Introduction
There is increasing interest in the use of natural
products for treatment and/or prevention of a variety of diseases
(1,2). Natural products, such as propolis
processed by honeybees, contain a wide variety of chemical
compounds that exert potent biological effects, and also exhibit
anticancer activity (3). The
authors have previously the growth inhibitory activity of ethanol
extracts from plant resin-derived propolis in human colon carcinoma
cell lines (3). The authors have
also previously found that the dietary administration of powdered
leaves of Peucedanum japonicum and Terminalia catappa
reduces the occurrence of azoxymethane-induced aberrant crypt foci
(ACF), preneoplastic lesions in rat colon carcinogenesis (4,5).
The medium-chain fatty acid (FA),
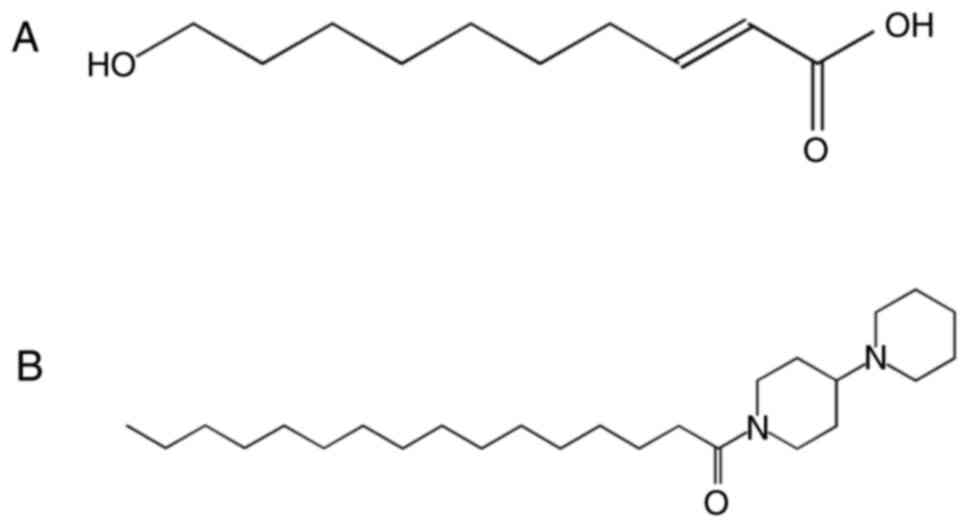
10-hydroxy-2-decenoic acid (10-HDA) (Fig. 1A), is the most abundant FA and a
major lipid component of the honeybee product, royal jelly (RJ)
(6,7). This unique FA exhibits a broad range
of biological and pharmacological properties (8-10).
For instance, there are several studies reporting the antibacterial
activity of RJ against Gram-positive and Gram-negative bacteria
(6,11-13).
However, limited experimental evidence is available for the
biological properties, including the anticancer and
anti-inflammatory activities of 10-HDA (14,15).
In the present study, inspired by the biological
profile of 10-HDA in continuation of a previous study (16) by the authors, a novel compound,
1-palmitoyl-4-piperidinopiperidine (PPI), that exhibits a
resemblance to 10-HDA in its backbone structure, was synthesized.
The aim of the present study was to examine the anticancer
activities of PPI. In addition, the present study aimed to
elucidate the molecular mechanisms through which it inhibits the
growth of human colon carcinoma cells, focusing on the possibility
that it may exert its effects, at least in part, by suppressing the
function of a signal transducer and activator of transcription 3
(STAT3) and its related molecules.
Materials and methods
Chemistry and chemicals
PPI (Fig. 1B) was
synthesized by the authors (17).
In brief, palmitic acid and 1-hydroxy-benzotriazole were dissolved
in dimethylformamide, and 4-piperidinopiperidine was added and
mixed (solution 1). 1-Ethyl-3-(3-dimethylaminopropyl)-carbodiimide
was dissolved in dimethylformamide (solution 2). Solutions 1 and 2
were mixed under cooled conditions (4°C) for 2 h and at room
temperature for 12 h. Chloroform was added to the reaction product
and followed by washing with hydrochloric acid twice and saturated
brine solution twice.
Cryptotanshinone (CTS) (Tokyo Chemical Industry Co.,
Ltd.), a specific inhibitor of the signal transducer and activator
of transcription 3 (STAT3) SH2 domain, and 5-fluorouracil (5-FU;
Sigma-Aldrich; Merck KGaA) were dissolved in dimethyl sulfoxide
(DMSO; Sigma-Aldrich; Merck KGaA). All chemicals were stored at
-20°C until use.
Silica gel column chromatography
The remaining chloroform solution was purified with
silica gel column chromatography, yielding PPI. PPI
(=1-(1,4′-bipiperidin-1′-yl)hexadecan-1-one): Mp. 189°C; EIMS
m/z (rel. int.): 406 [M]+ (42), 377 (12), 322 (11), 167 (46), 124 (100), 110 (20), 84 (30); 1H-NMR (400 MHz,
CDCl3) δ: 0.88 (3H, t, J=6.8 Hz), 1.25 (26H, br
s), 1.60-1.69 (5H, m), 2.00-3.40 (4H, br s), 2.17 (2H, m), 2.30
(2H, dd, J=8.8, 7.8 Hz), 2.53 (2H, m), 3.08 (2H, m), 3.26
(2H, m), 4.01 (1H, m), 4.86 (1H, m). PPI was dissolved in acetone
(FUJIFILM Wako Pure Chemical Corp.).
Cells and cell culture
HT29, SW480, SW837 and HCT116 human colorectal
carcinoma cell lines, and the FHC human colon normal epithelial
cell line were obtained from the American Type Culture Collection
(ATCC). Exponentially dividing cells were maintained in Dulbecco's
modified Eagle's medium (DMEM; FUJIFILM Wako Pure Chemical
Corporation) supplemented with 10% (v/v) fetal bovine serum
(BioWest S.A.S.) in an incubator with humidified air at 37°C with
5% CO2. Cells were plated in 10-cm culture dishes
(Thermo Fisher Scientific, Inc.), treated with 1.0-10 µM PPI
for 24 or 48 h and harvested. As an untreated vehicle control,
cells were treated with acetone or DMSO at a final concentration
≤0.5%.
Cell proliferation assays
Cell viability was determined using colony formation
and MTT assays performed as previously described (18). Carcinoma cells were plated into
6-well 35-mm-diameter culture plates (7.5×102
cells/well) and treated with 0.3% acetone (control) or various
concentrations (0.25-5 µM) of PPI for 7 days in DMEM plus
10% FBS. After washing with phosphate-buffered saline (PBS),
colonies were fixed with 100% methanol (FUJIFILM Wako Pure Chemical
Corporation) and stained with Giemsa solution at room temperature
for 30 min (Sigma-Aldrich; Merck KGaA) and then counted.
Carcinoma cells or colon normal epithelial cells
were seeded into 96 well plates (1-5×103 cells/well).
Cells were treated with acetone/DMSO (control) or increasing
concentrations of 0.07-12.5 µM PPI, 1.2-20 µM CTS or
1.2-20 µM 5-FU for 48 or 96 h. MTT reagent (50 µg)
was added to each well, and assayed using an MTT assay kit (Roche
Diagnostics GmbH). The absorbance was measured at 595 nm using a
spectrophotometric microplate reader (Model 680 series microplate
readers; Bio-Rad Laboratories, Inc.). The IC50 value was
calculated from kinetic parameters derived from the growth curve
data of each chemical. All assays were performed in duplicate or
triplicate and yielded similar results. In these two assay systems,
the relative surviving fraction, when compared with cells treated
with the vehicle, was plotted on the dose-response curve. A
viability of 100% corresponds to the control cells.
Flow cytometric analysis
Flow cytometric analysis was performed as previously
described (19). The HT29 and
SW837 cells were plated onto 10-cm dishes in DMEM containing 10%
FBS. After synchronizing the cells with serum starvation, the cells
were treated with 0.1% acetone (control) or 1-10 µM PPI for
48 h, harvested, fixed with 70% ethanol, centrifuged (750 × g for 5
min at room temperature), resuspended in 400 µl of PBS
containing 2 mg/ml RNase (Nacalai Tesque Inc., Kyoto, Japan), and
stained with 400 µl of 0.1 mg/ml propidium iodide (Nacalai
Tesque, Inc.). The cell suspension was filtered through a 40
µm nylon filter (Ikemoto Scientific Technology Co. Ltd.).
Samples of 20,000 cells were then analyzed for DNA histograms and
cell cycle phase distributions by flowcytometry using a FACSCalibur
instrument (BD Biosciences), and the data were analyzed by a
CELLQuest computer program (BD Biosciences), as previously
described (20).
Detection of apoptosis
In cell cultures, apoptosis was detected by
observing DNA fragmentation on agarose gel electrophoresis as
previously described (19). In
brief, following treatment of the HT29 cells with 10 µM PPI
for 48 h, the cells were harvested, centrifuged (750 × g for 5 min
at room tempera-ture) and washed twice with PBS. The cell pellet
was then homogenized in 50 mM SEDTA (0.1 M NaCl and 50 mM EDTA).
Following supplementation with 1% sodium dodecyl sulfate (SDS), the
homogenate was digested with proteinase K (FUJIFILM Wako Pure
Chemical Corporation) and extracted twice with phenol/chloroform,
and DNA was precipitated with ethanol. Following RNase treatment,
DNA fragmentation was visualized by agarose gel electrophoresis and
ethidium bromide (0.5 µg/ml) staining. Apoptosis was also
detected by flow cytometric analysis as described above.
Transient transfection reporter
assays
Reporter assays were performed as previously
described (18). The SW837 cells
were plated into 6-well 35-mm-diameter culture plates
(5×104 cells/well) in DMEM plus 10% FBS and cultured
overnight to allow for cell attachment. Subsequently, 1 µg
of STAT3 Luciferase Reporter Vector (Panomics, Inc.) was
transiently transfected into the cells using Lipofectamine 3000
transfection reagent (Thermo Fisher Scientific, Inc.). Following
transfection (approximately 24 h), the cells were incubated with
0.5% acetone (control) or 0.6-2.5 µM PPI in DMEM plus 10%
FBS for 24 h. Cell extracts were then prepared, each sample was
assayed in triplicate using a lucif-erase assay system (Promega
Corp.), and luciferase activity was measured using a TD-20/20
Luminometer (Promega Corp.). A CMV-driven β-galactosidase
expression plasmid (Promega Corp.) was co-transfected into the
cells to normalize the transfection efficiency.
Preparation of whole-cell lysates
Whole-cell lysates were prepared according to
previously established procedures (21). The HT29 and SW837 cells were
treated with 0.5% acetone (control) or 1.0-10 µM PPI for
24-48 h and harvested. The cells were then lysed with modified
radioimmunoprecipitation assay (RIPA) buffer [150 mM NaCl, 1% NP40,
0.1% SDS, 50 mM Tris-HCl (pH 8.0), 0.5% deoxycholic acid, 2 mM
EDTA, 2 mM EGTA, 1 mM DTT, and 25% glycerol].
Preparation of cytosolic and chromatin
fractions
The HT29 and SW837 cells (6.5×105
cells/10-cm diameter dish) were treated with 0.1% acetone (control)
or 1.2-2.5 µM PPI for 24 h, harvested and resuspended in
solution A (10 mM HEPES pH 7.9, 10 mM KCl, 1.5 mM MgCl2,
11% sucrose, 10% glycerol, 0.1% Triton X, 1 mM DTT, 0.1 mM
Na3VO4, 10 mM NaF, 20 µg/ml aprotinin,
20 µg/ml leupeptin and 20 µM PMSF). The cytoplasmic
fraction was separated from the nuclei by centrifugation (1,300 × g
for 4 min, 4°C). Isolated nuclei were lysed in solution B (3 mM
EDTA, 0.2 mM EGTA, 1 mM DTT, 20 µg/ml aprotinin, 20
µg/ml leupeptin and 20 µM PMSF). The chromatin
fraction was separated from the nuclei by centrifugation (1,700 × g
for 4 min, 4°C). Cytoplasmic and chromatin fractions were subjected
to SDS-PAGE (12% gel) and examined by western blot analysis.
Western blot analysis
The assay was performed according to previously
established procedures using Bio-Rad Protein Assay Dye Reagent
Concentration (Bio-Rad Laboratories, Inc.) (21). The whole-cell lysate, cytosolic
fractions and chromatin fractions (20-80 µg protein per
lane) were separated by SDS-PAGE (12.0-13.5% gel) and transferred
onto an Immobilon-P transfer membrane (EMD Millipore). The
membranes were then blocked at room temperature for 1 h with 3% BSA
in TBS-T [50 mM Tris-HCl (pH 7.5), 150 mM NaCl, and 0.2% Tween-20],
followed by incubation at room temperature for 1 h with primary
antibodies to β-actin (sc-1616R, Santa Cruz Biotechnology, Inc.,
1:200), histone H3 (ab1791, Abcam, 1:1,000), caspase-3 (sc-65497,
Santa Cruz Biotechnology, Inc., 1:200), caspase-7 (sc-6138, Santa
Cruz Biotechnology, Inc., 1:200), caspase-8 p18 (sc-7890, Santa
Cruz Biotechnology, Inc., 1:200), caspase-9 p10 (sc-7885, Santa
Cruz Biotechnology, Inc., 1:200), poly (ADP-ribose) polymerase
(PARP; #9542, Cell Signaling Technology, Inc., 1:1,000), STAT3
(sc-7179, Santa Cruz Biotechnology, Inc., 1:200), phosphorylated
(p)-STAT3 (Tyr705) (#9131, Cell Signaling Technology, Inc.,
1:1,000), cyclin D1 (06-137, EMD Millipore, 1:1,000), p53 (sc-126,
Santa Cruz Biotechnology, Inc., 1:200), Bcl-2 (sc-509, Santa Cruz
Biotechnology, Inc., 1:200), Bcl-xL (sc-8392, Santa Cruz
Biotechnology, Inc., 1:200), Bax (sc-526, Santa Cruz Biotechnology,
Inc., 1:200) and vascular endothelial growth factor (VEGF; sc-7269,
Santa Cruz Biotechnology, Inc., 1:200). The membranes were then
incubated at room temperature for 1 h with secondary anti-bodies
(anti-mouse IgG, NA931, Cytiva, 1:4,000 or anti-rabbit IgG, NA934,
Cytiva, 1:3,000) and each band was visualized with a
Light-CaptureII imaging analyzer (ATTO Corp.).
Molecular docking analysis
Computational work was performed using Discovery
Studio 2017R2 (Dassault Systèmes BIOVIA software programe, BIOVIA).
For calculation, the CHARMm force field was applied and the CDOCKER
algorithm was used to dock PPI into the protein. Using the CDOCKER
protocol, the binding affinity was evaluated by the -CDOCKER_ENERGY
(kcal/mol) obtained from the docking analysis of a compound of
interest. The crystal structure of the target protein was obtained
from RCSB Protein data bank (PDB) (https://www.rcsb.org/). The PDB code of STAT3 was
3CWG. The structure of PPI was sketched in ChemDraw computer
program (PerkinElmer, Inc.).
Chick chorioallantoic membrane (CAM)
assays
A modified CAM assay was performed to clearly
visualize blood vessels. PPI was dissolved in 1.0% methylcellulose
solution (methyl-cellulose is dissolved in PBS). As an untreated
vehicle control, acetone was added to the 1.0% methylcellulose
solution at a final concentration ≤0.5%. The fertilized chicken
eggs (Goto Furanjo, Kakamigahara, Japan) were kept in a humidified
incubator at 37°C. Ovalbumin was removed from 4-day-old embryonated
eggs. A small hole was drilled on eggshell and capped, and the eggs
were incubated at 37°C. Following 24 h of incubation, 0, 0.2, 1.0
or 5.0 µM PPI were applied to the center of silicon rings
that were placed on each CAM, and the eggs were incubated at 37°C.
Following a 2-day incubation, fat emulsion (Intralipos®;
Otsuka Pharmaceutical Co., Ltd.) was injected into the
chorioallantois. The blood vessels were photographed using Light
Capture II (ATTO Corp.) at a magnification of ×5. Angiogenesis was
quantified by measuring the total number of blood vessels, the
total number of branches of blood vessels seen in each silicon ring
and the total length of blood vessels per diameter of the ring. In
total, 6-7 eggs were used in each treatment group and the assay was
performed more than once to confirm the results.
Tumor xenograft assay
A total of 15 female BALB/cSlc-nu/nu mice,
aged 6 weeks old were purchased from Japan SLC, Inc. All mice were
quarantined for 1 week and housed in plastic cages (3-4 mice/cage,
weighing 16.7±0.15 g) with free access to tap water and a basal
diet (MF; Oriental Yeast Co. Ltd.) under controlled conditions of
humidity (50±10%), temperature (23±2°C) and lighting (12 h light/12
h dark cycle; 8:00 a.m. lights on, 8:00 p.m. lights off). Animal
experiments were performed with the approval of the Animal Ethics
Committee of the Nagoya City University (approval no. H25M-16) and
according to the guidelines of the committee. Viable HT29 human
colon carcinoma cells (2.5×106 cells/200 µl DMEM
without L-glutamine and phenol red) were subcutaneously injected
into the flanks of the mice. After confirming the visible tumor
mass, mice were assigned into 2 experimental groups. PPI was
dissolved in soybean oil (FUJIFILM Wako Pure Chemical Corporation).
The mice in group 1 (n=7, vehicle-control) and group 2 (n=8,
treatment) received intra-peritoneal (i.p.) injections of the
vehicle (soybean oil) and 50 mg/kg PPI, respectively, once per day
for 10 days (a total of 4 times) until the end of the experiment.
The animals were observed on a daily basis for tumor growth, body
weight and symptomatic adverse side-effects. Tumors were measured
twice a week. The mean volume per tumor was calculated using the
following formula: V (mm3)=LxWxDxπ/6 where 'V' is the
volume, and 'L' is the length, 'W' is the width, and 'D' is the
depth of the tumor (22). At 49
days after the inoculation, all animals were sacrificed by
decapitation following anesthesia with 3% isoflurane and a complete
autopsy was performed. Tumors were carefully removed, fixed with
10% buffered formalin and processed for histopathological
examination [hematoxylin (9131-2, Sakura Finetek Japan Co., Ltd.)
and eosin (1B-425, Nacalai Tesque, Inc.) (H&E) staining].
Tumors were stained with hematoxylin at room temperature for 2 min
and eosin at room temperature for 10 min. Furthermore, necrosis and
viable areas (mm2) in the H&E-stained tumor sections
(3 µm in thickness) were measured using an imaging system
(Digital Microscope VHX-5000, Keyence Corporation). The percentage
necrotic area was calculated, with 100% representing a total area
(necrosis plus viable areas) of the tumor.
Immunohistochemical analysis
The assay was performed using a BOND-MAX automated
immunohistochemistry system (Leica Biosystems GmbH) as previously
described (23). The
paraffin-embedded tumor sections (3-µm-thick) were boiled in
10 mM citric acid buffer solution (pH 6.0) at 100°C for antigen
retrieval, and incubated with primary antibodies to p-STAT3 (#9145,
1:50) at room temperature for 1 h, cleaved caspase-3 (#9661, 1:100)
at 4°C overnight, or CD34 (#3569, 1:100) (all from Cell Signaling
Technology, Inc.) at room temperature for 30 min. Primary antibody
was detected using biotinylated secondary antibody (VECTASTAIN ABC
Standard kit, PK-4000, Vector Laboratories, Inc.) and
diaminobenzidine (DAB). Incubation was performed at room
temperature for 5 min. The sections were counterstained with
hematoxylin at room temperature for 30 sec or 5 min. The number of
blood vessels was measured in a specific structure consisting of
CD34-positive vascular endothelial cells in the viable area of the
tumor. In total, >10 fields were examined in each section. The
p-STAT3-positive rate (%) was determined by calculating the ratio
of the p-STAT3-positive cells/total number of cells counted
(Olympus DP70, Olympus 3-CCD COLOR CAMERA CS530 MD, Olympus Corp.,
Tokyo, Japan). In this assay, >10 fields were examined in each
section. p-STAT3-positive cells were determined by setting a
consistent threshold for all slides using image J software
(National Institutes of Health). In the present study, the
apoptotic index was defined by calculating the number of cleaved
caspase-3-positive cells per mm2.
Rat model of colon ACF
This experiment was performed as described in
previous studies (4,24). Male F344 rats (n=18, 4 weeks old,
weighing 72.3±0.91 g, Japan SLC, Inc.) were used in this
experiment. All rats were quarantined for 1 week and maintained as
described in a mouse bioassay system in this section. A total of 18
rats were randomly divided into 4 groups. Rats in groups 1-3 were
administered a subcutaneous injections of 20 mg/kg azoxymethane
(AOM, CAS no. 25843-45-2, purity >95%, FUJIFILM Wako Pure
Chemical Corporation) twice a week. At 2 days after the final
injection of AOM, rats in group 1 received i.p. injections of
soybean oil (FUJIFILM Wako Pure Chemical Corporation) and rats in
groups 2 and 3 received i.p. injections of 2 and 10 mg/kg PPI,
respectively, once per 10 days (a total of 4 times until the end of
the experiment). The rats in group 4 were administered the basal
diet alone throughout the experiment and served as an untreated
control. Animals were observed on a daily basis for body weight
changes and symptomatic adverse side-effects during the experiment.
At the week 7, all animals were sacrificed by decapitation
following anesthesia with 3% isoflurane and a complete autopsy was
performed. Colon tissues were removed, cut longitudinally and fixed
with 10% buffered formalin. After fixing, colon tissues (≤1 mm in
thickness) were stained with 0.2% methylene blue solution (M9410,
Sigma-Aldrich; Merck KGaA) at room temperature for 30 sec. Using a
microscope system (Leica Application Suite ver. 4, Leica Biosystems
GmbH), the numbers of ACF were counted according to the criteria
described by Bird (25). The
number of ACF per colon (multiplicity) and the number of crypts per
one focus were determined as previously described by the authors
(24). These animal experiments
were performed with the approval of the Animal Ethics Committee of
the Nagoya City University (approval no. H25M-44) and according to
the guidelines of the committee.
Statistical analysis
Statistical analysis was performed with EZR (Saitama
Medical Center, Jichi Medical University) (26). Comparisons between the
vehicle-treated control group and the PPI-treated group in the
xenograft model were made using a Student's t-test, Welch's t-test,
or Mann-Whitney U test. As regards the data of the luciferase
reporter assay, CAM assay and the animal experiments, ANOVA or the
Kruskal-Wallis test, as needed were used, and the Bonferroni/Dunn,
Steel's-Dwass test, or Tukey's multiple comparison tests were
applied to evaluate statistical significance. All results are
expressed as the means ± SE. Differences between groups at
P<0.05 were considered statistically significant.
Results
PPI inhibits the growth of human colon
carcinoma cells
As shown by the colony formation assay, PPI induced
a marked and dose-dependent inhibition of the growth of the HT29,
SW480, SW837 and HCT116 cell lines, with IC50 values of
approximately 0.5, 1.0, 1.9 and 2.2 µM, respectively
(Fig. 2A). To examine the
cytotoxic effects of PPI on the FHC human colon normal epithelial
cell line, the cells were treated with increasing concentrations
(0.07-12.5 µM) of PPI or acetone (≤0.5%, control) for 96 h,
and cell growth was then determined by MTT assays (Fig. 2B). When the HT29 colon carcinoma
cells were treated with 1.5 µM PPI, approximately >90% of
the cells died but at the same concentration of PPI, but
approximately 80% of the normal colon epithelial cells survived
without any significant morphological abnormalities, indicating
that this drug selectively kills carcinoma cells at an effective
dose level. As shown in Fig. 2C,
in the HT29, SW480, SW837 and HCT116 cell lines, PPI induced a
marked growth inhibitory effect in a dose-dependent manner, with
IC50 values of approximately 0.8, 1.4, 2.0 and 2.1
µM, respectively, when the cells were treated with
increasing concentrations (0.25-5.0 µM) of PPI or 0.1%
acetone (control) for 48 h, and cell growth was then measured by
MTT assays. As observed in the growth curves, the IC50
value of PPI in each cell line was markedly lower than that of 5-FU
or CTS, indicating that PPI exerted more potent inhibitory effects
in this assay system (Fig.
2C).
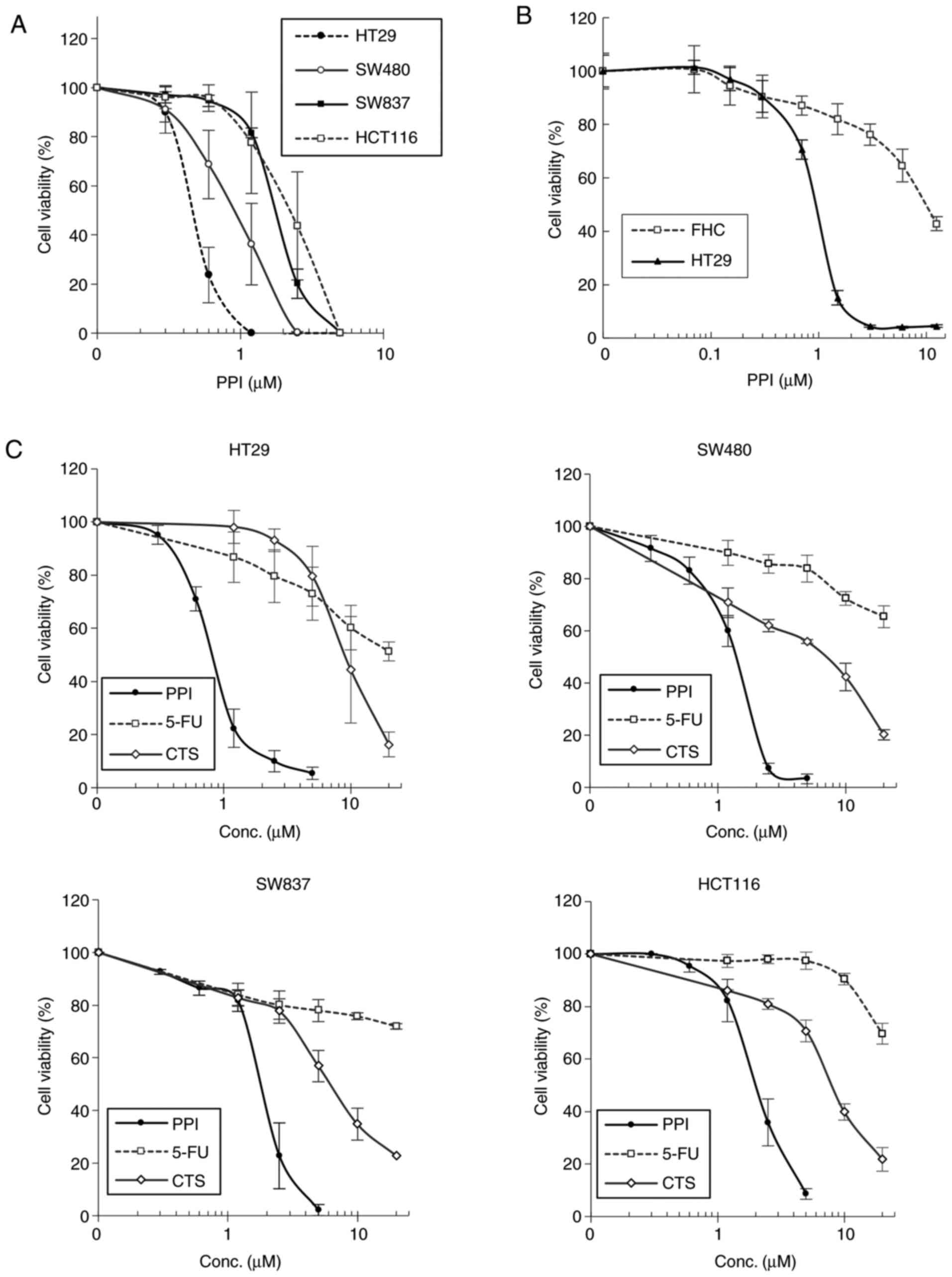 | Figure 2Inhibition of cell growth by PPI in
human colon carcinoma cell lines. (A) Colony formation assays.
These colon carcinoma cell lines were tested. Exponentially
dividing cells were treated with 0.25-5.0 µM PPI for 7 days
in DMEM containing 10% FBS, and colonies were then stained and
counted. (B) MTT assays. Growth curves of HT29 and FHC cell lines
were indicated Cells were treated with 0.07-12.5 µM PPI for
96 h in DMEM containing 10% FBS. (C) MTT assays. Three agents (PPI,
5-FU and CTS) were tested in each cell line (HT29, SW480, SW837 and
HCT116). Cells were treated with 0.25-5.0 µM PPI, 1.2-20
µM 5-FU, and 1.2-20 µM CTS for 48 h in DMEM
containing 10% FBS. Error bars indicate the standard error (SE) in
each panel. For additional details, please see the 'Materials and
methods'. PPI, palmitoyl piperidinopiperidine; 5-FU,
5-fluorouracil; CTS, cryptotanshinone. |
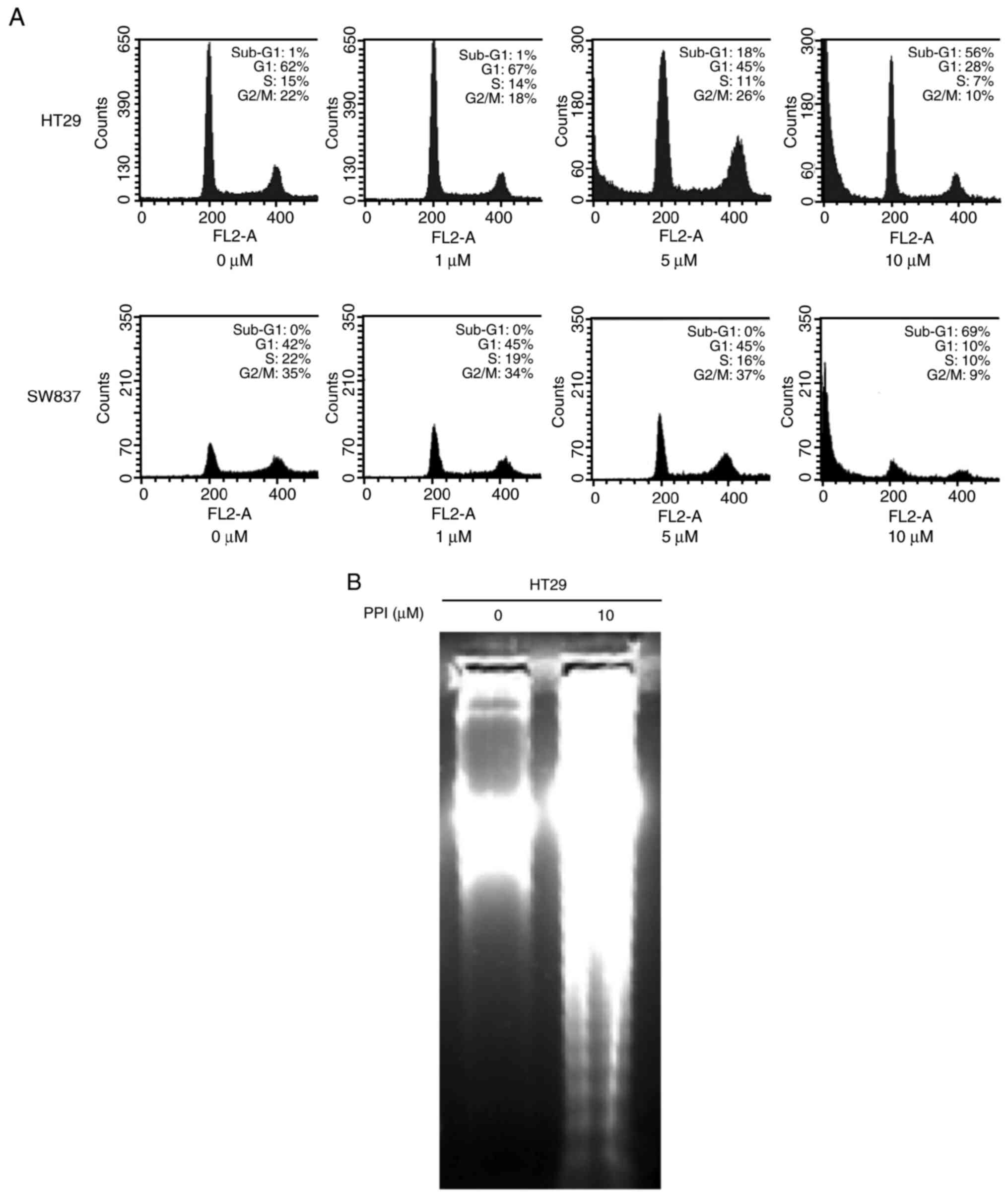
PPI induces an increase in the number of
cells in the G1 phase of the cell cycle, and induces sub-G1
fractions of cells at a higher concentration level
Cell cycle analysis was performed to examine whether
the PPI-treated cells arrest in a specific phase of the cell cycle
and whether PPI induces the sub-G1 fractionation of cells. Flow
cytometric analysis indicated that when the HT29 and SW837 cells
were treated with 1 µM PPI for 48 h, the percentage of cells
in the G1 phase increased by 5 and 3%, respectively, and this was
associated with a concomitant decrease in the number of cells in
the S and G2-M phase of the cell cycle (Fig. 3A). Only when the HT29 and SW837
cells were treated with higher concentrations (5 or 10 µM)
of PPI for 48 h, they began to detach from the culture dish and
displayed evidence of apoptosis by an increase in the sub-G1
population of cells (Fig. 3A).
Additional experiments detecting DNA fragmentation were performed
with the HT29 cells, as described below.
PPI induces the fragmentation of DNA in
HT29 cells
The appearance of a ʻDNA ladderʼ was observed in a
sample treated with 10 µM PPI on agarose gel electrophoresis
(Fig. 3B), demonstrating the
induction of apoptosis under the current experimental
condition.
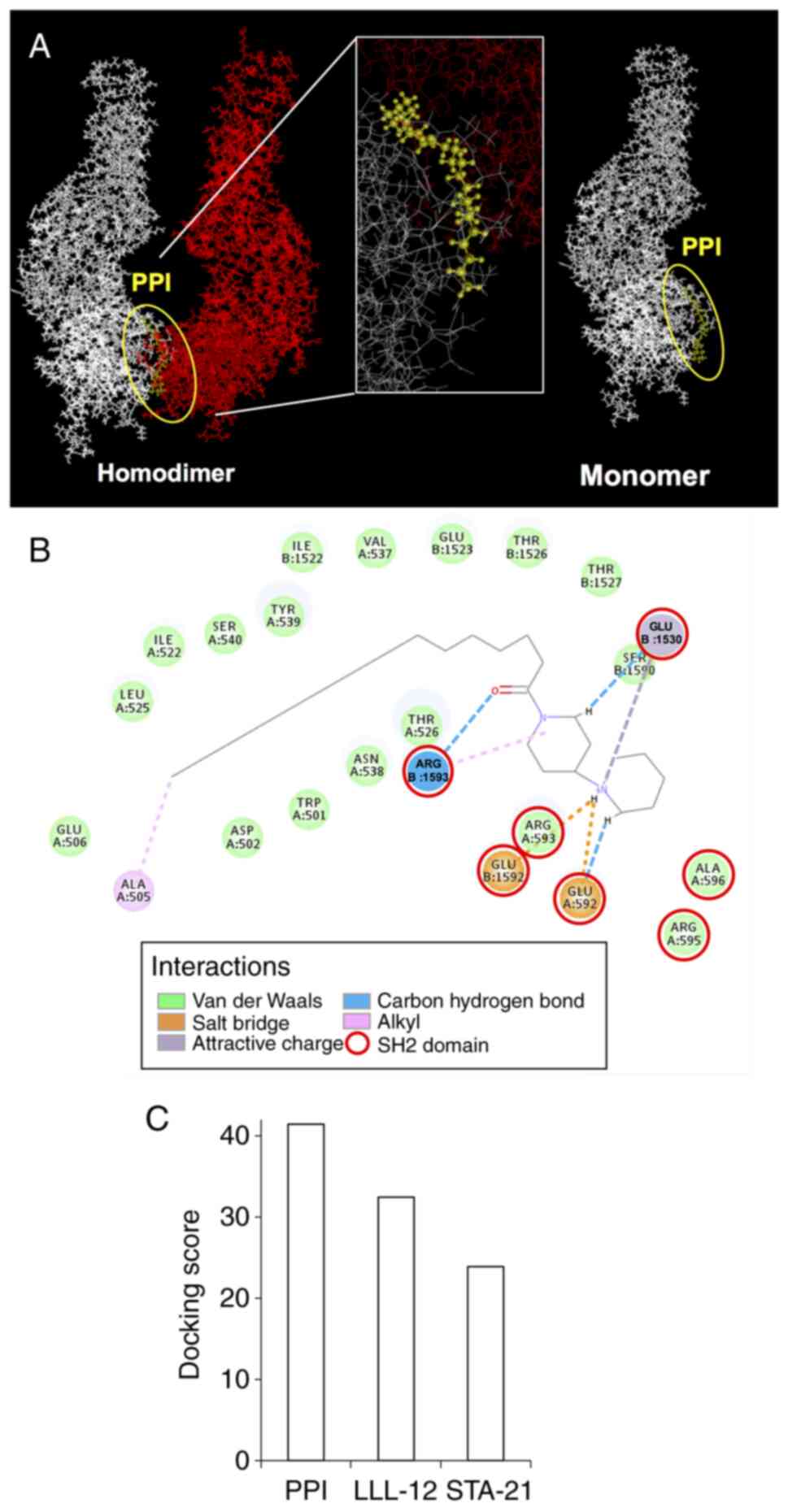
Binding mode of PPI
Previous research has demonstrated that the STAT3
transcription factor plays an important role in the development and
progression of a wide range of cancers, including colon cancer, by
regulating cell proliferation, cell cycle progression, cell
survival, angiogenesis, immune evasion and epithelial-mesenchymal
transition (27). In fact,
constitutive activation of STAT3 is frequently seen in human
cancers including colorectal cancer (28). The present study also found that
PPI inhibited the growth of human colon carcinoma cell lines as
described above. Furthermore, a critical step in STAT3 activation
is the dimerization between two STAT3 monomers, and this step is
dependent on the reciprocal binding of the SH2 domain of one STAT3
monomer to the other monomer (29). Thus, to understand the interaction
between PPI and the protein STAT3, PPI was docked in the active
site of STAT3 (PDB code: 3CWG). As shown in Fig. 4A, PPI exhibited shape
complementarity with the binding pocket of the SH2 domain of STAT3.
The carbon, oxygen, nitrogen, or hydrogen atoms of PPI formed
interactions, such as alkyl, salt bridge, carbon hydrogen bond, van
der Waals or attractive charge, and came into close contact with
the amino acid residues, Trp, Asp, Ala, Glu, Ile, Leu, Thr, Val,
Asn, Tyr, Ser, or Arg (Fig. 4B).
The binding affinity was evaluated by the -CDOCKER_ENERGY
(kcal/mol) obtained from the docking analysis of a compound of
interest. As a result of this calculation, PPI exhibited a higher
-CDOCKER_ENERGY than that of the conventional specific inhibitors,
LLL-12 and STA-21, of the SH2 domain of STAT3 (Fig. 4C).
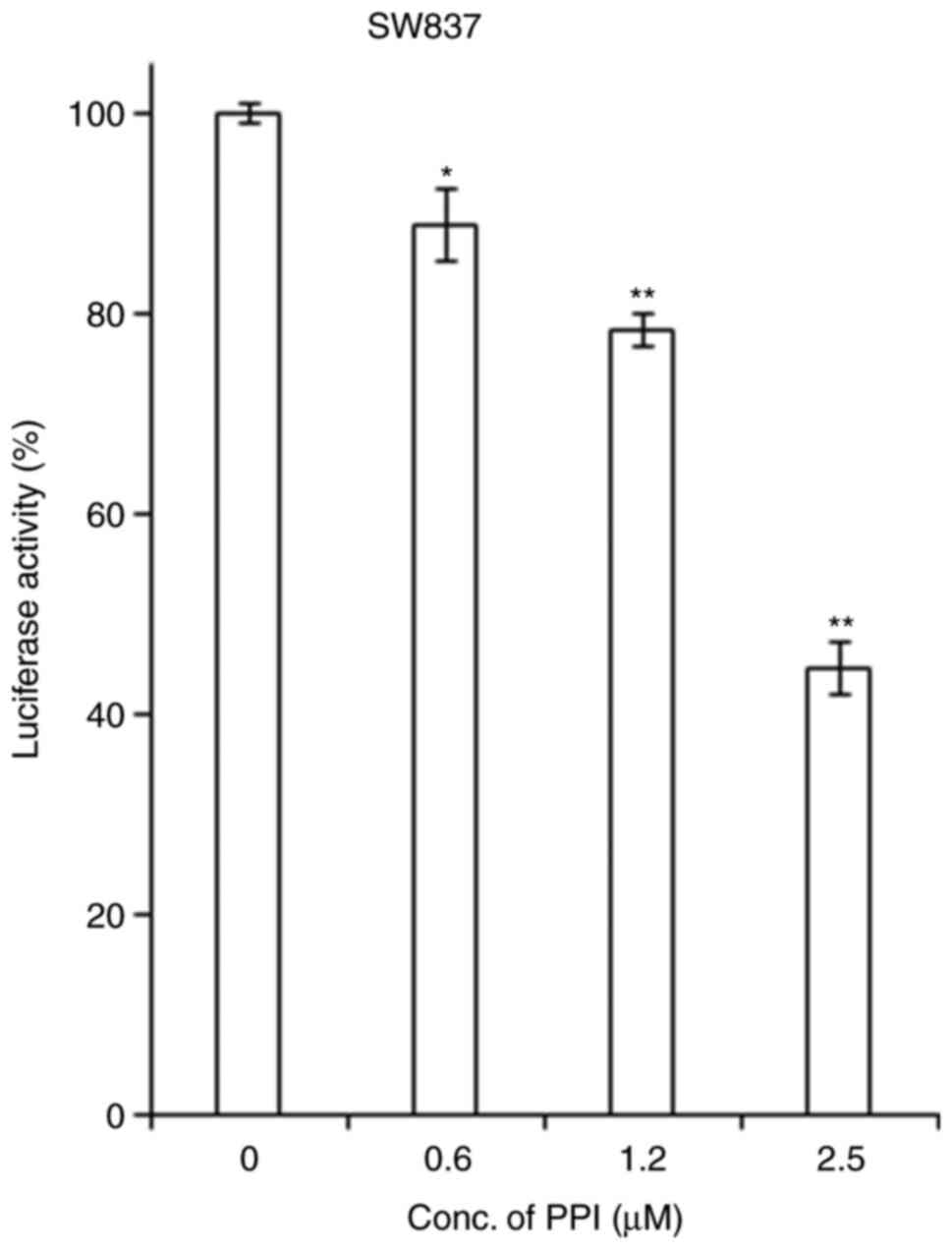
PPI inhibits the transcriptional activity
of STAT3
Based on the above-mentioned finding that PPI can
bind to SH2 domain of STAT3 in a molecular docking analysis, the
present study then examined the effects of PPI on the
transcriptional activity of the STAT3 in transient transfection
luciferase reporter assays using a STAT3 reporter. It was found
that treatment with PPI led to a dose-dependent decrease in the
activity of the STAT3 reporter in SW837 cells (Fig. 5).
Effects of PPI on the expression levels
of STAT3 and p-STAT3 in whole lysate samples and cytosol/chromatin
fraction samples
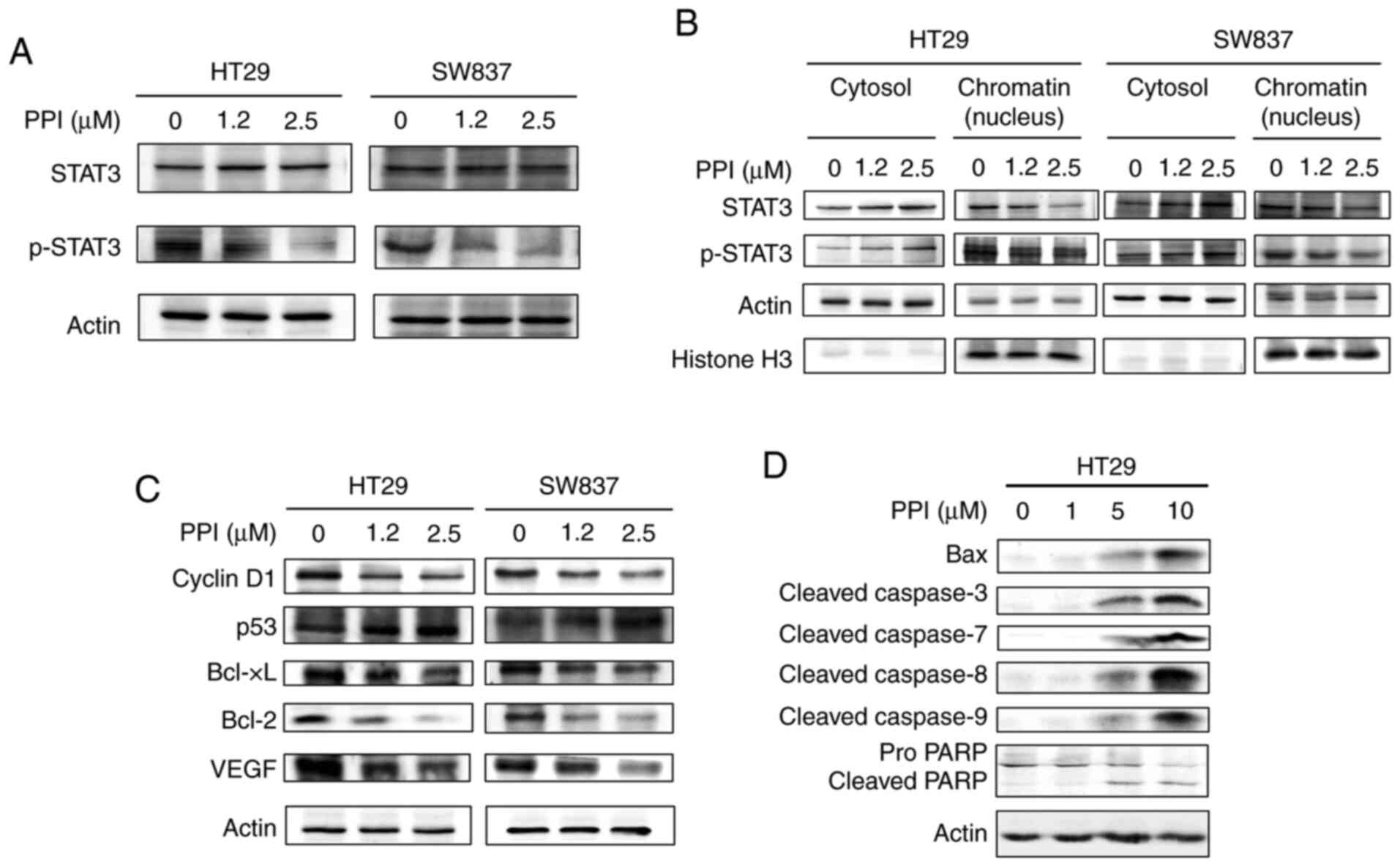
As it was found that PPI inhibited the
transcriptional activity of STAT3 in a dose-dependent manner,
western blot analysis was performed to determine whether treatment
of the HT29 and SW837 cells with PPI alters the cytosolic or
nuclear levels of the STAT3 and phosphorylated form of STAT3
(p-STAT3) in 3 different fractions of cell lysates. In the whole
cell lysate samples, it was found that treatment of the HT29 and
SW837 cells with 1.2 or 2.5 µM PPI led to a marked decrease
in the expression levels of p-STAT3, but not those of STAT3 in
these cells (Fig. 6A). In the HT29
and SW837 cell lines, treatment of the cells with 1.2 or 2.5
µM PPI led to a decrease in the expression levels of both
STAT3 and p-STAT3 in the chromatin fraction (Fig. 6B). In the HT29 and SW837 cells,
treatment of these cells with 1.2 or 2.5 µM PPI led to an
increase in the expression levels of both STAT3 and p-STAT3 in the
cytosolic fraction (Fig. 6B).
Effects of PPI on the expression levels
of cell cycle-related and STAT3-driven molecules
Due to the finding that PPI induced G1 arrest in the
cell cycle (Fig. 3A) and that PPI
inhibited the expression levels of p-STAT3 (Fig. 6A), the present study wished to
determine whether treatment of the HT29 and SW837 cells with 1.2 or
2.5 µM PPI alters the cellular levels of the G1 cell cycle
control protein cyclin D1, the cell cycle inhibitor protein p53 and
STAT3-driven molecules. It was found that treatment with PPI led to
an increase, in p53 and a decrease in cyclin D1, Bcl-xL, Bcl-2 and
VEGF expression (Fig. 6C). These
changes occurred in a dose-dependent manner.
Effects of PPI on the levels of
expression of apoptosis-related molecules
From the above-mentioned findings, it could be
inferred that PPI induced the apoptosis of the HT29 cell line.
Thus, it was of interest to determine whether PPI affects the
cellular levels of apoptosis-related molecules. HT29 cells were
treated with 0.2% acetone (control) or with increasing
concentrations (1, 5 and 10 µM) of PPI for 48 h. PPI induced
a marked and dose-dependent increase in the expression levels of
Bax, cleaved caspase-3, cleaved caspase-7, cleaved caspase-8,
cleaved caspase-9 and cleaved PARP (Fig. 6D). PPI also induced a concomitant
decrease in the expression levels of pro PARP when the carcinoma
cells were treated with 5 or 10 µM (Fig. 6D).
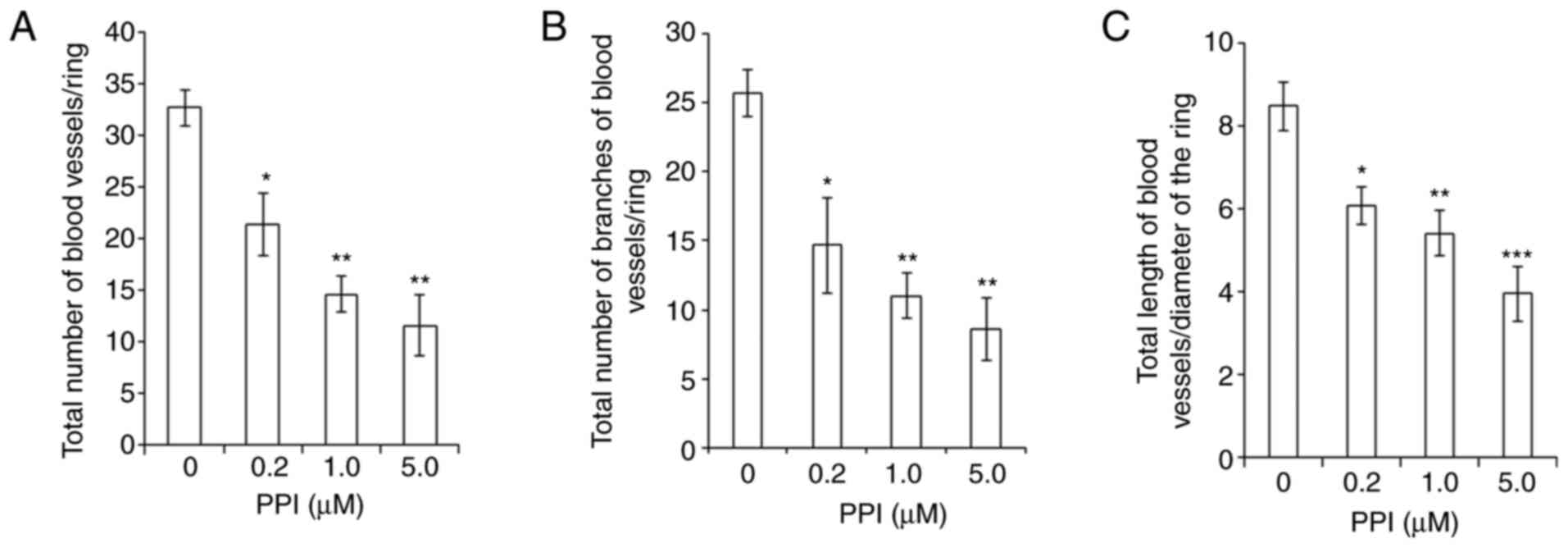
Inhibitory effects of PPI on the
angiogenesis of the CAM
From the above-presented results of western blot
analyses, it was found that PPI inhibited the expression level of
VEGF (Fig. 6C), a key mediator of
angiogenesis. Thus, the anti-angiogenic effect of PPI was also
examined in vivo using CAM assays. A marked and
dose-dependent inhibition of the angiogenesis of the CAM was
observed (Fig. 7). There was a
marked and dose-dependent decrease in the total number of blood
vessels/ring (P<0.001; Fig.
7A), the total number of branches of blood vessels/ring
(P<0.001; Fig. 7B) and the
total length of blood vessels/diameter of the ring (P<0.001;
Fig. 7C).
Growth inhibitory and anti-angiogenic
effects of PPI in a xenograft model
As the above-described in vitro assays
provided evidence that PPI inhibits the in vitro growth of
human colon carcinoma cells (Fig.
2) and the angiogenesis of CAM (Fig. 7), it was of interest to determine
whether PPI also exerts potent inhibitory effects on tumor growth
and angiogenesis in vivo. All the BALB/cSlc-nu/nu
mice in the present study survived (the experimental protocol is
illustrated in Fig. 8A). Treatment
of the mice with PPI did not cause significant body weight loss
during the experiment (Fig. 8B).
Body weight (g) at the end of each treatment was 17.9±0.40 (group
1) and 18.1±0.29 (group 2). After 4 experimental weeks, treatment
of the mice with 50 mg/kg PPI (group 2) resulted in a significant
decrease in tumor volume when compared to the control
vehicle-treated mice (group 1) (P<0.001; Fig. 8C). Treatment of the mice with 50
mg/kg PPI resulted in a significant decrease in the number of blood
vessels compared to the controls (Fig.
8D). Collectively, these results demonstrate that PPI exerts a
potent inhibitory effect on angiogenesis in vivo.
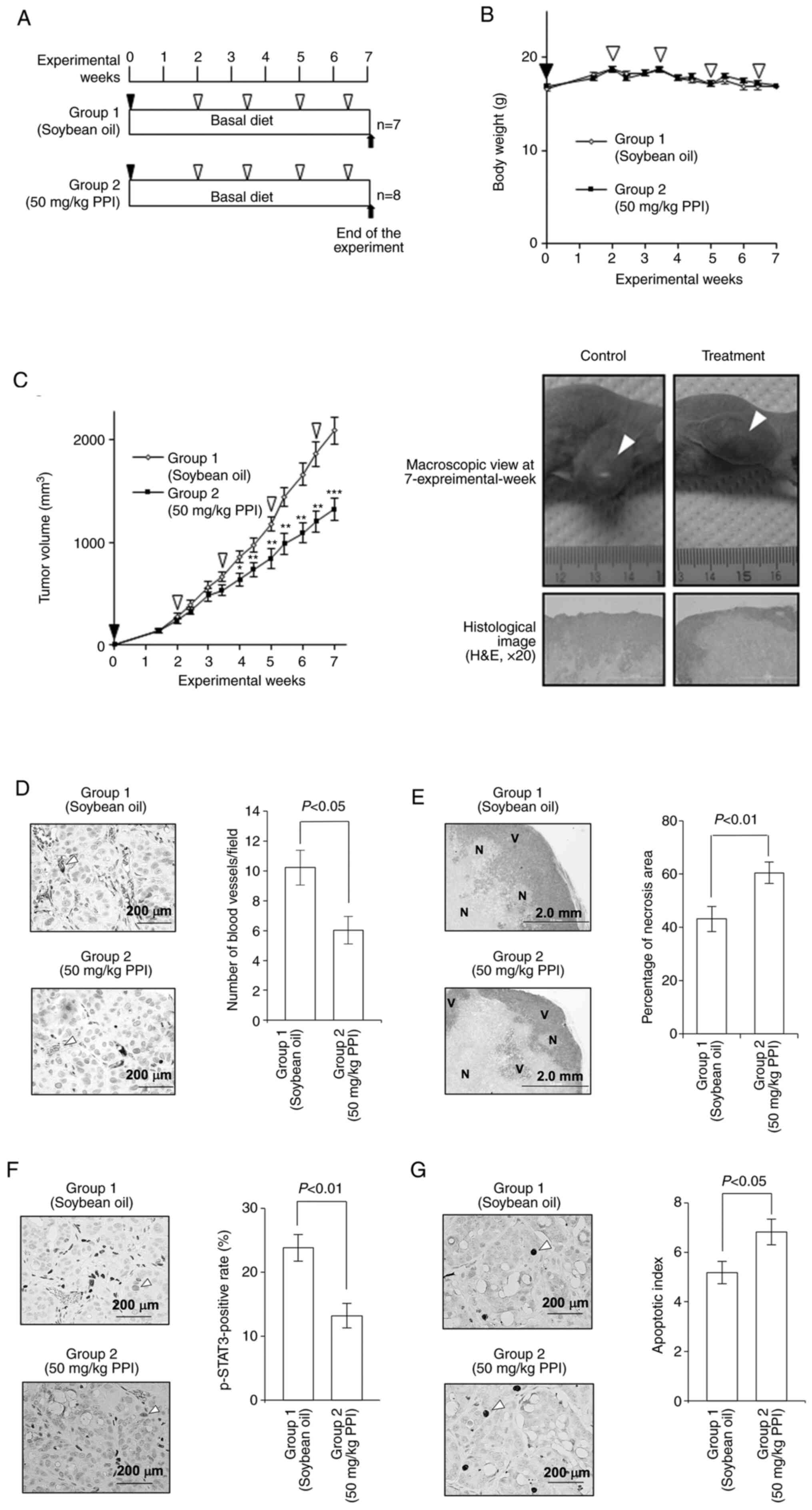 | Figure 8Effects of PPI in a mouse xenograft
model. (A) Experimental protocol. After inoculation of viable HT29
human colon carcinoma cells into the flanks of mice, the animals
were administered intraperitoneal injections of soybean oil
(control) or 50 mg/kg PPI once per 10 days (a total of 4 times)
until the end of the experiment. (B) Time-course record of body
weight during the experiment. Treatment of mice with PPI did not
cause a significant body weight loss during experiment. Black
arrowheads indicate the inoculation of HT29 cells; white arrowheads
indicate intraperitoneal injections of soybean oil (group 1) or 50
mg/kg PPI (group 2). (C) Tumor volume during the experiment (left
panel). A marked decrease in tumor volume was observed after 4
experimental weeks (*P<0.05, **P<0.01
and ***P<0.001). Representative images of animals
with tumors are also shown (right panel). Arrowheads indicate the
tumor mass in the right flank of the mice, and histological images
are also shown. The tumor of the control measured 17.2 mm in
length, 16.4 mm in width and 12.2 mm in depth. The tumor of the
treatment group measured 17.8 mm in length, 12.9 mm in width and
9.4 mm in depth. (D) Representative images of CD34
immunohistochemical staining of the tumor. Vehicle control (soybean
oil, left upper panel), PPI treatment (left lower panel), and the
number of CD34-positive blood vessels (right panel) are shown. (E)
Vehicle control (soybean oil, left upper panel), PPI treatment
(left lower panel) and percentage of necrosis area are shown. This
value was calculated, with 100% representing a total area (necrosis
plus viable areas) of the tumor. 'N' and 'V' indicate necrosis and
viable areas, respectively. (F and G) p-STAT3-positive rate (%) and
apoptotic index are shown, respectively. Representative images of
(F) p-STAT3 and (G) cleaved caspase-3 immunohistochemical staining
of the tumor are demonstrated in vehicle-control (soybean oil, left
upper panel), PPI treatment (left lower panel). Arrowheads indicate
positively stained cells with each antibody. Error bars indicate
the SE in each panel. PPI, palmitoyl piperidinopiperidine; STAT3,
signal transducer and activator of transcription 3. |
PPI induces an increase in the necrotic
area in implanted tumors
As it was found that there was a significant
decrease in tumor volume in the PPI-treated group, the present
study then investigated its effects on the induction of apoptosis
by determining the area in which cells underwent apoptosis with a
microscope imaging system. There was a significant increase in the
necrotic area of the PPI-treated tumors compared to that of the
vehicle-treated tumors (P<0.01; Fig. 8E).
Effects of PPI on the immunohistochemical
expression levels of p-STAT3 and apoptotic index in the tumor
xenograft model
In view of the above-mentioned findings that PPI
induced apoptosis, as shown by flow cytometric analysis and DNA
fragmentation assay, the present study then examined the mechanisms
through which PPI plays a role in controlling the apoptotic process
in the tumor. For this purpose, the effects of PPI were assessed by
conducting immunohistochemical analysis using p-STAT3 and cleaved
caspase-3 antibodies. Treatment of the mice with PPI led to a
significant decrease in the p-STAT3 positive rate of the tumor
compared to the control (Fig. 8F)
and also led to a significant increase in the apoptotic index
compared to the control (Fig.
8G).
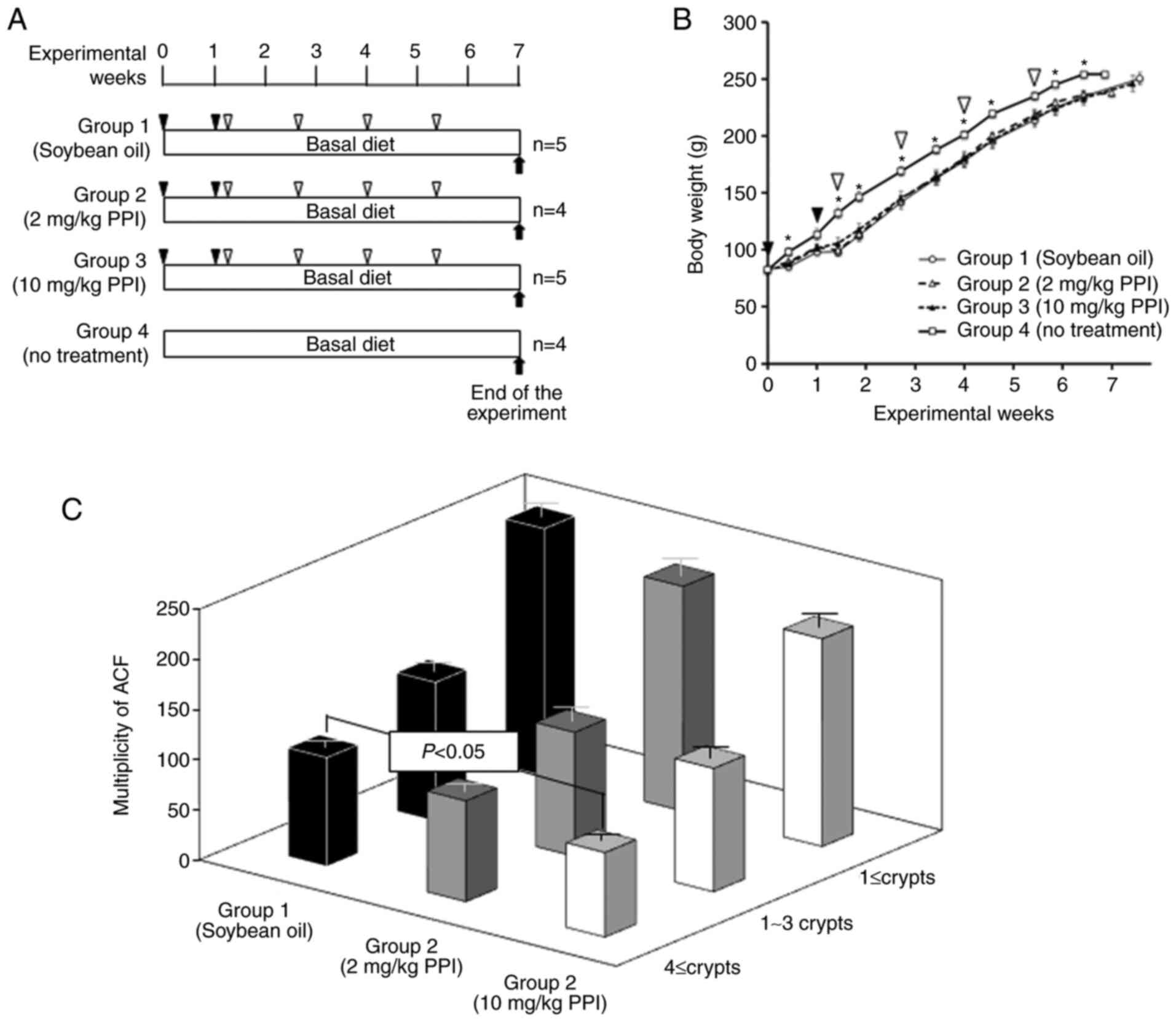
Inhibition of the formation of ACF by
PPI
To examine whether PPI exerts inhibitory effects in
the post-initiation phase of AOM-induced colon carcinogenesis, the
well-established and short-term protocol of the rat ACF model
system was used (4,24). All rats in the present study
survived (the experimental protocol is illustrated in Fig. 9A) and no significant body weight
loss was observed between the PPI-treated groups (groups 2 and 3)
and the vehicle-treated control group (group 1) during the
experiment (Fig. 9B). However,
there was a significant decrease in body weight of the PPI-treated
groups (groups 1-3) when compared to that of the no treatment group
(group 4) due to the effects of the administration of the
carcinogen, AOM (Fig. 9B). Body
weight (g) at the start of the experiment was 83.0±1.52 (group 1),
84.3±2.53 (group 2), 82.2±2.27 (group 3) and 82.5±2.22 (group 4).
Body weight (g) at week 7 was 250.4±5.46 (group 1), 238.0±3.49
(group 2), 246.2±7.14 (group 3) and 254.0±3.08 (group 4). ACF were
mainly found in the middle portion of the colon. PPI significantly
inhibited the multiplicity of larger ACF consisting of ≥4 aberrant
crypts (Fig. 9C). There was a
dose-dependent decrease observed in the multiplicity of ACF,
although this finding was not statistically significant. These
findings indicate that the inhibitory effect on tumor promotion was
at least in part via the formation of larger ACF in a
carcinogen-induced rat ACF model system.
Discussion
The fact that natural products have been
traditionally used worldwide in the prevention and/or treatment of
several chronic diseases among various ethnic societies was the
inspiration for the present study. It was hypothesized that it
would be possible to develop a novel anticancer drug derived from
10-HDA as an initial lead compound by creating a structure-based
pharmacophore model as it was found that 10-HDA inhibited the
growth of human colon carcinoma cells (Fig. S1) (16). After synthesizing >100
derivatives of 10-HDA (Fig. 1A)
and optimizing their structures by quantitative structure-activity
relationship (QSAR), the present study eventually obtained one
candidate compound, named PPI (Fig.
1B). Several polyamine derivatives resembling PPI in structure
were demonstrated in a previous study; however, none of these
exhibited potent growth inhibitory activity against leukemia P-388
or human epidermoid carcinoma of the nasopharynx KB cell lines
(30). When carcinoma cells were
treated with the effective concentration level of PPI,
approximately >90% of carcinoma cells died, but at the same
concentration, >80% of normal colon epithelial cells survived
(Fig. 2B), indicating that this
drug selectively kills carcinoma cells under appropriate
experimental conditions.
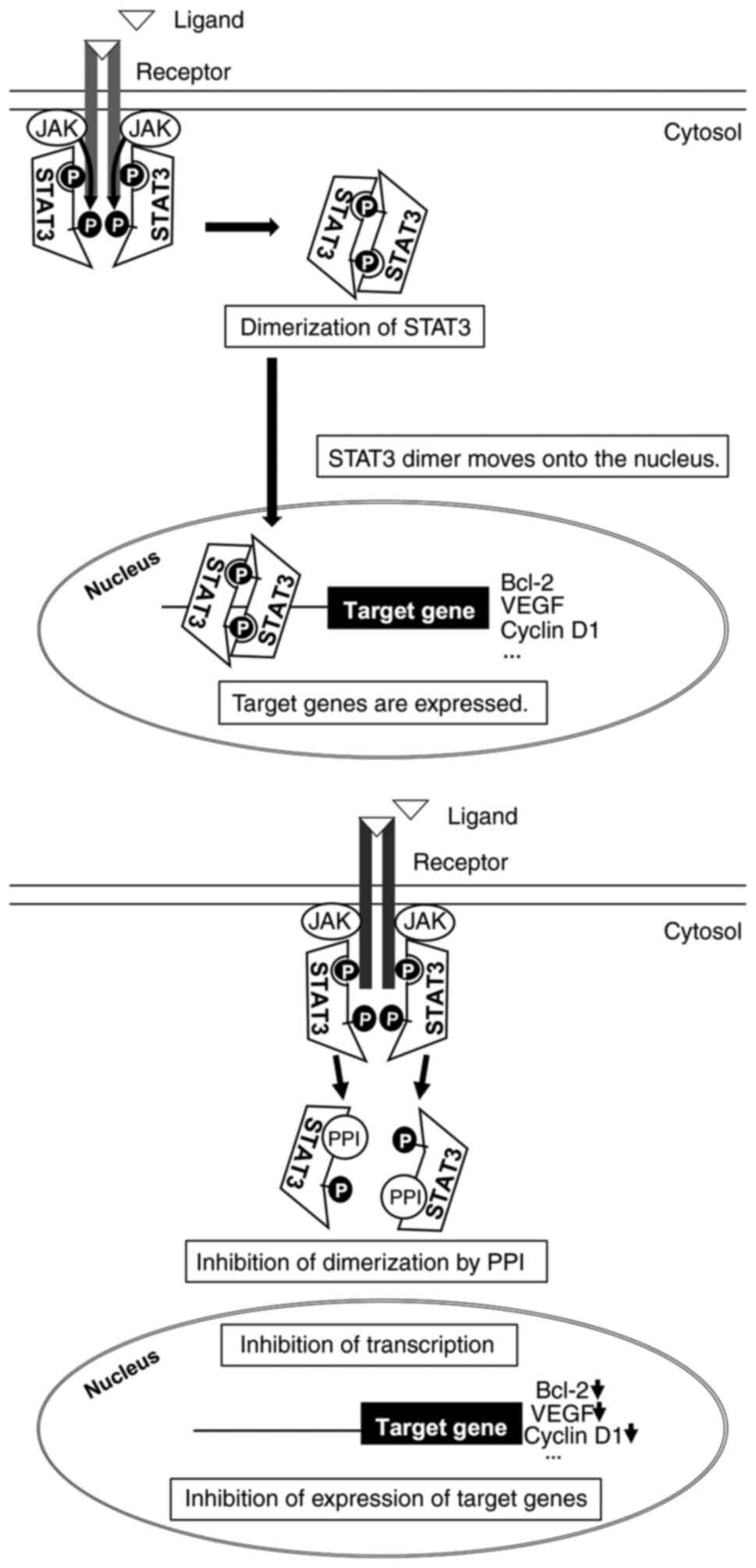
The present study focused on the transcription
factor, STAT3, that is highly expressed in colon carcinoma cells or
tissues (28) and can drive
carcinogenesis- or angiogenesis-related genes (31). STAT3 is activated by
phosphorylation at tyrosine residue 705 (Tyr-705), which leads to
the dimerization, nucleus translocation, recognition of STAT3
specific DNA binding site (32),
and the activation of target gene transcription, including Bcl-2,
VEGF and cyclinD1 (31). The
dimerization of STAT3 is dependent on the reciprocal binding of the
SH2 domain of one monomer to the Pro-pTyr-Leu-Lys-Thr-Lys segment
of the other STAT3 monomer (33).
In silico docking simulation in the present study exhibited
that PPI can bind to SH2 domain of STAT3, suggesting that this drug
blocks dimerization of STAT3, thereby inactivating its
function.
The present study then focused on the mechanisms
through which PPI kills human colon carcinoma cells. It was found
that PPI inhibits the transcriptional activity of STAT3 and
decreases the expression level of the phosphorylated form of STAT3
(p-STAT3). PPI also led to a decrease in the expression level of
STAT3 and p-STAT3 in the chromatin fraction (Fig. 6B), suggesting the inhibition of the
translocation of these molecules. However, further investigations
are necessary to draw firm conclusions on this aspect. Treatment
with PPI led to an increase in p53, and a decrease in cyclin D1,
Bcl-2, and Bcl-xL expression. Furthermore, there was a marked
increase in the levels of expression of Bax, cleaved caspase-3,
cleaved caspase-7, cleaved caspase-8, cleaved caspase-9 and cleaved
PARP, with a concomitant decrease in the levels of pro PARP.
Together with the findings of flow cytometric analysis, PPI induced
the dose-dependent inhibition of carcinoma cell growth, and this
anti-proliferative effect appears to be due to its ability to
induce, at least in part, G1-phase arrest and apoptotic cell death.
There are two different pathways that lead to apoptosis: The
mitochondrial and death receptor path-ways (34). Both pathways utilize caspase to
induce apoptosis. In the present study, it was found that PPI
induced changes in the protein expression levels of caspases and
Bcl-2 family proteins (Fig. 6C and
D), indicating at least in part, the involvement of these
molecules in apoptosis. In addition, the finding that p53-mutant
cell lines HT29 and SW837 are sensitive to the treatment of PPI
warrants further investigation. In the present study, STAT3
specific inhibitors (LLL-12 and STA-2) were used (Fig. 4). It has been reported that the
IC50 values of LLL-12 are 0.16-3.09 µM in several
human tumor cell lines (35). In a
previous study, treatment of the MDA-MB-468 cells with 20 or 30
µM STA-21 led to a potent inhibitory effect on the growth
and survival of these cells (36).
In the present study, the IC50 values of PPI were
0.5-2.2 µM, suggesting that PPI exerts more potent growth
inhibitory effects than LLL-12 or STA-21 in several cell lines
tested (35,36).
By performing CAM assay, the present study examined
whether this drug inhibits angiogenesis. It was found that PPI
inhibited the total number (maximum effect, 65%), length (maximum
effect, 53%) and the number of branch of blood vessels (maximum
effect, 67%) in CAM, in a dose-dependent manner. In chick CAM, it
has been demonstrated that VEGF plays an important role in
angiogenesis (37) and that the
anti-angiogenic effect may be associated with the expression levels
of VEGF (37). In addition, the
present study found that treatment of human colon carcinoma cells
with PPI led to a marked decrease in the expression level of VEGF.
Collectively, the transcriptional inhibition of STAT3 by PPI may be
one possible mechanism, where the functional performance of
molecules related to apoptosis, angiogenesis and cell cycle
progression are affected, and eventually contribute to growth
inhibition (Fig. 10). Further
studies are in progress to examine the range of anticancer activity
of PPI in a spectrum of the current four human colon cancer cell
lines.
In a mouse xenograft model, at the end of the
experiment, an approximately 40% inhibition in the size of the
tumor was observed. PPI induced a significant decrease in the
p-STAT3 positive rate by 40%. Furthermore, it was found that the
apoptotic index in the tumor tissue increased and the number of
CD34-positive blood vessels decreased, indicating the growth
inhibition, the induction of apoptosis and the inhibition of
angiogenesis in vivo. Finally, a rat ACF model system was
used to evaluate inhibitory effect of PPI in the post-initiation
phase of AOM-induced colon carcinogenesis. ACF are cryptic lesions
distinguished by their increased size and thicker epithelial lining
(24). Furthermore, ACF have only
been observed in the colon of carcinogen-treated mice and rats and
recognized as preneoplastic lesions (24). In the present study, F344 rats
received subcutaneous injections of the carcinogen, AOM, and were
then treated with the vehicle soybean oil or PPI. By counting the
number of precancerous lesions, ACF, and comparing it between
control and treatment groups, the preventive effects of PPI on the
post-initiation phase of AOM-induced colon carcinogenesis were
determined. A study of longer duration may be of use to evaluate
the inhibitory effect on colon carcinogenesis and toxicity or the
adverse side-effects of PPI in clinical trials. In the present
study, PPI induced a significant decrease in the multiplicity (no.
of ACF per colon) of larger ACF consisting of ≥4 aberrant crypts,
presumably demonstrating the inhibitory effect on tumor promotion
at least in part via a formation of larger ACF in a
carcinogen-induced rat ACF model system. ACF range in size and have
from 1 to 412 aberrant crypts per focus (38-41).
The size in the topographic view and histologically dysplastic
feature of ACF are critical factors when ACF are distinguished as
preneoplastic lesions (23). In
histological slides, large ACF exhibit dysplasia and can thus be
termed as microadenoma (24).
Therefore, PPI may target more malignant population of affected
cells, although this aspect warrants further investigation.
Supplementary Data
Funding
The present study was supported in part by grants
from the Ministry of Education, Culture, Sports, Science and
Technology of Japan (grant nos. 22501050, 25430154 and 17K07223 to
MS).
Availability of data and materials
All data generated or analyzed during the current
study are included in the current article.
Authors' contributions
SA, MI and MS designed the study. SA, EY, KF, HM, MI
and MS performed the experiments and acquired the data. SA, EY, KF,
HM, MI and MS analyzed and interpreted the data. SA, KF, MI and MS
wrote, reviewed and/or revised the manuscript. MI and MS
contributed to the material management. MS supervised the study.
All the authors are fully aware of the contents of this paper, and
all authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were performed with the
approval of the Animal Ethics Committee of the Nagoya City
University (approval nos. H25M-16 and H25M-44) and according to the
guidelines of the committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Dr Sachi Sri Kantha
(Gifu University, Gifu Pharmaceutical University) for providing
critical discussions and comments; Mr. Kenta Moriwaki, Mr. Yasuaki
Isoda, Mr. Keigo Sato (Graduate School of Medical Sciences and
Medical School, Nagoya City University) and Dr Munehiro Yamada (NOF
Corp., Chemicals Research Laboratory, Amagasaki, Japan) for their
excellent technical assistance; and Dr Takamitsu Morioka, Dr
Shizuko Kakinuma and Dr Yoshiya Shimada for the use of the
analyzing device (Department of Radiation Effects Research,
National Institute of Radiological Sciences, National Institutes
for Quantum and Radiological Science and Technology, Chiba, Japan).
The authors also wish to acknowledge the assistance of the Research
Equipment Sharing Center at the Nagoya City University.
References
|
1
|
Surh YJ: Cancer chemoprevention with
dietary phytochemicals. Nat Rev Cancer. 3:768–780. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee KW, Bode AM and Dong Z: Molecular
targets of phytochemicals for cancer prevention. Nat Rev Cancer.
11:211–218. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ishihara M, Naoi K, Hashita M, Itoh Y and
Suzui M: Growth inhibitory activity of ethanol extracts of Chinese
and Brazilian propolis in four human colon carcinoma cell lines.
Oncol Rep. 22:349–354. 2009.PubMed/NCBI
|
|
4
|
Morioka T, Suzui M, Nabandith V, Inamine
M, Aniya Y, Nakayama T, Ichiba T, Mori H and Yoshimi N: The
modifying effect of Peucedanum japonicum, a herb in the Ryukyu
Islands, on azoxymethane-induced colon preneoplastic lesions in
male F344 rats. Cancer Lett. 205:133–141. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Morioka T, Suzui M, Nabandith V, Inamine
M, Aniya Y, Nakayama T, Ichiba T and Yoshimi N: Modifying effects
of Terminalia catappa on azoxymethane-induced colon carcino-genesis
in male F344 rats. Eur J Cancer Prev. 14:101–105. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Blum MS, Novak AF and Taber S III:
10-Hydroxy-delta 2-decenoic acid, an antibiotic found in royal
jelly. Science. 130:452–453. 1959. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lerkcer G, Capella P, Conte LS, Ruini F
and Giordani G: Components of royal Jelly: I. Identification of the
organic acids. Lipids. 16:912–919. 1981. View Article : Google Scholar
|
|
8
|
Sugiyama T, Takahashi K and Mori H: Royal
jelly acid, 10-hydroxy-trans-2-decenoic acid, as a modulator of the
innate immune responses. Endocr Metab Immune Disord Drug Targets.
12:368–376. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li X, Huang C and Xue Y: Contribution of
lipids in honeybee (Apis mellifera) royal jelly to health. J Med
Food. 16:96–102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen YF, Wang K, Zhang YZ, Zheng YF and Hu
FL: In vitro anti-inflammatory effects of three fatty acids from
royal jelly. Mediators Inflamm. 2016:35836842016. View Article : Google Scholar :
|
|
11
|
Yatsunami K and Echigo T: Antibacterial
action of Royal jelly. Bull Fac Agr Tamagawa Univ. 25:13–22.
1985.
|
|
12
|
Alreshoodi FM and Sultanbawa Y:
Antimicrobial activity of royal jelly. Anti-Infective Agents.
13:50–59. 2015. View Article : Google Scholar
|
|
13
|
Sediva M, Laho M, Kohutova L, Mojzisova A,
Majtan J and Klaudiny J: 10-HDA, A major fatty acid of Royal jelly,
exhibits pH dependent growth-inhibitory activity against different
strains of Paenibacillus larvae. Molecules. 23:32362018. View Article : Google Scholar
|
|
14
|
Townsend GF, Morgan JF, Tolnai S, Hazlett
B, Morton HJ and Shuel RW: Studies on the in vitro antitumor
activity of fatty acids. I 10-Hydroxy-2-decenoic acid from royal
jelly. Cancer Res. 20:503–510. 1960.PubMed/NCBI
|
|
15
|
Yang YC, Chou WM, Widowati DA, Lin IP and
Peng CC: 10-hydroxy-2-decenoic acid of royal jelly exhibits
bactericide and anti-inflammatory activity in human colon cancer
cells. BMC Complement Altern Med. 18:2022018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suzui M: An anticancer agent derived from
natural fatty acid. Chemical Industry. 66:22–27. 2015.In
Japanese.
|
|
17
|
Suzui M and Iinuma M: Anticancer agent.
Japan Patent 5597427. 2014
|
|
18
|
Suzui M, Masuda M, Lim JTE, Albanese C,
Pestell RG and Weinstein IB: Growth inhibition of human hepatoma
cells by acyclic retinoid is associated with induction of p21(CIP1)
and inhibition of expression of cyclin D1. Cancer Res.
62:3997–4006. 2002.PubMed/NCBI
|
|
19
|
Suzui M, Inamine M, Kaneshiro T, Morioka
T, Yoshimi N, Suzuki R, Kohno H and Tanaka T: Indole-3-carbinol
inhibits the growth of human colon carcinoma cells but enhances the
tumor multiplicity and volume of azoxymethane-induced rat colon
carcinogenesis. Int J Oncol. 27:1391–1399. 2005.PubMed/NCBI
|
|
20
|
Suzui M, Sunagawa N, Chiba I, Moriwaki H
and Yoshimi N: Acyclic retinoid, a novel synthetic retinoid,
induces growth inhibition, apoptosis, and changes in mRNA
expression of cell cycle- and differentiation-related molecules in
human colon carcinoma cells. Int J Oncol. 28:1193–1199.
2006.PubMed/NCBI
|
|
21
|
Suzui M, Shimizu M, Masuda M, Lim JTE,
Yoshimi N and Weinstein IB: Acyclic retinoid activates retinoic
acid receptor beta and induces transcriptional activation of
p21(CIP1) in HepG2 human hepatoma cells. Mol Cancer Ther.
3:309–316. 2004.PubMed/NCBI
|
|
22
|
Kawamori T, Rao CV, Seibert K and Reddy
BS: Chemopreventive activity of celecoxib, a specific
cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer
Res. 58:409–412. 1998.PubMed/NCBI
|
|
23
|
Futakuchi M, Nitanda T, Ando S, Matsumoto
H, Yoshimoto E, Fukamachi K and Suzui M: Therapeutic and preventive
effects of osteoclastogenesis inhibitory factor on osteolysis,
proliferation of mammary tumor cell and induction of cancer stem
cells in the bone microenvironment. Int J Mol Sci. 19:8882018.
View Article : Google Scholar :
|
|
24
|
Suzui M, Morioka T and Yoshimi N: Colon
preneoplastic lesions in animal models. J Toxicol Pathol.
26:335–341. 2013. View Article : Google Scholar
|
|
25
|
Bird RP: Observation and quantification of
aberrant crypts in the murine colon treated with a colon
carcinogen: preliminary findings. Cancer Lett. 37:147–151. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kanda Y: Investigation of the freely
available easy-to-use soft-ware 'EZR' for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar
|
|
27
|
Yu H and Jove R: The STATs of cancer-new
molecular targets come of age. Nat Rev Cancer. 4:97–105. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin Q, Lai R, Chirieac LR, Li C, Thomazy
VA, Grammatikakis I, Rassidakis GZ, Zhang W, Fujio Y, Kunisada K,
et al: Constitutive activation of JAK3/STAT3 in colon carcinoma
tumors and cell lines: Inhibition of JAK3/STAT3 signaling induces
apoptosis and cell cycle arrest of colon carcinoma cells. Am J
Pathol. 167:969–980. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shuai K, Horvath CM, Huang LH, Qureshi SA,
Cowburn D and Darnell JE Jr: Interferon activation of the
transcription factor Stat91 involves dimerization through
SH2-phosphotyrosyl peptide interactions. Cell. 76:821–828. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Weinstock LT, Rost WJ and Cheng CC:
Synthesis of new poly-amine derivatives for cancer chemotherapeutic
studies. J Pharm Sci. 70:956–959. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Carpenter RL and Lo HW: STAT3 target genes
relevant to human cancers. Cancers. 6:897–925. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Reich NC and Liu L: Tracking STAT nuclear
traffic. Nat Rev Immunol. 6:602–612. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fletcher S, Turkson J and Gunning PT:
Molecular approaches towards the inhibition of the signal
transducer and activator of transcription 3 (Stat3) protein. Chem
Med Chem. 3:1159–1168. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pfeffer CM and Singh ATK: Apoptosis: A
target for anticancer therapy. Int J Mol Sci. 19:4482018.
View Article : Google Scholar :
|
|
35
|
Lin L, Hutzen B, Li PK, Ball S, Zuo M,
DeAngelis S, Foust E, Sobo M, Friedman L, Bhasin D, et al: A novel
small molecule, LLL12, inhibits STAT3 phosphorylation and
activities and exhibits potent growth-suppressive activity in human
cancer cells. Neoplasia. 12:39–50. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Song H, Wang R, Wang S and Lin J: A
low-molecular-weight compound discovered through virtual database
screening inhibits Stat3 function in breast cancer cells. Proc Natl
Acad Sci USA. 102:4700–4705. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Baum O, Suter F, Gerber B, Tschanz SA,
Buergy R, Blank F, Hlushchuk R and Djonov V: VEGF-A promotes
intussusceptive angiogenesis in the developing chicken
chorioallantoic membrane. Microcirculation. 17:447–457.
2010.PubMed/NCBI
|
|
38
|
Fenoglio-Preiser CM and Noffsinger A:
Aberrant crypt foci: A review. Toxicol Pathol. 27:632–642. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Di Gregorio C, Losi L, Fante R, Modica S,
Ghidoni M, Pedroni M, Tamassia MG, Gafà L, Ponz de Leon M and
Roncucci L: Histology of aberrant crypt foci in the human colon.
Histopathology. 30:328–334. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nucci MR, Robinson CR, Longo P, Campbell P
and Hamilton SR: Phenotypic and genotypic characteristics of
aberrant crypt foci in human colorectal mucosa. Hum Pathol.
28:1396–1407. 1997. View Article : Google Scholar
|
|
41
|
Shpitz B, Bomstein Y, Mekori Y, Cohen R,
Kaufman Z, Neufeld D, Galkin M and Bernheim J: Aberrant crypt foci
in human colons: Distribution and histomorphologic characteristics.
Hum Pathol. 29:469–475. 1998. View Article : Google Scholar : PubMed/NCBI
|