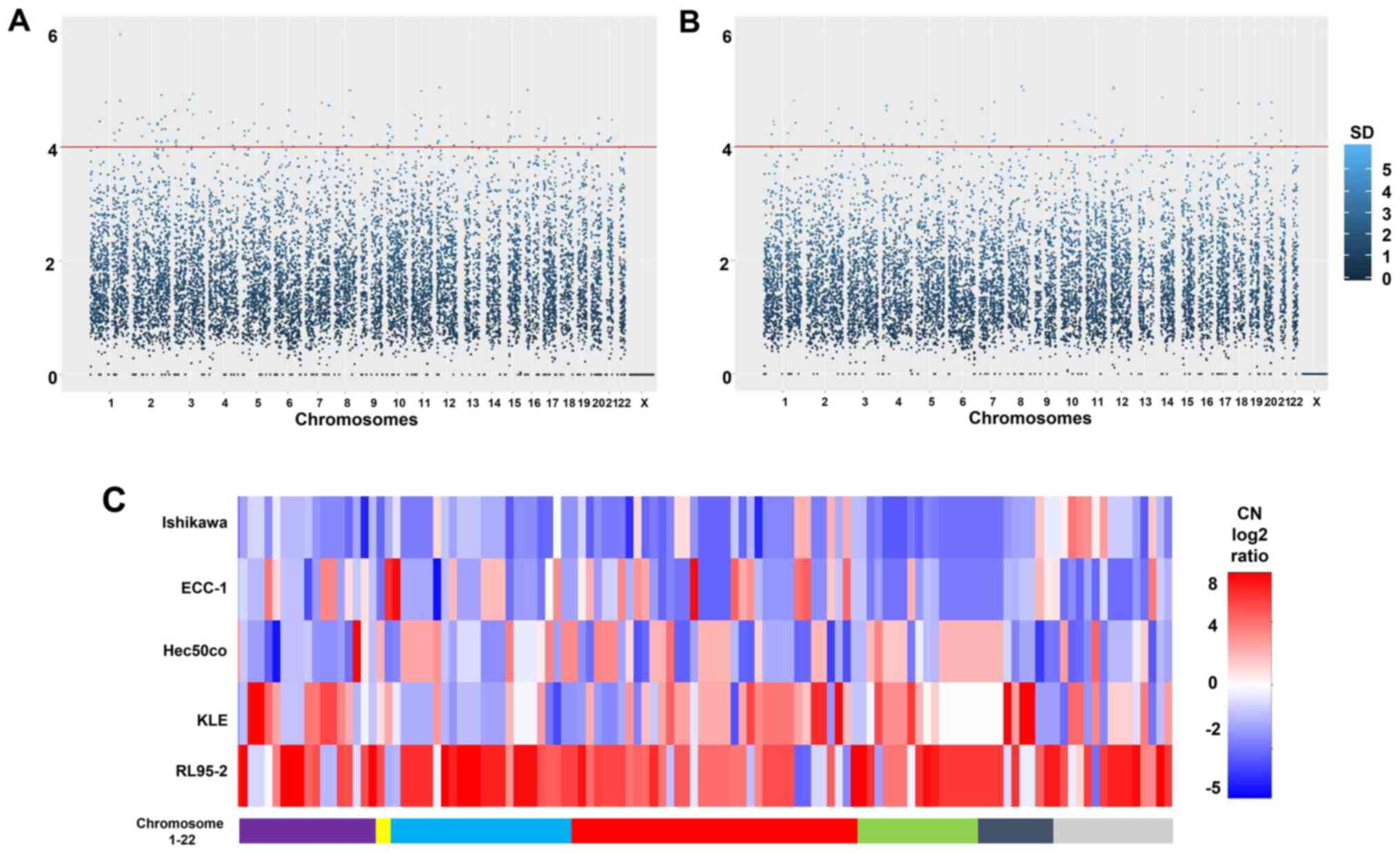
The overall pattern of CNV for both chromosomal
segments and individual genes is presented in Fig. 3A and B. In total, 75,640 chromosome
segments were analyzed and 404 of these reached the cut-off of 3×
mean standard deviation (Fig. 3A).
Similarly, 28,918 genes were assessed, with 117 of these at or
above the 3× mean standard deviation threshold. The identity of
these 117 genes along with their chromosome location and relative
copy numbers are shown in Fig. 3C
and Table SII. In terms of simply
comparing the cell lines to each other, it was clear that Ishikawa
cells are more prone to copy number loss compared with the other
cell lines, whereas RL95-2 cells would be termed 'copy number high'
according to TCGA classification schemes (13). There was a similarity in copy
number patterns between Ishikawa and ECC-1 cells (Fig. 3C), but this is not as notable as it
is with other aforementioned analysis (STR genotyping).
Overall, CNVs are not as pronounced in endometrial
cancer as with other cancer types. Notable exceptions include focal
amplifications of the MYC proto-oncogene (8q24.12), Erb-B2 receptor
tyrosine kinase (17q12) and cyclin E1 (19q12), which appear to be
characteristic of serous endometrial cancer (13-15).
However, none of these were observed in the five present cell
lines. On the other hand, a substantial region of chromosome 4,
4p16-4p15, was amplified in Hec50co and RL95-2 cells (Fig. 3C). This region contains several
significantly altered loci surrounding the locus fibroblast growth
factor receptor 3 (4p16.3), which has previously been noted in
endometrial cancer (13).
Another locus, insulin-like growth factor 1 receptor
(IGF1R; 15q26.3) has been identified as a focal amplification in
endometrial cancer (13,15). In the present study, the 15q26.3
region was amplified in KLE and ECC-1 cells but did not reach
significance as established by the cut-off of 3× the mean standard
deviation (Fig. 3C). Meanwhile,
arrestin domain containing 4 (ARRDC4; 15q26.2) did reach
significance in ECC-1 cells (Fig.
3C). This was one of a number of CNVs where Ishikawa and ECC-1
cells did not present the same profile (Fig. 3C), suggesting that prolonged
culture has led to development of unique features that are not
present in the original Ishikawa cells.
Discussion
The present study attempted a genomic
characterization of five commonly used endometrial cancer cell
lines: Ishikawa, ECC-1, Hec50co, KLE And RL95-2 through a
combination of STR genotyping, RNA sequencing, methylation-specific
PCR and MSI testing. The data presented showed that these cells are
a mixture of characteristics. None of these cell lines are wholly
representative of any one of the four TGCA-based clusters (13-16).
This does not come as a surprise since several datasets in TCGA and
Pan-Cancer Analysis of Whole Genomes have suggested that any
individual cell line or tumor will likely present characteristics
of two or more clusters, thus making such static categorizations
problematic (12-16). For example, the current study
demonstrated that the Ishikawa cell line contains a non-activating
POLE mutation a TP53 mutation of unknown function and a
wild-type PTEN gene and is MSI-High and copy number low.
Also, Ishikawa cells originated from a patient with a well
differentiated grade 2 adenocarcinoma (19). Thus, Ishikawa cells display
characteristics of cluster 2, 3 and 4 but in vivo will form
endometrioid tumors in mice (21).
A similar set of inconclusive arguments can be made for each of the
five cell lines. For example, RL95-2 cells do not have a
POLE mutation but do have a PTEN termination mutant,
are MSI-Low and are copy number high. These features would place
them closest to cluster 4, but they originated from a grade 2
moderately differentiated adenosquamous tumor, not a serous
adenocarcinoma (29). KLE cells
are wild-type for POLE, MSI-Stable and possess a well-known
TP53 gain-of-function mutation. The tumor from which they
originated was a poorly differentiated grade 3 adenocarcinoma,
though precise histology was not provided (28). However, KLE cells are also close to
cluster 4. Finally, Hec50co cells form serous adenocarcinomas in
mice (26), are MSI-Stable and p53
null. These cells have long been regarded as the archetype of the
former type II endometrial carcinoma (12). While their copy number profile is
intermediate among these cell lines, their other features of
MSI-Stable and TP53-mutated best place them in cluster
4.
Finally, the similarities displayed in the present
study between ECC-1 and Ishikawa cells support the notion that
ECC-1 cells are, in fact, Ishikawa cells. In addition to data
presented herein, Korch et al provided data from two
additional loci, Penta C and Penta D, that indicates these cells
came from the same individual (8).
However, there were differences that warrant regarding ECC-1 cells
as similar but not identical to Ishikawa cells. Apart from the
mutation profile itself, both Ishikawa and ECC-1 cells are
MSI-positive, yet Ishikawa cells have an unmethylated MLH1 promoter
while ECC-1 cells have a methylated promotor. There is a well-known
association between MSI and MLH1 hyper-methylation (44,45);
however, such inconsistencies have been previously reported.
Endometrial tumors that are MSI-positive and MLH1 unmethylated have
been identified (46). In such
tumors there are inactivating mutations in the MutS homolog 6
(MSH6) gene (42). Ishikawa cells
do display a previously unreported MSH6 mutation, L398R. This is
not seen in ECC-1 cells; however, whether or not this mutation can
explain the MLH1 methylation discrepancy between the two cell lines
is unknown. Rather than simply dismissing ECC-1 cells as an
Ishikawa contaminant as commercial sources have done, it might be
useful to examine the differences between the two cell lines in
order to assess the effect of those differences against a
background of genomic similarity.
Of course, the endometrial cancer cell line story
does not end with these five cell lines. The information on an
additional 122 endometrial cancer cell lines was assembled. While
the five cell lines reported in the present study may meet the
criteria for both mechanistic and pre-clinical endometrial cancer
models, the existence of numerous other, less well characterized,
cell lines suggests that better representatives may be available.
Further, other cell lines may be better suited to different types
of studies relevant to endometrial cancer. For example, recent
publications have documented the fact that, while endometrial
cancer incidence in general is on the rise, the cancer that is seen
in African American women seems to be of a different nature in
terms of incidence, histology and prognosis (3,47).
Accordingly, the 22 uterine serous papillary carcinoma ARK serous
cells originated from a population of patients, among whom 13 were
identified as Caucasian and nine were identified as African
American (48,49). However, it is not clear if these
cells are immortalized or are commercially available. Perhaps these
or other novel cell lines could be used help improve our
under-standing of how the cancer afflicting African American women
is distinct from endometrial cancer in other ethnic groups, and if
those differences could be used to clinical advantage as has
recently been suggested (47).
Additionally, the present study indicated that, while the majority
of endometrial cancer cell lines originate from adenocarcinomas,
other histological types are in short supply, with only six cell
lines originating from patients diagnosed with a malignant mixed
Müllerian tumor, three from a clear cell histology and nine
sarcomas. Among the 122 endometrial cancer cell lines for which we
provide information herein, the original histology and source
information is often lacking or confusing. Therefore, it would
benefit the study of endometrial cancer if more detailed
information were to be made available along with expanded genomic
information. Ultimately, this would provide investigators with a
wider field from which to select cells for in vitro
pre-clinical studies.
In conclusion, the present study provides a
comprehensive characterization of the five most commonly studied
endometrial cancer cell lines, leveraging technological advances in
high-throughput sequencing to define mutations, copy number
variations and HRR proficiency. The data indicated that these cell
lines do not cleanly fit into any of the four clusters as defined
by TCGA, highlighting the limitation of studying cell lines that
have been immortalized and passaged for, in several cases, decades.
Therefore, researchers should be aware of the limitations
associated with studies of cancer cells cultured in monolayers
(2-dimensional) as was performed in the present study. Future
studies in endometrial cancer could be expanded to 3-dimensional
models using fresh patient tumors, as our group has recently
reported using organoid models for ovarian cancer (50).
Supplementary Data
Acknowledgments
Not applicable.
Funding
The study was funded by The National Institutes of
Health/National Cancer Institute (grant nos. R01 CA99908 and R01
CA184101) and by The Basic Research Fund of the University of Iowa
Carver College of Medicine Department of Obstetrics and
Gynecology.
Availability of data and materials
RNA sequencing data have been deposited in the
National Institutes of Health Gene Expression Omnibus (GEO
[https://www.ncbi.nlm.nih.gov/geo/]),
(accession number GSE151207). The additional datasets analyzed
during the current study are available from The Cancer Genome Atlas
[https://portal.gdc.cancer.gov/],
International Agency for Research on Cancer TP53 repositories
[www.iarc.fr], ClinVar database [www.ncbi.nlm.nih.gov/clinvar/], dbSNP [www.ncbi.nlm.nih.gov/snp/] and ARUP Laboratories
[www.aruplab.com/].
Authors' contributions
EJD carried out the cell cultures, purified total
cellular RNAs and genomic DNAs, performed the bisulfite conversions
and bisPCRs, and was a major contributor in writing the manuscript.
JGB analyzed and interpreted the RNA sequencing data and was a
major contributor in writing the manuscript. KWT was a major
contributor in study design, data analysis and writing and editing
the manuscript. KKL was the senior investigator, was the recipient
of the National Institutes of Health grants and was a major
contributor in conceptualization of the study, data analysis and
writing the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
KWT is a co-founder of Immortagen, Inc. All other
authors certify that they have no affiliations with or involvement
in any organization or entity with any financial interests in the
subject matter or materials discussed in this manuscript.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cote ML, Ruterbusch JJ, Olson SH, Lu K and
Ali-Fehmi R: The growing burden of endometrial cancer: A major
racial disparity affecting black women. Cancer Epidemiol Biomarkers
Prev. 24:1407–1415. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sheikh MA, Althouse AD, Freese KE, Soisson
S, Edwards RP, Welburn S, Sukumvanich P, Comerci J, Kelley J,
LaPorte RE and Linkov F: USA endometrial cancer projections to
2030: Should we be concerned? Future Oncol. 10:2561–2568. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gey GO: Tissue culture studies of the
proliferative capacity of cervical carcinoma and normal epithelium.
Cancer Res. 12:264–265. 1952.
|
|
6
|
Borrell B: How accurate are cancer cell
lines? Nature. 463:8582010. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gillet JP, Calcagno AM, Varma S, Marino M,
Green LJ, Vora MI, Patel C, Orina JN, Eliseeva TA, Singal V, et al:
Redefining the relevance of established cancer cell lines to the
study of mechanisms of clinical Anti-cancer drug resistance. Proc
Natl Acad Sci USA. 108:18708–18713. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Korch C, Spillman MA, Jackson TA, Jacobsen
BM, Murphy SK, Lessey BA, Jordan VC and Bradford AP: DNA profiling
analysis of endometrial and ovarian cell lines reveals
misidentification, redundancy and contamination. Gynecol Oncol.
127:241–248. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Domcke S, Sinha R, Levine DA, Sander C and
Schultz N: Evaluating cell lines as tumour models by comparison of
genomic profiles. Nat Commun. 4:21262013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Anglesio MS, Wiegand KC, Melnyk N, Chow C,
Salamanca C, Prentice LM, Senz J, Yang W, Spillman MA, Cochrane DR,
et al: Type-specific cell line models for type-specific ovarian
cancer research. PLoS One. 8:e721622013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Beaufort CM, Helmijr JC, Piskorz AM,
Hoogstraat M, Ruigrok-Ritstier K, Besselink N, Murtaza M, van
IJcken WF, Heine AA, Smid M, et al: Ovarian cancer cell line panel
(OCCP): Clinical importance of in vitro morphological subtypes.
PLoS One. 9:e1039882014.PubMed/NCBI
|
|
12
|
Albitar L, Pickett G, Morgan M, Davies S
and Leslie KK: Models representing type I and type II human
endometrial cancers: Ishikawa H and Hec50co cells. Gynecol Oncol.
106:52–64. 2007.PubMed/NCBI
|
|
13
|
Cancer Genome Atlas Research Network;
Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H,
Robertson AG, Pashtan I, Shen R, et al: Integrated genomic
characterization of endometrial carcinoma. Nature. 497:67–73.
2013.PubMed/NCBI
|
|
14
|
Murali R, Soslow RA and Weigelt B:
Classification of endometrial carcinoma: More than two types.
Lancet Oncol. 15:e268–e278. 2014.PubMed/NCBI
|
|
15
|
Berger AC, Korkut A, Kanchi RS, Hegde AM,
Lenoir W, Liu W, Liu Y, Fan H, Shen H, Ravikumar V, et al: A
Comprehensive Pan-cancer molecular study of gynecologic and breast
cancers. Cancer Cell. 33:690–705.e9. 2018.PubMed/NCBI
|
|
16
|
Hoadley KA, Yau C, Hinoue T, Wolf DM,
Lazar AJ, Drill E, Shen R, Taylor AM, Cherniack AD, Thorsson V, et
al: Cell-of-Origin patterns dominate the molecular classification
of 10,000 tumors from 33 types of cancer. Cell. 173:291–304.e6.
2018.PubMed/NCBI
|
|
17
|
Bairoch A: The cellosaurus, a cell-line
knowledge resource. J Biomol Tech. 29:25–38. 2018.PubMed/NCBI
|
|
18
|
Robin T, Capes-Davis A and Bairoch A:
CLASTR: The cello-saurus STR similarity search tool-A precious help
for cell line authentication. Int J Cancer. 146:1299–1306.
2020.
|
|
19
|
Nishida M, Kasahara K, Kaneko M, Iwasaki H
and Hayashi K: Establishment of a new human endometrial
adenocarcinoma cell line, Ishikawa cells, containing estrogen and
progesterone receptors. Nihon Sanka Fujinka Gakkai Zasshi.
37:1103–1111. 1985.In Japanese. PubMed/NCBI
|
|
20
|
Nishida M, Kasahara K, Oki A, Satoh T,
Arai Y and Kubo T: Establishment of eighteen clones of Ishikawa
cells. Hum Cell. 9:109–116. 1996.PubMed/NCBI
|
|
21
|
Nishida M: The Ishikawa cells from birth
to the present. Hum Cell. 15:104–117. 2002. View Article : Google Scholar
|
|
22
|
Satyaswaroop PG, Zaino RJ and Mortel R:
Human endometrial adenocarcinoma transplanted into nude mice:
Growth regulation by estradiol. Science. 219:58–60. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mo B, Vendrov AE, Palomino WA, DuPont BR,
Apparao KB and Lessey BA: ECC-1 cells: A well-differentiated
Steroid-responsive endometrial cell line with characteristics of
luminal epithelium. Biol Reprod. 75:387–394. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dai D, Wolf DM, Litman ES, White MJ and
Leslie KK: Progesterone inhibits human endometrial cancer cell
growth and invasiveness: Down-regulation of cellular adhesion
molecules through progesterone B receptors. Cancer Res. 62:881–886.
2002.PubMed/NCBI
|
|
25
|
Kuramoto H, Nishida M, Morisawa T, Hamano
M, Hata H, Kato Y, Ohno E and Iida T: Establishment and
characterization of human endometrial cancer cell lines. Ann N Y
Acad Sci. 622:402–421. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dai D, Albitar L, Nguyen T, Laidler LL,
Singh M and Leslie KK: A therapeutic model for advanced endometrial
cancer: Systemic progestin in combination with local
adenoviral-mediated progesterone receptor expression. Mol Cancer
Ther. 4:169–175. 2005.PubMed/NCBI
|
|
27
|
Liu Z, Wan G, Heaphy C, Bisoffi M,
Griffith JK and Hu CA: A novel loss-of-function mutation in TP53 in
an endometrial cancer cell line and uterine papillary serous
carcinoma model. Mol Cell Biochem. 297:179–187. 2007. View Article : Google Scholar
|
|
28
|
Richardson GS, Dickersin GR, Atkins L,
MacLaughlin DT, Raam S, Merk LP and Bradley FM: KLE: A cell line
with defective estrogen receptor derived from undifferentiated
endometrial cancer. Gynecol Oncol. 17:213–230. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Way DL, Grosso DS, Davis JR, Surwit EA and
Christian CD: Characterization of a new human endometrial carcinoma
(RL95-2) established in tissue culture. In Vitro. 19:147–158. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cabrera CM, Cobo F, Nieto A, Cortés JL,
Montes RM, Catalina P and Concha A: Identity tests: Determination
of cell line Cross-contamination. Cytotechnology. 51:45–50. 2006.
View Article : Google Scholar
|
|
31
|
Schroeder A, Mueller O, Stocker S,
Salowsky R, Leiber M, Gassmann M, Lightfoot S, Menzel W, Granzow M
and Ragg T: The RIN: An RNA integrity number for assigning
integrity values to RNA measurements. BMC Mol Biol. 7:32006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dobin A, Davis CA, Schlesinger F, Drenkow
J, Zaleski C, Jha S, Batut P, Chaisson M and Gingeras TR: STAR:
Ultrafast universal RNA-seq aligner. Bioinformatics. 29:15–21.
2013. View Article : Google Scholar
|
|
33
|
Boland CR, Thibodeau SN, Hamilton SR,
Sidransky D, Eshleman JR, Burt RW, Meltzer SJ, Rodriguez-Bigas MA,
Fodde R, Ranzani GN and Srivastava S: A National cancer institute
Workshop on microsatellite instability for cancer detection and
familial predisposition: Development of international criteria for
the determination of microsatellite instability in colorectal
cancer. Cancer Res. 58:5248–5257. 1998.PubMed/NCBI
|
|
34
|
Li H, Handsaker B, Wysoker A, Fennell T,
Ruan J, Homer N, Marth G, Abecasis G and Durbin R; 1000 Genome
Project Data Processing Subgroup: The sequence Alignment/Map format
and SAMtools. Bioinformatics. 25:2078–2079. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kuilman T, Velds A, Kemper K, Ranzani M,
Bombardelli L, Hoogstraat M, Nevedomskaya E, Xu G, de Ruiter J,
Lolkema MP, et al: CopywriteR: DNA copy number detection from
off-target sequence data. Genome Biol. 16:492015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hoppe MM, Sundar R, Tan DSP and
Jeyasekharan AD: Biomarkers for homologous recombination deficiency
in cancer. J Natl Cancer Inst. 110:704–713. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Satyaswaroop PG: Endometrial Cancer. Human
cell culture. Masters JRW and Palsson B: Springer; Dordrecht: pp.
71–78. 1999
|
|
38
|
van Gool IC, Bosse T and Church DN: POLE
proofreading mutation, immune response and prognosis in endometrial
cancer. Oncoimmunology. 5:e10726752015. View Article : Google Scholar
|
|
39
|
Chen Y, Dey R and Chen L: Crystal
structure of the p53 core domain bound to a full consensus site as
a self-assembled tetramer. Structure. 18:246–256. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Brachova P, Thiel KW and Leslie KK: The
consequence of oncomorphic TP53 mutations in ovarian cancer. Int J
Mol Sci. 14:19257–19275. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Brachova P, Mueting SR, Devor EJ and
Leslie KK: Oncomorphic TP53 mutations in gynecologic cancers lose
the normal protein:protein interactions with the microRNA
microprocessing complex. J Cancer Ther. 5:506–516. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Brachova P, Mueting SR, Carlson MJ,
Goodheart MJ, Button AM, Mott SL, Dai D, Thiel KW, Devor EJ and
Leslie KK: TP53 oncomorphic mutations predict resistance to
platinum and Taxane-based standard chemotherapy in patients
diagnosed with advanced serous ovarian carcinoma. Int J Oncol.
46:607–618. 2015. View Article : Google Scholar
|
|
43
|
Walsh CS: Two decades beyond BRCA1/2:
Homologous recombination, hereditary cancer risk and a target for
ovarian cancer therapy. Gynecol Oncol. 137:343–350. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Esteller M, Levine R, Baylin SB, Ellenson
LH and Herman JG: MLH1 promoter hypermethylation is associated with
the microsatellite instability phenotype in sporadic endometrial
carcinomas. Oncogene. 17:2413–2417. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Simpkins SB, Bocker T, Swisher EM, Mutch
DG, Gersell DJ, Kovatich AJ, Palazzo JP, Fishel R and Goodfellow
PJ: MLH1 promoter methylation and gene silencing is the primary
cause of microsatellite instability in sporadic endometrial
cancers. Hum Mol Genet. 8:661–666. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Goodfellow PJ, Buttin BM, Herzog TJ, Rader
JS, Gibb RK, Swisher E, Look K, Walls KC, Fan MY and Mutch DG:
Prevalence of defective DNA mismatch repair and MSH6 mutation in an
unselected series of endometrial cancers. Proc Natl Acad Sci USA.
100:5908–5913. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dubil EA, Tian C, Wang G, Tarney CM,
Bateman NW, Levine DA, Conrads TP, Hamilton CA, Maxwell GL and
Darcy KM: Racial disparities in molecular subtypes of endometrial
cancer. Gynecol Oncol. 149:106–116. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Varughese J, Cocco E, Bellone S, de Leon
M, Bellone M, Todeschini P, Schwartz PE, Rutherford TJ, Pecorelli S
and Santin AD: Uterine serous papillary carcinomas overexpress
human trophoblast-cell-surface marker (Trop-2) and are highly
sensitive to immunotherapy with hRS7, a humanized anti-Trop-2
monoclonal antibody. Cancer. 117:3163–3172. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
English DP, Bellone S, Cocco E, Bortolomai
I, Pecorelli S, Lopez S, Silasi DA, Schwartz PE, Rutherford T and
Santin AD: Oncogenic PIK3CA gene mutations and HER2/neu gene
amplifications determine the sensitivity of uterine serous
carcinoma cell lines to GDC-0980, a selective inhibitor of Class I
PI3 kinase and mTOR kinase (TORC1/2). Am J Obstet Gynecol.
209:465.e1–e9. 2013. View Article : Google Scholar
|
|
50
|
Bi J, Thiel KW, Litman JM, Zhang Y, Devor
EJ, Newtson AM, Schnieders MJ, Gonzalez Bosquet J and Leslie KK:
Characterization of a TP53 somatic variant of unknown function from
an ovarian cancer patient using organoid culture and computational
modeling. Clin Obstet Gynecol. 63:109–119. 2020. View Article : Google Scholar
|