Recent progress in the molecular biology of
non-small cell lung cancer (NSCLC) has led to the identification of
diverse molecular mutations based on driver oncogenes, such as
epidermal growth factor receptor (EGFR) and anaplastic
lymphoma kinase (ALK) translocation. EGFR is one of
the most common mutations, and a crucial therapeutic target in
NSCLC. Sensitizing mutations in exon 21 (L858R exon 21-point
mutation) and exon 19 (exon 19 deletions) activate the tyrosine
kinase domain in EGFR, which promote the continuous
uncontrolled cell growth, proliferation and metastasis of tumor
cells in NSCLC. The prevalence of EGFR mutation (EGFRm) is lower
among Caucasian (15-18%) than among Asian (36-40%) and Indian
(22-26%) populations (1-3).
EGFR-tyrosine kinase inhibitors (TKIs) demonstrate
clinical responsiveness by potentially blocking the cell signaling
pathways responsible for EGFR-mutated tumor cell growth (4). The first- and second-generation
EGFR-TKIs have exhibited efficacy as first-line therapy for
patients with NSCLC with EGFRm; however, the emergence of
resistance in patients is inevitable (5,6). The
T790M mutation in exon 20 of EGFR is the most common (50-70%
of tumors) mechanism for secondary resistance to first-line
EGFR-TKIs (7,8). In addition, human epidermal growth
factor receptor (HER2) amplification and HER2 mutation have also
been reported in a subset of EGFR-TKI-resistant lung tumors
(9). Osimertinib is a
third-generation, irreversible, oral EGFR-TKI that potently and
selectively inhibits both EGFRm and EGFR T790M. It has demonstrated
efficacy in NSCLC with central nervous system (CNS) metastases
(10). Previous clinical trials on
EGFR-TKIs have primarily focused on response rates and
progression-free survival (PFS) as endpoints in NSCLC. Although,
currently approved first-, second-, and third-generation EGFR-TKIs
have demonstrated favorable response rates and PFS in NSCLC, the
overall survival (OS) benefits have been marginal with most of the
TKIs (11). OS, historically
considered as the gold standard endpoint for establishing the
efficacy in medical oncology due to its objectivity, reliability
and precision, is defined as the time from randomization to
mortality (12). An increase in
PFS may not necessarily result in an increase in OS among patients
with locally advanced or metastatic NSCLC. However, recent
developments with the use of third-generation TKIs have provided
promising results in terms of OS benefits in NSCLC. The present
narrative review compares the OS benefits of first-, second- and
third-generation EGFR-TKIs for patients with stage IV EGFRm NSCLC
and discusses their role in disease management. Relevant
publications in the English language that reported the clinical
efficacy and safety of EGFR-TKIs were identified by searching the
PubMed, Google Scholar and Embase databases. Articles on clinical
trials and real-world evidence, along with publications from major
oncology societies, such as European Society of Medical Oncology
(ESMO) and National Comprehensive Cancer Network (NCCN), were
included in the present review.
Treatment algorithms for NSCLC have markedly changed
over the past few years with the introduction of targeted
therapies. The current scenario of treatment for stage IV NSCLC
will continue to evolve with emerging clinical and preclinical data
explaining the mechanisms responsible for the observed clinical
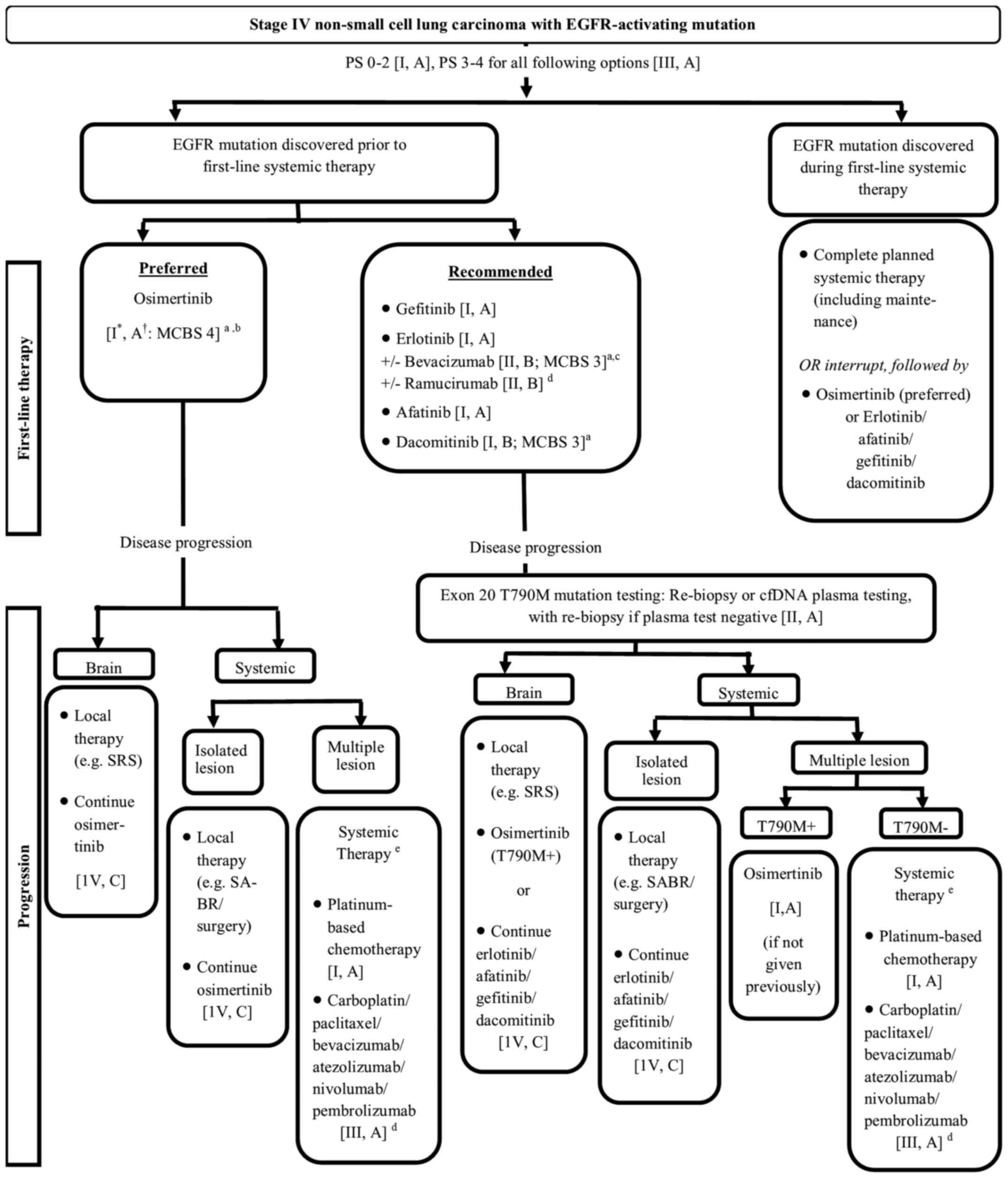
outcomes. The treatment algorithm for stage IV NSCLC with
EGFR-activating mutation as recommended by the ESMO 2019 and NCCN
2020 guidelines is presented in Fig.
1 (13,14).
LUX lung 7, a global randomized trial, revealed the
superiority of afatinib compared with gefitinib as the first-line
treatment in terms of improved PFS and time to treatment failure.
The ARCHER 1050 study reported a favorable PFS for dacomitinib
compared with gefitinib. A higher magnitude of PFS benefit was
observed with dacomitinib as demonstrated in the ARCHER 1050 study
(Table II) (34,36).
Despite the PFS benefit, a greater number of
clinical trials for first- and second-generation EGFR-TKIs have
reported either a null or a marginal benefit for OS compared with
chemotherapy. The NEJ002 trial reported a similar median OS for
gefitinib and carboplatin-paclitaxel [27.7 months vs. 26.6 months;
hazard ratio (HR), 0.887; P=0.483], whereas the EURTAC trial
revealed a marginal (not statistically signifi-cant) OS benefit
with erlotinib vs. chemotherapy [22.9 months vs. 19.6 months; HR,
0.92; 95% confidence interval (CI), 0.63-1.35; P=0.68] (Table I) (23,27).
The LUX-Lung 3 (HR, 0.78; 95% CI, 0.58-1.06; P=0.11) and LUX-Lung 6
(HR, 0.83; 95% CI, 0.62-1.09; P=0.18) trials did not demonstrate an
improved OS with afatinib compared with standard chemo-therapy
(Table I) (30). The LUX-Lung 7 study revealed that
the median OS was numerically higher favoring afatinib (27.9
months), albeit not statistically significant when compared with
gefitinib (25.0 months) (HR, 0.86; P=0.2580) (Table II) (35). The ARCHER 1050 study assessed
dacomitinib compared with gefitinib in treatment-naïve patients and
reported a median OS of 34.1 months with dacomitinib and 26.8
months with gefitinib, with an estimated HR of 0.760 (95% CI,
0.582-0.993; P=0.044) (Table II)
(37). However, the endpoint of OS
was third in hierarchy for statistical analysis following PFS and
ORR. In addition, although the study demonstrated a significant PFS
benefit compared with the control group, the ORR endpoint was not
met. Hence, this OS benefit cannot be considered significant per
the hierarchical approach of hypothesis testing. The United States
FDA also reported that the findings of ARCHER 1050 were not
consistent with an improvement in OS for dacomitinib (38). The improvement in OS may be the
effect of subsequent therapy following the discontinuation of study
drugs, approximately 50% of patients from the dacomitinib group and
62% from the gefitinib group received additional treatment,
primarily chemotherapy, whereas few patients received
third-generation EGFR-TKIs as subsequent therapy (osimertinib,
olmutinib, rociletinib, avitinib and unspecified EGFR-TKIs). The
patients receiving subsequent third-generation EGFR-TKIs appeared
to survive longer than patients who received chemotherapy.
Resistance, brain metastasis and adverse events
(AEs). Despite the initial benefit, in at least half of patients
treated with first- and second-generation EGFR-TKIs, disease
ultimately progresses (after a median of 10-14 months) due to
acquired resistance, primarily in patients with the T790M mutation
encoded by exon 20 of EGFR (Tables
I and II) (20,22,29,31,34,37).
The T790M mutation occurs in approximately 50-70% of tumors with
acquired resistance to EGFR-TKIs (7,8,39-43).
Other biological resistance mechanisms include MET amplification,
EGFR amplification, phosphatidylinositol-4,5-bisphosphate 3-kinase
catalytic subunit alpha (PIK3CA) amplification, HER2 amplification
and histological transformation to small cell lung cancer (44). Alongside the challenge of
resistance, a significant proportion of patients with EGFRm NSCLC
(25%) exhibit brain metastases at diagnosis, which further
escalates during the course of disease (45), leading to poor survival with
significant impairments in the quality of life (46,47).
An insufficient crossing of EGFR-TKIs to sanctuary sites in CNS is
a crucial deficiency resulting in disease progression in CNS with
first- and second-generation EGFR-TKIs. In patients carrying CNS
metastases, both first- and second-generation EGFR-TKIs exhibit
limited efficacy due to the inadequate ability to penetrate the
blood-brain barrier, leading to low concentrations in the
cerebrospinal fluid and the relapse of CNS metastases (48-51).
Unfavorable toxicity profile with AEs, including but not limited
to, fatigue, rash, stomatitis, diarrhea and elevated levels of
alanine aminotransferase, is yet another challenge associated with
first- and second-generation TKIs and may warrant dose reduction or
drug discontinuation (11). The
higher toxicity to first- and second-generation EGFR-TKIs is
attributed to their higher affinity for the wild-type EGFR. A
comparative overview of the AEs for different EGFR-TKIs has been
provided in the studies by Doval et al (52), and Shah and Shah (53).
The limitations associated with first- and
second-generation EGFR-TKIs have paved the way for the development
of third-generation EGFR-TKIs, including osimertinib. The
third-generation agents are pyrimidine-based compounds designed to
target EGFR activating the T790M mutation in a selective and
irreversible manner, facilitating improved potency, better safety
and superior penetration into sanctuary sites in CNS compared with
earlier-generation EGFR-TKIs (54). These TKIs exhibit better tolerance
and a lower epithelial toxicity due to poor wild-type EGFR
activity compared with earlier-generation EGFR-TKIs (55,56).
Osimertinib inhibits EGFR carrying T790M, del19 and L858R
mutations, with least activity against the wild-type EGFR.
Evidence from pre-clinical studies has also demonstrated the
antitumor efficacy of osimertinib against multiple HER2 aberrations
in lung cancer, either as a single agent or in combination with the
BET inhibitor JQ1 (57).
Initially, osimertinib received accelerated approval
by the FDA (2015) for T790M mutation-positive NSCLC following
resistance to first-line EGFR-TKI therapy based on promising
evidence from the AURA1 and AURA2 studies (58-61).
The AURA3 study demonstrated the superior efficacy and safety of
osimertinib compared with pemetrexed plus carboplatin or cisplatin
following progression with first-line EGFR-TKIs. The median PFS was
significantly longer with osimertinib than with chemotherapy (10.1
vs. 4.4 months; HR, 0.30; P<0.001) (Table I) (32). Based on these results, osimertinib
received regular approval from the FDA (2017) for disease
progression on or after EGFR-TKI therapy (62). Furthermore, the AURA3 study also
revealed a longer time to deterioration of key symptoms and a
higher improvement in the global health status or quality of life
of patients treated with osimertinib than with chemotherapy
(63). In line with the AURA
studies, the FLAURA study revealed a higher PFS with osimertinib
compared with standard of care (SoC) (18.9 months vs. 10.2 months;
HR, 0.46; P<0.001), maintained consistently across all subgroups
(including race and different mutation types) (Table II) (64). This led to its approval as the
first-line treatment for metastatic NSCLC with EGFR exon 19
deletions or exon 21 L858R mutations (65). Consistent with the overall FLAURA
results, a subset of the Asian population demonstrated clinically
meaningful efficacy outcomes and a better safety profile for
osimertinib compared with the SoC EGFR-TKI group (higher median
PFS, 16.5 months vs. 11.0 months; HR, 0.54; P<0.0001), higher
ORR (80 vs. 75%) and fewer AEs of grade 3 or higher (40 vs. 48%)
(66). The OS data for osimertinib
were immature when it received approval from the FDA; however, the
OS was continuously monitored. The two endpoints of OS and CNS PFS
were tested after the primary PFS analysis in a hierarchical
procedure at the time of PFS analysis.
Currently, robust data demonstrating the benefits of
OS with EGFR-TKIs are limited. Recent accelerated drug approvals
have been primarily based on ORRs and PFS. Although PFS is
considered as the designated surrogate endpoint for OS, its
validity has been questioned due to the high risk of bias,
particularly when the magnitude of the PFS benefit is minimal
(67,68). OS is precise and easily measurable
and provides an unambiguous yardstick to evaluate efficacy.
Furthermore, OS is a reliable endpoint with a standardized
definition and no risk of bias, and it does not require any
validated instrument or frequent radiological assessment (69). Hence, continued OS monitoring is
crucial to demonstrate direct clinical benefit of any drug.
International bodies, such as the American Society of Clinical
Oncology, have highlighted the need for clinically meaningful
outcomes, including OS, quality of life and the AE profile in order
to ensure accurate treatment effects (70). Mature data from the FLAURA study
revealed a statistically significant and clinically meaningful
improvement in OS with osimertinib (71). This is the first time an EGFR-TKI
has translated PFS to a significant OS benefit. The median OS in
the osimertinib group was extended by 6.8 months, representing a
20% reduction in the risk of mortality (osimertinib, 38.6; 95% CI,
34.5-41.8 vs. standard EGFR-TKIs 31.8; 95% CI, 26.6-36.0; HR for
mortality, 0.80; 95% CI, 0.64-1.00; P=0.046) (71). The OS results for osimertinib and
comparator EGFR-TKIs at months 12, 24 and 36 were as follows: Month
12, 89 vs. 83%; month 24, 74 vs. 59%; and month 36, 54 vs. 44%. In
addition, there was an improvement in OS with osimertinib across
the key patient subgroups. However, the benefit varied in different
subgroups, and the largest numerical between-group differences were
observed between Asian and non-Asian patients (71) (Table
III). Recent evidence from the AURA3 study reported no
statistically significant benefit in the OS of patients with
advanced NSCLC with the T790M mutation for osimertinib vs.
pemetrexed plus carboplatin or cisplatin (median OS, 26.8 vs. 22.5
months; HR, 0.87; 95% CI, 0.67-1.12; P=0.277), possibly reflecting
the high crossover rate (73%) of patients from platinum-pemetrexed
to osimertinib (Table I) (33). The analysis after crossover
adjustment revealed an HR of 0.54 (95% CI, 0.18-1.6). However, the
time to first subsequent therapy or mortality revealed a clinically
meaningful advantage towards osimertinib (HR, 0.21; 95% CI,
0.16-0.28; P<0.001) (33).
Previous studies, such as the LUX-Lung 3 and 6
studies have primarily assessed systemic PFS in patients with NSCLC
carrying the EGFRm with CNS metastasis. Osimertinib is the first
EGFR-TKI to be evaluated for both systemic and intra-cranial PFS in
patients with CNS metastasis. The AURA3 study demonstrated a longer
PFS (8.5 vs. 4.2 months), a better CNS response rate (70 vs. 31%)
and a longer duration of response (8.9 vs. 5.7 months) with
osimertinib compared to chemotherapy in patients with CNS
metastases (72). Similarly, the
FLAURA study revealed a longer CNS PFS (irrespective of T790M) with
osimertinib than with standard EGFR-TKIs (gefitinib or erlotinib),
which increased the time patients with CNS metastases lived without
CNS disease progression or time to mortality (median CNS PFS was
not reached with osimertinib and was 13.9 months with standard
EGFR-TKI therapy), alongside a reduced risk of CNS progression by
52%; (HR, 0.48; 95% CI, 0.26-0.86; P=0.014) (73). In addition, the CNS ORRs were 66
and 43% in patients with measurable and/or non-measurable CNS
lesions [odds ratio (OR) 2.5; 95% CI, 1.2-5.2; P=0.011] treated
with osimertinib and standard EGFR-TKIs, respectively. CNS
progression was lower in the osimertinib group (20%) than in the
standard EGFR-TKI group (39%), whereas CNS progres-sion from new
CNS lesions was reported in 12% patients in the osimertinib group
and 30% patients in the standard EGFR-TKI group. Thus, unlike other
EGFR-TKIs, osimertinib not only decreases, but also prevents CNS
progression. Having demonstrated a better efficacy and comparable
tolerability in patients with CNS metastases, osimertinib can defer
the need for whole-brain radiotherapy, which is associated with AEs
and may not improve survival or quality of life (74,75).
Empirical evidence from a real-world study revealed clinically
meaningful CNS efficacy of osimertinib, with more than half of
patients with EGFR T790M NSCLC and CNS metastases responding to
treatment (response rate 59%; 95% CI, 55-62%) (76). Similarly, a recent meta-analysis
demonstrated the effectiveness and safety of osimertinib for
patients with intracranial metastatic disease with CNS ORR of 64%,
CNS disease control rate of 90%, complete intracranial response
rates of 7-23%, and a median best decrease in intracranial lesion
size of -40 to -64% (77).
Leptomeningeal metastases (LM), occurring in approximately 9% of
EGFRm cases, (double of that among NSCLC population), further
intensify the burden of CNS metastasis. The phase I, open-label
BLOOM study demonstrated that osimertinib exhibited clinically
meaningful efficacy and manageable safety in patients with EGFRm
NSCLC and cytologically confirmed LM who had progressed on
EGFR-TKIs. The study reported an ORR of 41% with a median duration
of response of 15.2 months. The median PFS and OS were 8.6 months
(95% CI, 5.4-13.7 months) and 11.0 months (95% CI, 8.0-18.0
months), respectively. Osimertinib also led to an improvement of
neurological symptoms and CSF clearance in 57 and 28% of the
patients, respectively (78).
Similar results were elucidated by the AURA LM analysis, which
exhibited a median LM PFS and OS of 11.1 months (95% CI 4.6-Not
calculable) and 18.8 months (95% CI, 6.3-NC), respectively
(79). Recent evidence from phase
II and real-world studies also suggest that osimertinib may be a
promising treatment option for EGFRm NSCLC with brain metastases
and LM, regardless of the T790M mutation status (80,81).
Alongside better clinical efficacy, osimertinib has
demonstrated a favorable and consistent toxicity profile in the
FALURA study. Despite almost twice the length of therapy (median
exposure: Osimertinib, 20.7 months; EGFR-TKI, 11.5 months), fewer
patients in the osimertinib group experienced grade ≥3 AEs compared
with the comparator EGFR-TKI (42 vs. 47%) or discontinued treatment
due to AEs (15 vs. 18%) (71). The
most common AEs in patients treated with osimertinib were diarrhea
(60%), rash (59%), nail toxicity (39%), dry skin (38%) and
stomatitis (29%). Interstitial lung disease (ILD) was reported in
11 patients (4%) in the osimertinib group and 6 (2%) in the
standard EGFR-TKI group; however, no fatal events of ILD were
reported in either group. Severe AEs of ILD occurred in 6 patients
(2%) in the osimertinib arm and in 4 (1%) in the comparator arm.
Few patients had a fatal AE; 9 (3%) in the osimertinib group and 10
(4%) in the comparator arm. However, none of the deaths in the
osimertinib arm and 2 in the comparator arm were related to the
treatment.
The proportion of patients remaining on first-line
study treatment after 3 years was higher for osimertinib (28%) than
for standard EGFR-TKIs (9%) (Table
IV) (71). The median time
(months) to first subsequent treatment was longer for osimertinib
(25.5 months; 95% CI 22.0-29.1) than for standard EGFR-TKIs (13.7
months; 95% CI 12.3-15.7) (HR 0.48; 95% CI 0.393-0.581;
P<0.0001) (Table IV) (71). This is a crucial indirect measure
highlighting how long patients can potentially benefit from
first-line osimertinib, and can remain on a well-tolerated
treatment. Moreover, 31% patients in the osimertinib group did not
receive any subsequent cancer treatment, while 48% received first
subsequent anticancer treatment, primarily chemotherapy (Table IV) (71). A higher proportion (65%) in the
standard EGFR-TKI group received first subsequent anticancer
treatment, of which 47% received osimertinib. Additionally, 72
patients (26%) in the osimertinib group and 92 patients (33%) in
the comparator group received a second subsequent therapy.
To maximize the clinical benefit of the different
EGFR-TKIs, it is imperative to strategize their optimal sequencing
(82,83). There is currently no evidence to
ascertain at diagnosis the patients who are likely to develop T790M
following treatment with first- or second-generation EGFR-TKIs. In
many developing countries, T790M testing is not routinely
conducted. Moreover, not all patients who progress on first-line
EGFR-TKIs will receive a subsequent second-line treatment because
of declining functional status. The results from previous EGFR-TKI
trials have revealed that only few patients received
post-progression treatment; the FLAURA study demonstrated that 30%
patients in both groups received no subsequent therapy.
Additionally, tissue or liquid biopsies are not always feasible or
successful owning to challenges of tissue accessibility and patient
performance status (84). In this
regard, the dual-pronged approach of osimertinib, including a
beneficial first-line therapeutic strategy for TKI-naive patients
with NSCLC and as second-line standard therapy in patients with
EGFR T790M mutations, irrespective of CNS metastasis, can be
favorable (85). A network
meta-analysis of 25 studies revealed that osimertinib seemed to be
the most preferable first-line treatment in advanced EGFR-mutated
NSCLC (86). Compelling evidence
from the FLAURA study has demonstrated that the clinically and
statistically significant PFS and intracranial efficacy benefit of
osimertinib is compounded by an extended median OS, with a 20%
reduction in risk of death and 52% reduction in risk of CNS
progression (71). Longer duration
to first subsequent treatment, along with acceptable toxicity and
better quality of life outcomes, has placed osimertinib as a
favorable option in the first-line setting for patients with EGFRm
NSCLC. The recently released guide-lines by both ESMO and NCCN have
also recommended first-line osimertinib as a preferred option for
patients with EGFRm, regardless of T790M mutation (Fig. 1) (13,14).
A cost-effectiveness analysis of osimertinib in the first-line
treatment of advanced EGFRm NSCLC using a Markov cohort model
estimated that osimertinib was more effective in terms of
quality-adjusted life-year gained than comparators
(erlotinib-gefitinib) (87). A
real-world study among EGFRm patients has demonstrated comparable
health utility scores and toxicity profiles between osimertinib and
gefitinib. This supports the more favorable safety profile of
osimertinib for guiding economic analyses going forward (88).
In this era of precision medicine, optimized
treatment sequencing and enhanced patient selection accounting for
clinical and molecular characterization form the corner-stone for
improved patient outcomes. The development of patient-centric
strategies comprising of potent therapies, such as osimertinib,
alone or together with other combination drugs, is crucial for
attaining the ultimate goal of clinical outcomes. Furthermore,
monitoring disease evolution using liquid biopsy is important to
evaluate changes in circulating tumor DNA, mutation burden, the
detection of cancer progression, and the development of drug
resistance (89). Met
amplification is the most common resistance mechanism to
osimertinib therapy (first-line, 7-15%; second-line, 5-50%)
alongside other mechanisms, such as C797S (first-line, 7%;
second-line, 10-26%) and PIK3CA mutations (first-line, 7%;
second-line, 5%) (90). The
identification of targeted treatment options following the failure
of osimertinib and T790M-independent acquired resistance to first-
and second-generation EGFR-TKIs is an unmet medical need. The
current NCCN guidelines recommended continuing osimertinib or
switching to first-line systemic therapy for patients progressing
on first-line osimertinib (Fig. 1)
(14). An enhanced understanding
on the resistance mechanisms with first-line osimertinib and
potential combination strategies may help delay the resistance and
provide therapeutic benefits after resistance is acquired. Apart
from EGFR, the upregulation of other oncogenic pathways acts
as a common resistance mechanism to tyrosine-kinase inhibition.
Multiple clinical studies are evaluating EGFR-TKIs (including
osimertinib) in combination with agents targeting pathways, such as
MET, MAPK, BCL-2, and JAK activation. The NEJ009 trial, a single
country study from Japan, demonstrated a significantly better PFS,
with a longer median survival time (52.2 months) for the
combination therapy of gefitinib with carboplatin and pemetrexed,
compared with gefitinib monotherapy (38.8 months; HR, 0.695;
P=0.013) (Table V) (91). Similarly, the RELAY study reported
a significantly prolonged PFS with the combination therapy of
ramucirumab plus erlotinib (Table
V) (92). However, few studies
of combination therapies with first-generation TKIs have reported
PFS benefit at the cost of increased toxicity (Table V) (93,94).
In this regard, the combination with osimertinib may be deemed
favorable. Osimertinib has been combined with JAK 1 inhibitors,
interrupting signaling of the JAK/STAT pathway, in a second-line
study in T790M-mutant patients (95). Several other phase 1 and 2 clinical
trials are currently evaluating the efficacy of osimertinib with
combination drugs such as dasatinib, sapanisertib, glutaminase
inhibitor CB-839 hydrochloride, necitumumab, navitoclax, and
anlotinib (96-101). ORCHARD, a phase 2 platform study
in patients with advanced NSCLC and disease progression on
first-line osimertinib therapy, is evaluating the efficacy and
safety of osimertinib with savolitinib, gefi-tinib, and necitumumab
(102). An early phase study to
assess combination therapy of osimertinib with brigatinib that
prolong the C797S/T790M/activating-mutation-mediated resistance to
osimertinib is underway (103).
Furthermore, studies to assess combination therapies with
osimertinib with chemotherapy (FLAURA 2) in patients with
metastases, and savolitinib (SAVANNAH) to address MET resistance
(after prior osimertinib therapy) are ongoing (104,105).
A better understanding of the involved genomic
mechanisms in NSCLC has paved the way for target pathways and
multiple treatment approaches. Patient characterization, precision
therapy tailored according to the patient risk, regular monitoring
for disease progression, and overcoming resistance are imperative
to improve survival in patients with advanced NSCLC. While a number
of the recent clinical trials for NSCLC have PFS as the designated
surrogate endpoint for OS, its validity has been questioned,
particularly when the magnitude of PFS benefit is limited. OS is an
unambiguous and reliable endpoint providing confirmatory evidence
of drug efficacy for improving patient survival. The FLAURA study
with osimertinib is the first trial that has demonstrated
clinically and statistically significant PFS, intracranial
efficacy, and a statistically significant OS benefit compared with
standard EGFR-TKIs. The median OS benefit of greater than 3 years
sets a new benchmark for osimertinib and provides a window of
opportunity for the management of patients with stage IV NSCLC with
sensitizing mutation. This reaffirms the importance of osimertinib
as the first-line therapy. In addition, osimertinib may be a
promising treatment option for EGFR-mutated NSCLC with brain
metastases and LM, regardless of T790M mutation status. Combination
approaches with first-line osimertinib along with anticancer drugs
may help address the issue of resistance to EGFR-TKIs.
The present manuscript was funded by AstraZeneca
Pharma India Ltd.
Not applicable.
AKV and AG conceptualized synthesized and
interpreted the literature evidences for the manuscript. GM
interpreted the literature evidences, reviewed, revised, and edited
the manuscript for scientific content. All authors have read and
approved the final manuscript.
Not applicable.
Not applicable.
GM is an employee of AstraZeneca Pharma India
Ltd.
The authors would like to thank AstraZeneca Pharma
India Ltd. and Dr Piyalee Pal and Mrs. Neelam Joglekar from Covance
Scientific Services & Solutions Pvt. Ltd., Pune for providing
medical writing support that was funded by AstraZeneca in
accordance with GPP3 guidelines.
|
1
|
Zhang YL, Yuan JQ, Wang KF, Fu XH, Han XR,
Threapleton D, Yang ZY, Mao C and Tang JL: The prevalence of EGFR
mutation in patients with non-small cell lung cancer: A systematic
review and meta-analysis. Oncotarget. 7:78985–78993. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Midha A, Dearden S and McCormack R: EGFR
mutation incidence in non-small-cell lung cancer of adenocarcinoma
histology: A systematic review and global map by ethnicity
(mutMapII). Am J Cancer Res. 5:2892–2911. 2015.PubMed/NCBI
|
|
3
|
Chantharasamee J, Poungvarin N,
Danchaivijitr P and Techawatanawanna S: Clinical outcome of
treatment of meta-static non-small cell lung cancer in patients
harboring uncommon EGFR mutation. BMC Cancer. 19:7012019.
View Article : Google Scholar
|
|
4
|
Seshacharyulu P, Ponnusamy MP, Haridas D,
Jain M, Ganti AK and Batra SK: Targeting the EGFR signaling pathway
in cancer therapy. Expert Opin Ther Targets. 16:15–31. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Morgillo F, Della Corte CM, Fasano M and
Ciardiello F: Mechanisms of resistance to EGFR-targeted drugs: Lung
cancer. ESMO Open. 1:e0000602016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Barnes TA, O'Kane GM, Vincent MD and
Leighl NB: Third-generation tyrosine kinase inhibitors targeting
epidermal growth factor receptor mutations in non-small cell lung
cancer. Front Oncol. 7:1132017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nosaki K, Satouchi M, Kurata T, Yoshida T,
Okamoto I, Katakami N, Imamura F, Tanaka K, Yamane Y, Yamamoto N,
et al: Re-biopsy status among non-small cell lung cancer patients
in Japan: A retrospective study. Lung Cancer. 101:1–8. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Arcila ME, Oxnard GR, Nafa K, Riely GJ,
Solomon SB, Zakowski MF, Kris MG, Pao W, Miller VA and Ladanyi M:
Rebiopsy of lung cancer patients with acquired resistance to EGFR
inhibitors and enhanced detection of the T790M mutation using a
locked nucleic acid-based assay. Clin Cancer Res. 17:1169–1180.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takezawa K, Pirazzoli V, Arcila ME, Nebhan
CA, Song X, de Stanchina E, Ohashi K, Janjigian YY, Spitzler PJ,
Melnick MA, et al: HER2 amplification: A potential mechanism of
acquired resistance to EGFR inhibition in EGFR-mutant lung cancers
that lack the second-site EGFRT790M mutation. Cancer Discov.
2:922–933. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Scott LJ: Osimertinib as first-line
therapy in advanced NSCLC: A profile of its use. Drugs Ther
Perspect. 34:351–357. 2018. View Article : Google Scholar
|
|
11
|
Kujtan L and Subramanian J: Epidermal
growth factor receptor tyrosine kinase inhibitors for the treatment
of non-small cell lung cancer. Expert Rev Anticancer Ther.
19:547–559. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Imai H, Kaira K and Minato K: Clinical
significance of post-progression survival in lung cancer. Thorac
Cancer. 8:379–386. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Planchard D, Popat S, Kerr K, Novello S,
Smit EF, Faivre-Finn C, Mok TS, Reck M, Van Schil PE, Hellmann MD,
et al: Metastatic non-small-cell lung cancer: ESMO Clinical
Practice Guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 29(Suppl 4): iv192–iv237. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ettinger DS, Wood DE, Aggarwal C, Aisner
DL, Akerley W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac
LR, et al: NCCN Guidelines Insights: Non-small cell lung cancer,
version 1.2020. J Natl Compr Canc Netw. 17:1464–1472. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rajappa S, Krishna MV and Narayanan P:
Integrating osimertinib in clinical practice for non-small cell
lung cancer treatment. Adv Ther. 36:1279–1290. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hsu WH, Yang JC, Mok TS and Loong HH:
Overview of current systemic management of EGFR-mutant NSCLC. Ann
Oncol. 29(Suppl 1): i3–i9. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
U.S. Food and drug administration: Center
for Drug Evaluation and Research: Approval letter IRESSA,
NDA21-399. 2003, https://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/21-399_IRESSA_Approv.pdf.
|
|
18
|
Mok TS, Wu YL, Thongprasert S, Yang CH,
Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et
al: Gefitinib or carboplatin-paclitaxel in pulmonary
adenocarcinoma. N Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fukuoka M, Wu YL, Thongprasert S,
Sunpaweravong P, Leong SS, Sriuranpong V, Chao TY, Nakagawa K, Chu
DT, Saijo N, et al: Biomarker analyses and final overall survival
results from a phase III, randomized, open-label, first-line study
of gefitinib versus carboplatin/paclitaxel in clinically selected
patients with advanced non-small-cell lung cancer in Asia (IPASS).
J Clin Oncol. 29:2866–2874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mitsudomi T, Morita S, Yatabe Y, Negoro S,
Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H and Hirashima T:
Gefitinib versus cisplatin plus docetaxel in patients with
non-small-cell lung cancer harbouring mutations of the epidermal
growth factor receptor (WJTOG3405): An open label, randomised phase
3 trial. Lancet Oncol. 11:121–128. 2010. View Article : Google Scholar
|
|
21
|
Yoshioka H, Mitsudomi T, Morita S, Yatabe
Y, Negoro S, Okamoto I, Seto T, Satouchi M, Tada H, Hirashima T, et
al: Final overall survival results of WJTOG 3405, a randomized
phase 3 trial comparing gefitinib (G) with cisplatin plus docetaxel
(CD) as the first-line treatment for patients with non-small cell
lung cancer (NSCLC) harboring mutations of the epidermal growth
factor receptor (EGFR). J Clin Oncol. 32(Suppl 15): S81172014.
View Article : Google Scholar
|
|
22
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al: Gefitinib or chemotherapy for non-small-cell lung cancer
with mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Inoue A, Kobayashi K, Maemondo M, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al: Updated overall survival results from a randomized phase III
trial comparing gefitinib with carboplatin-paclitaxel for
chemo-naïve non-small cell lung cancer with sensitive EGFR gene
mutations (NEJ002). Ann Oncol. 24:54–59. 2013. View Article : Google Scholar
|
|
24
|
Zhou C, Wu YL, Chen G, Feng J, Liu XQ,
Wang C, Zhang S, Wang J, Zhou S, Ren S, et al: Erlotinib versus
chemotherapy as first-line treatment forpatients with advanced EGFR
mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802):
A multicentre, open-label, randomised, phase 3 study. Lancet Oncol.
12:735–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou C, Wu YL, Chen G, Feng J, Liu XQ,
Wang C, Zhang S, Wang J, Zhou S, Ren S, et al: Final overall
survival results from a randomised, phase III study of erlotinib
versus chemotherapy as first-line treatment of EGFR
mutation-positive advanced non-small-cell lung cancer (OPTIMAL,
CTONG-0802). Ann Oncol. 26:1877–1883. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rosell R, Carcereny E, Gervais R,
Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia Gomez R,
Pallares C, Sanchez JM, et al: Erlotinib versus standard
chemotherapy as first-line treatment for European patients with
advanced EGFR mutation-positive non-small-cell lung cancer
(EURTAC): A multicentre, open-label, randomised phase 3 trial.
Lancet Oncol. 13:239–246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leon LF, Golsorkhi A, Liu S, Drozdowskyj A
and Rosell R: Overall survival analyses of first-line erlotinib
versus chemotherapy in the EURTAC study population controlling for
the use of post-study therapy. Ann Oncol. 25(Suppl 4): iv426–iv470.
2014. View Article : Google Scholar
|
|
28
|
Wu YL, Zhou C, Liam CK, Wu G, Liu X, Zhong
Z, Lu S, Cheng Y, Han B, Chen L, et al: First-line erlotinib versus
gemcitabine/cisplatin in patients with advanced EGFR
mutation-positive non-small-cell lung cancer: Analyses from the
phase III, randomized, open-label, ENSURE study. Ann Oncol.
26:1883–1889. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sequist LV, Yang JC, Yamamoto N, O'Byrne
K, Hirsh V, Mok T, Geater SL, Orlov S, Tsai CM, Boyer M, et al:
Phase III study of afatinib or cisplatin plus pemetrexed in
patients with metastatic lung adenocarcinoma with EGFR mutations. J
Clin Oncol. 31:3327–3334. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang JC, Wu YL, Schuler M, Sebastian M,
Popat S, Yamamoto N, Zhou C, Hu CP, O'Byrne K, Feng J, et al:
Afatinib versus cisplatin-based chemotherapy for EGFR
mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6):
Analysis of overall survival data from two randomised, phase 3
trials. Lancet Oncol. 16:141–151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang
Y, Li W, Hou M, Shi JH, Lee KY, et al: Afatinib versus cisplatin
plus gemcitabine for first-line treatment of Asian patients with
advanced non-small-cell lung cancer harbouring EGFR mutations
(LUX-Lung 6): An open-label, randomised phase 3 trial. Lancet
Oncol. 15:213–222. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim
HR, Ramalingam SS, Shepherd FA, He Y, Akamatsu H, Theelen WS, et
al: Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung
cancer. N Engl J Med. 376:629–640. 2017. View Article : Google Scholar
|
|
33
|
Papadimitrakopoulou VA, Mok TS, Han JY,
Ahn MJ, Delmonte A, Ramalingam SS, Kim SW, Shepherd FA, Laskin J,
He Y, et al: Osimertinib versus platinum-pemetrexed for patients
with EGFR T790M advanced NSCLC and progression on a prior
EGFR-tyrosine kinase inhibitor: AURA3 overall survival analysis.
Ann Oncol. S0923-7534:42155–42156. 2020.
|
|
34
|
Park K, Tan EH, O'Byrne K, Zhang L, Boyer
M, Mok T, Hirsh V, Yang JC, Lee KH, Lu S, et al: Afatinib versus
gefitinib as first-line treatment of patients with EGFR
mutation-positive non-small-cell lung cancer (LUX-Lung 7): A phase
2B, open-label, randomised controlled trial. Lancet Oncol.
17:577–589. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Paz-Ares L, Tan EH, O'byrne K, Zhang L,
Hirsh V, Boyer M, Yang JH, Mok T, Lee KH, Lu S, et al: Afatinib
versus gefitinib in patients with EGFR mutation-positive advanced
non-small-cell lung cancer: Overall survival data from the phase
IIb LUX-Lung 7 trial. Ann Oncol. 28:270–277. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa
K, Niho S, Tsuji F, Linke R, Rosell R, Corral J, et al: Dacomitinib
versus gefitinib as first-line treatment for patients with
EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): A
randomised, open-label, phase 3 trial. Lancet Oncol. 18:1454–1466.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mok TS, Cheng Y, Zhou X, Lee KH, Nakagawa
K, Niho S, Lee M, Linke R, Rosell R, Corral J, et al: Improvement
in overall survival in a randomized study that compared dacomitinib
with gefitinib in patients with advanced non-small-cell lung cancer
and EGFR-activating mutations. J Clin Oncol. 36:2244–2250. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
U.S. Food and Drug Administration, Center
for Drug Evaluation and Research: NDA multi-disciplinary review and
evaluation, 211288Orig1s000, VIZIMPRO/dacomitinib. 2018, https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/211288Orig1s000MultidisciplineR.pdf.
|
|
39
|
Sequist LV, Waltman BA, Dias-Santagata D,
Digumarthy S, Turke AB, Fidias P, Bergethon K, Shaw AT, Gettinger
S, Cosper AK, et al: Genotypic and histological evolution of lung
cancers acquiring resistance to EGFR inhibitors. Sci Transl Med.
3:75ra262011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yu HA, Arcila ME, Rekhtman N, Sima CS,
Zakowski MF, Pao W, Kris MG, Miller VA, Ladanyi M and Riely GJ:
Analysis of tumor specimens at the time of acquired resistance to
EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers.
Clin Cancer Res. 19:2240–2247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Koyyala VP, Batra U, Jain P, Sharma M,
Goyal P, Medisetty P, Jajodia A and Maheshwari UD: Frequency of
T790M mutations after progression on epidermal growth factor
receptor tyrosine kinase inhibitor in metastatic non-small cell
lung cancer in Indian patients: Real-time data from tertiary cancer
hospital. Lung India. 35:390–394. 2018. View Article : Google Scholar
|
|
42
|
Oxnard GR, Arcila ME, Sima CS, Riely GJ,
Chmielecki J, Kris MG, Pao W, Ladanyi M and Miller VA: Acquired
resis-tance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung
cancer: Distinct natural history of patients with tumors harboring
the T790M mutation. Clin Cancer Res. 17:1616–1622. 2011. View Article : Google Scholar
|
|
43
|
Jaiswal R, Pinninti R, Krishna Mohan MV,
Santa A, Boyella PK, Nambaru L, Murthy SS, Chowdary KV and Rajappa
S: T790M mutation and clinical outcomes with osimertinib in
patients with epidermal growth factor receptor-mutant non-small
cell lung cancer. Indian J Med Paediatr Oncol. 40:73–78. 2019.
View Article : Google Scholar
|
|
44
|
Westover D, Zugazagoitia J, Cho BC, Lovly
CM and Paz-Ares L: Mechanisms of acquired resistance to first- and
second-generation EGFR tyrosine kinase inhibitors. Ann Oncol.
29(Suppl 1): i10–i19. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rangachari D, Yamaguchi N, VanderLaan PA,
Folch E, Mahadevan A, Floyd SR, Uhlmann EJ, Wong ET, Dahlberg SE,
Huberman MS and Costa DB: Brain metastases in patients with
EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung
Cancer. 88:108–111. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ali A, Goffin JR, Arnold A and Ellis PM:
Survival of patients with non-small-cell lung cancer after a
diagnosis of brain metastases. Curr Oncol. 20:e300–e306. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Peters S, Bexelius C, Munk V and Leighl N:
The impact of brain metastasis on quality of life, resource
utilization and survival in patients with non-small-cell lung
cancer. Cancer Treat Rev. 45:139–162. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Heon S, Yeap BY, Lindeman NI, Joshi VA,
Butaney M, Britt GJ, Costa DB, Rabin MS, Jackman DM and Johnson BE:
The impact of initial gefitinib or erlotinib versus chemotherapy on
central nervous system progression in advanced non-small cell lung
cancer with EGFR mutations. Clin Cancer Res. 18:4406–4414. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Omuro AM, Kris MG, Miller VA, Franceschi
E, Shah N, Milton DT and Abrey LE: High incidence of disease
recurrence in the brain and leptomeninges in patients with nonsmall
cell lung carcinoma after response to gefitinib. Cancer.
103:2344–2348. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen YH, Chen YF, Chen CY, Shih JY and Yu
CJ: Clinical factors associated with treatment outcomes in EGFR
mutant non-small cell lung cancer patients with brain metastases: A
case-control observational study. BMC Cancer. 19:10062019.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hoffknecht P, Tufman A, Wehler T, Pelzer
T, Wiewrodt R, Schütz M, Serke M, Stöhlmacher-Williams J, Märten A,
Maria Huber R, et al: Efficacy of the irreversible ErbB family
blocker afatinib in epidermal growth factor receptor (EGFR)
tyrosine kinase inhibitor (TKI)-pretreated non-small-cell lung
cancer patients with brain metastases or leptomeningeal disease. J
Thorac Oncol. 10:156–163. 2015. View Article : Google Scholar
|
|
52
|
Doval DC, Desai CJ and Sahoo TP:
Molecularly targeted therapies in non-small cell lung cancer: The
evolving role of tyrosine kinase inhibitors. Indian J Cancer.
56(Suppl): S23–S30. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Shah RR and Shah DR: Safety and
tolerability of epidermal growth factor receptor (EGFR) tyrosine
kinase inhibitors in oncology. Drug Saf. 42:181–198. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tan CS, Kumarakulasinghe NB, Huang YQ, Ang
YLE, Choo JR, Goh BC and Soo RA: Third generation EGFR TKIs:
Current data and future directions. Mol Cancer. 17:292018.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Finlay MR, Anderton M, Ashton S, Ballard
P, Bethel PA, Box MR, Bradbury RH, Brown SJ, Butterworth S,
Campbell A, et al: Discovery of a potent and selective EGFR
inhibitor (AZD9291) of both sensitizing and T790M resistance
mutations that spares the wild type form of the receptor. J Med
Chem. 57:8249–8267. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Murtuza A, Bulbul A, Shen JP, Keshavarzian
P, Woodward BD, Lopez-Diaz FJ, Lippman SM and Husain H: Novel
third-generation EGFR tyrosine kinase inhibitors and strategies to
overcome therapeutic resistance in lung cancer. Cancer Res.
79:689–698. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Liu S, Li S, Hai J, Wang X, Chen T, Quinn
MM, Gao P, Zhang Y, Ji H, Cross DAE and Wong KK: Targeting HER2
aberrations in non-small cell lung cancer with osimertinib. Clin
Cancer Res. 24:2594–2604. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
U.S. Food and Drug Administration, Center
for Drug Evaluation and Research: Application number:
208065Orig1s000, Tagrisso/osimertinib. https://www.accessdata.fda.gov/drug-satfda_docs/nda/2015/208065Orig1s000SumR.pdf.
Accessed September 4, 2019.
|
|
59
|
Jänne PA, Yang JC, Kim DW, Planchard D,
Ohe Y, Ramalingam SS, Ahn MJ, Kim SW, Su WC, Horn L, et al: AZD9291
in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J
Med. 372:1689–1699. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Goss G, Tsai CM, Shepherd FA, Bazhenova L,
Lee JS, Chang GC, Crino L, Satouchi M, Chu Q, Hida T, et al:
Osimertinib for pretreated EGFR Thr790Met-positive advanced
non-small-cell lung cancer (AURA2): A multicentre, open-label,
single-arm, phase 2 study. Lancet Oncol. 17:1643–1652. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yang JC, Ahn MJ, Kim DW, Ramalingam SS,
Sequist LV, Su WC, Kim SW, Kim JH, Planchard D, Felip E, et al:
Osimertinib in pretreated T790M-positive advanced non-small-cell
lung cancer: AURA study phase II extension component. J Clin Oncol.
35:1288–1296. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
U.S. Food and Drug Administration:
Osimertinib (TAGRISSO): https://www.fda.gov/drugs/resources-information-approved-drugs/osimertinib-tagrisso.
|
|
63
|
Lee CK, Novello S, Rydén A, Mann H and Mok
T: Patient-reported symptoms and impact of treatment with
osimertinib versus chemotherapy in advanced non-small-cell lung
cancer: The AURA3 trial. J Clin Oncol. 36:1853–1860. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Soria JC, Ohe Y, Vansteenkiste J,
Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura
F, Nogami N, Kurata T, et al: Osimertinib in untreated EGFR-mutated
advanced non-small-cell lung cancer. N Engl J Med. 378:113–125.
2018. View Article : Google Scholar
|
|
65
|
U.S. Food and Drug Administration: FDA
approves osimer-tinib for first-line treatment of metastatic NSCLC
with most common EGFR mutations. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-osimertinib-first-line-treatment-metastatic-nsclc-most-common-egfr-mutations.
Accessed September 5, 2019.
|
|
66
|
Cho BC, Chewaskulyong B, Lee KH,
Dechaphunkul A, Sriuranpong V, Imamura F, Nogami N, Kurata T,
Okamoto I, Zhou C, et al: Osimertinib versus standard of care EGFR
TKI as first-line treatment in patients with EGFRm advanced NSCLC:
FLAURA Asian subset. J Thorac Oncol. 14:99–106. 2019. View Article : Google Scholar
|
|
67
|
Soria JC, Massard C and Le Chevalier T:
Should progression-free survival be the primary measure of efficacy
for advanced NSCLC therapy? Ann Oncol. 21:2324–2332. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Pasalic D, McGinnis GJ, Fuller CD,
Grossberg AJ, Verma V, Mainwaring W, Miller AB, Lin TA,
Jethanandani A, Espinoza AF, et al: Progression-free survival is a
suboptimal predictor for overall survival among metastatic solid
tumour clinical trials. Eur J Cancer. 136:176–185. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
U.S. Food and Drug Administration:
Clinical trial endpoints for the approval of cancer drugs and
biologics. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-trial-endpoints-approval-cancer-drugs-and-biologics.
Accessed September 5, 2019.
|
|
70
|
Ellis LM, Bernstein DS, Voest EE, Berlin
JD, Sargent D, Cortazar P, Garrett-Mayer E, Herbst RS, Lilenbaum
RC, Sima C, et al: American society of clinical oncology
perspec-tive: Raising the bar for clinical trials by defining
clinically meaningful outcomes. J Clin Oncol. 32:1277–1280. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ramalingam SS, Vansteenkiste J, Planchard
D, Cho BC, Gray JE, Ohe Y, Zhou C, Reungwetwattana T, Cheng Y,
Chewaskulyong B, et al: Overall survival with osimertinib in
untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 382:41–50.
2020. View Article : Google Scholar
|
|
72
|
Mok T, Ahn MJ, Han JY, Kang JH, Katakami
N, Kim H, Hodge R, Ghiorghiu DC, Cantarini M, Wu YL, et al: CNS
response to osimertinib in patients (pts) with T790M-positive
advanced NSCLC: Data from a randomized phase III trial (AURA3). J
Clin Oncol. 35(15_Suppl): S90052017. View Article : Google Scholar
|
|
73
|
Reungwetwattana T, Nakagawa K, Cho BC,
Cobo M, Cho EK, Bertolini A, Bohnet S, Zhou C, Lee KH, Nogami N, et
al: CNS response to osimertinib versus standard epidermal growth
factor receptor tyrosine kinase inhibitors in patients with
untreated EGFR-mutated advanced non-small-cell lung cancer. J Clin
Oncol. JCO20187831182018. View Article : Google Scholar : Epub ahead of
print. PubMed/NCBI
|
|
74
|
Monaco EA III, Faraji AH, Berkowitz O,
Parry PV, Hadelsberg U, Kano H, Niranjan A, Kondziolka D and
Lunsford LD: Leukoencephalopathy after whole-brain radiation
therapy plus radiosurgery versus radiosurgery alone for metastatic
lung cancer. Cancer. 119:226–232. 2013. View Article : Google Scholar
|
|
75
|
Mulvenna P, Nankivell M, Barton R,
Faivre-Finn C, Wilson P, McColl E, Moore B, Brisbane I, Ardron D,
Holt T, et al: Dexamethasone and supportive care with or without
whole brain radiotherapy in treating patients with non-small cell
lung cancer with brain metastases unsuitable for resection or
stereotactic radiotherapy (QUARTZ): Results from a phase 3,
non-inferiority, randomised trial. Lancet. 388:2004–2014. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Metro G, Provencio M, Kim DW, Cho BC, Park
K, Pan Y, Shi Y, Migliorino R, Tiseo M, Yu J, et al: Osimertinib in
epidermal growth factor receptor (EGFR) T790M advanced non-small
cell lung cancer (NSCLC): Analysis of patients with central nervous
system (CNS) metastases in a real-world study (ASTRIS). Ann Oncol.
30(Suppl 5): v6242019. View Article : Google Scholar
|
|
77
|
Erickson AW, Brastianos PK and Das S:
Assessment of effectiveness and safety of osimertinib for patients
with intracranial metastatic disease: A systematic review and
meta-analysis. JAMA Netw Open. 3:e2016172020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Yang JCH, Kim SW, Kim DW, Lee JS, Cho BC,
Ahn JS, Lee DH, Kim TM, Goldman JW, Natale RB, et al: Osimertinib
in patients with epidermal growth factor receptor mutation-positive
non-small-cell lung cancer and leptomeningeal metastases: The BLOOM
study. J Clin Oncol. 38:538–547. 2020. View Article : Google Scholar
|
|
79
|
Ahn MJ, Chiu CH, Cheng Y, Han JY, Goldberg
SB, Greystoke A, Crawford J, Zhao Y, Huang X, Johnson M, et al:
Osimertinib for patients with leptomeningeal metastases associated
with EGFR T790M-positive advanced NSCLC: The AURA leptomeningeal
metastases analysis. J Thorac Oncol. 15:637–648. 2020. View Article : Google Scholar
|
|
80
|
Park S, Lee MH, Seong M, Kim ST, Kang JH,
Cho BC, Lee KH, Cho EK, Sun JM, Lee SH, et al: A Phase II,
multicenter, two cohort study of 160 mg osimertinib in EGFR
T790M-positive non-small cell lung cancer patients with brain
metastases or leptomeningeal disease who progressed on prior EGFR
TKI therapy. Ann Oncol. 31:1397–1404. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Lee J, Choi Y, Han J, Park S, Jung HA, Su
JM, Lee SH, Ahn JS, Park K and Ahn MJ: Osimertinib improves overall
survival in patients with EGFR-Mutated NSCLC with leptomeningeal
metastases regardless of T790M mutational status. J Thorac Oncol.
15:1758–1766. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Girard N: Optimizing outcomes in EGFR
mutation-positive NSCLC: Which tyrosine kinase inhibitor and when?
Future Oncol. 14:1117–1132. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Gelatti ACZ, Drilon A and Santini FC:
Optimizing the sequencing of tyrosine kinase inhibitors (TKIs) in
epidermal growth factor receptor (EGFR) mutation-positive non-small
cell lung cancer (NSCLC). Lung Cancer. 137:113–122. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Kawamura T, Kenmotsu H, Taira T, Omori S,
Nakashima K, Wakuda K, Ono A, Naito T, Murakami H, Mori K, et al:
Rebiopsy for patients with non-small-cell lung cancer after
epidermal growth factor receptor-tyrosine kinase inhibitor failure.
Cancer Sci. 107:1001–1005. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Shah R and Lester JF: Tyrosine kinase
inhibitors for the treatment of EGFR mutation-positive
non-small-cell lung cancer: A clash of the generations. Clin Lung
Cancer. 21:e216–e228. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Zhang H, Chen J, Liu T, Dang J and Li G:
First-line treatments in EGFR-mutated advanced non-small cell lung
cancer: A network meta-analysis. PLoS One. 14:e02235302019.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Aguilar-Serra J, Gimeno-Ballester V,
Pastor-Clerigues A, Milara J, Marti-Bonmati E, Trigo-Vicente C,
Alós-Almiñana M and Cortijo J: Osimertinib in first-line treatment
of advanced EGFR-mutated non-small-cell lung cancer: A
cost-effectiveness analysis. J Comp Eff Res. 8:853–863. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Jiang SX, Walton RN, Hueniken K, Baek J,
McCartney A, Labbé C, Smith E, Chan SW, Chen R, Brown C, et al:
Real-world health utility scores and toxicities to tyrosine kinase
inhibitors in epidermal growth factor receptor mutated advanced
non-small cell lung cancer. Cancer Med. 8:7542–7555. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Schuler M: Liquid biopsies for dynamic
monitoring of EGFR mutations in lung cancer. J Thorac Oncol.
12(Suppl): S852017. View Article : Google Scholar
|
|
90
|
Leonetti A, Sharma S, Minari R, Perego P,
Giovannetti E and Tiseo M: Resistance mechanisms to osimertinib in
EGFR-mutated non-small cell lung cancer. Br J Cancer. 121:725–737.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Nakamura A, Inoue A, Morita S, Hosomi Y,
Kato T, Fukuhara T, Gemma A, Takahashi K, Fujita Y, Harada T, et
al: Phase III study comparing gefitinib monotherapy (G) to
combination therapy with gefitinib, carboplatin, and pemetrexed
(GCP) for untreated patients (pts) with advanced non-small cell
lung cancer (NSCLC) with EGFR mutations (NEJ009). J Clin Oncol.
36(15_suppl): S90052018. View Article : Google Scholar
|
|
92
|
Nakagawa K, Garon EB, Seto T, Nishio M,
Santiago PA, Chiu CH, Park K, Novello S, Nadal E, Imamura F, et al:
RELAY: A multi-national, double-blind, randomized Phase 3 study of
erlotinib (ERL) in combination with ramucirumab (RAM) or placebo
(PL) in previously untreated patients with epidermal growth factor
receptor mutation-positive (EGFRm) metastatic non-small cell lung
cancer (NSCLC). J Clin Oncol. 37(15_Suppl): 90002019. View Article : Google Scholar
|
|
93
|
Noronha V, Joshi A, Patil VM, Chougule A,
Mahajan A, Janu A, Purandare N, Kumar R, More S, Goud S, et al:
Phase III randomized trial comparing gefitinib to gefitinib with
pemetrexed-carboplatin chemotherapy in patients with advanced
untreated EGFR mutant non-small cell lung cancer (gef vs gef+ C). J
Clin Oncol. 37(15_Suppl): S90012019. View Article : Google Scholar
|
|
94
|
Saito H, Fukuhara T, Furuya N, Watanabe K,
Sugawara S, Iwasawa S, Tsunezuka Y, Yamaguchi O, Okada M, Yoshimori
K, et al: Erlotinib plus bevacizumab versus erlotinib alone in
patients with EGFR-positive advanced non-squamous non-small-cell
lung cancer (NEJ026): Interim analysis of an open-label,
randomised, multicentre, phase 3 trial. Lancet Oncol. 20:625–635.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
ClinicalTrials.gov: Assessing an oral
janus kinase inhibitor, AZD4205, in combination with osimertinib in
patients who have advanced non-small cell lung cancer (JACKPOT1).
ClinicalTrials.gov Identifier: NCT03450330.
https://clinical-trials.gov/ct2/show/NCT03450330.
Accessed March 1, 2018.
|
|
96
|
ClinicalTrials.gov: Dasatinib and
osimertinib (AZD9291) in advanced non-small cell lung cancer with
EGFR mutations. ClinicalTrials.gov Identifier:
NCT02954523. https://clinical-trials.gov/ct2/show/NCT02954523.
Accessed November 3, 2016.
|
|
97
|
ClinicalTrials.gov: Testing the
Combination of MLN0128 (TAK-228) and AZD9291 in Advanced EGFR
(Epidermal Growth Factor Receptor) Mutation Positive Non-small Cell
Lung Cancer. ClinicalTrials.gov Identifier:
NCT02503722. https://clinicaltrials.gov/ct2/show/NCT02503722?term=osimertinib&cond=NSCLC+Stage+IV&rank=3.
Accessed July 21, 2015.
|
|
98
|
ClinicalTrials.gov: Telaglenastat
Hydrochloride and Osimertinib in Treating Patients With
EGFR-Mutated Stage IV Non-small Cell Lung Cancer. ClinicalTrials.gov Identifier: NCT03831932 https://clinicaltrials.gov/ct2/show/NCT03831932?term=osimertinib&cond=NSCLC+Stage+IV&rank=6.
Accessed February 6, 2019.
|
|
99
|
ClinicalTrials.gov: Osimertinib and
Necitumumab in Treating Patients With EGFR-Mutant Stage IV or
Recurrent Non-small Cell Lung Cancer Who Have Progressed on a
Previous EGFR Tyrosine Kinase Inhibitor. ClinicalTrials.gov Identifier: NCT02496663.
https://clinicaltrials.gov/ct2/show/NCT02496663?term=osimertinib&cond=NSCLC+Stage+IV&rank=7.
Accessed July 14, 2015.
|
|
100
|
ClinicalTrials.gov: Study of anlotinib
combined with osimer-tinib as second-line treatment in stage
IIIb-IV NSCLC with confirmed EGFRm and T790M (ALTN-03). ClinicalTrials.gov Identifier: NCT04029350.
https://clinicaltrials.gov/ct2/show/NCT04029350?term=osimertinib&cond=NSCLC+Stage+IV&rank=12
Accessed July 23, 2019.
|
|
101
|
ClinicalTrials.gov: Osimertinib and
Navitoclax in treating patients with EGFR-positive previously
treated advanced or metastatic non-small cell lung cancer.
ClinicalTrials.Gov Identifier: NCT02520778.
https://clinicaltrials.gov/ct2/show/NCT02520778
Accessed August 13, 2015.
|
|
102
|
ClinicalTrials.gov: Phase 2 Platform Study
in patients with advanced non-small lung cancer who progressed on
first-line osimertinib therapy (ORCHARD) (ORCHARD). ClinicalTrials.gov Identifier: NCT03944772.
https://www.clinicaltrials.gov/ct2/show/NCT03944772.
Accessed May 10, 2019.
|
|
103
|
Uchibori K, Inase N, Araki M, Kamada M,
Sato S, Okuno Y, Fujita N and Katayama R: Brigatinib combined with
anti-EGFR antibody overcomes osimertinib resistance in EGFR-mutated
non-small-cell lung cancer. Nat Commun. 8:147682017. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
ClinicalTrials.gov: A Study of Osimertinib
With or Without Chemotherapy as 1st Line Treatment in Patients With
Mutated Epidermal Growth Factor Receptor Non-Small Cell Lung Cancer
(FLAURA2) (FLAURA2). ClinicalTrials.gov Identifier: NCT04035486.
https://clinicaltrials.gov/ct2/show/NCT04035486?term=flaura+2&draw=2&rank=1
Accessed July 29, 2019.
|
|
105
|
Clinical trial.gov: Osimertinib Plus
Savolitinib in EGFRm+/MET+ NSCLC Following Prior Osimertinib
(SAVANNAH). ClinicalTrials.gov Identifier:
NCT03778229. https://clinical-trials.gov/ct2/show/NCT03778229?term=SAVANNAH&draw=2&rank=1
Accessed December 19, 2018.
|