Introduction
Osteosarcoma (OS) is one of the most common
malignant bone cancers, originating from mesenchymal tissue, with
two high-incidence groups in adolescent and elderly individuals.
The annual incidence rate of OS is approximately 4.4/1 million
(1). In the early 1970s, with the
advent of neoadjuvant chemotherapy combined with surgical
resection, the 5-year survival rate of OS was increased from 20 to
70% (2-4). Unfortunately, the survival rate of
OS has not improved in the past 30 years. Therefore, it is
extremely urgent to study the pathogenesis of OS and explore new
therapeutic targets.
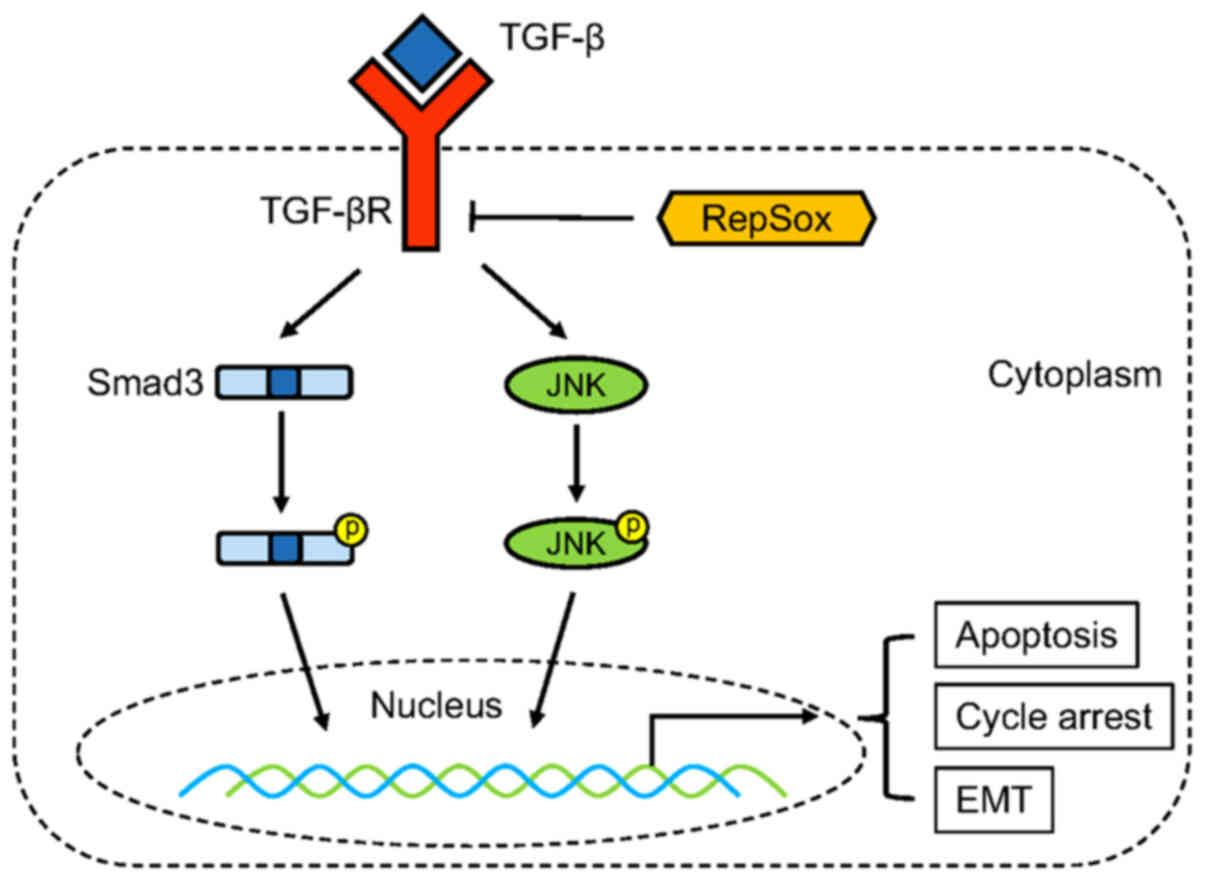
Transforming growth factor-β (TGF-β) participates in
the regulation of a variety of cellular biological processes
through its TGF-β type I and type II receptors, including cell
proliferation, differentiation, apoptosis, adhesion and migration,
and acts on the downstream Smad pathway (classical) and non-Smad
pathway (nonclassical) (5,6).
Dysfunction of TGF-β leads to serious consequences, even
tumorigenesis (7). Accumulating
evidence has revealed that there is a close relationship between
elevated TGF-β expression and the progression of tumors, such as
lung, colorectal, prostate and gastric cancer. Additionally, the
high expression of TGF-β in colorectal, prostate and breast cancer
is positively correlated with tumor metastasis (8-10).
Moreover, the serum TGF-β concentration in patients with OS lung
metastasis is higher than that in patients without pulmonary
metastasis (11,12). Therefore, it was hypothesized that
the inhibition of the TGF-β signaling pathway may play a protective
role in the occurrence and development of OS.
RepSox is a small molecule compound that acts as an
inhibitor of TGF-βI receptor kinase (13,14). A recent study indicated that
RepSox could inhibit osteoclast formation and bone resorption
through the Smad3 and JNK/AP-1 pathway (15). Moreover, it has been reported that
RepSox can induce mouse fibroblasts to reprogram into
cardiomyocytes (16), improve the
antitumor effects of antigen-presenting and immune-effector cells
that are impaired in acute myeloid leukemia (17), and inhibit skin fibrosis (18). To date, no studies have been
performed reporting the role of RepSox on tumors and its underlying
mechanism. Therefore, the effects caused by RepSox on bone tumors
remain unknown.
In the present study, the effects of RepSox on the
proliferation, cell cycle, apoptosis and metastasis of OS cell
lines in vitro and its effect on a tumor xenograft mouse
model was therefore analyzed.
Materials and methods
Media and reagents
RepSox and dimethyl sulfoxide (DMSO) were purchased
from Sigma-Aldrich; Merck KGaA. Dulbecco's modified Eagle's medium
(DMEM), fetal bovine serum (FBS) and penicillin-streptomycin were
purchased from Gibco; Thermo Fisher Scientific, Inc. Cell Counting
Kit-8 (CCK-8) reagent was obtained from Dojindo Molecular
Technologies, Inc. Primary antibodies against JNK2 (product no.
9258S), phosphorylated (p)-JNK (product no. 4668S), ERK1/2 (product
no. 5013S), p-ERK1/2 (product no. 4370S), p38 (product no. 8690S),
p-p38 (product no. 4511S), Smad3 (product no. 9513S), p-Smad3
(product no. 9520S) and GAPDH (product no. 3683S) were purchased
from Cell Signaling Technology, Inc. Primary antibodies against
matrix metalloproteinase (MMP)-2 (cat. no. 10373-2-AP) and MMP-9
(cat. no. 10375-2-AP) were purchased from ProteinTech Group, Inc.
Primary antibodies against Bax (product no. 5023S), Bcl-2 (product
no. 3498S), E-cadherin (product no. 3195S), N-cadherin (product no.
4061S) and vimentin (product no. 5741S) were purchased from Cell
Signaling Technology, Inc.
Cell culture
HOS and 143B cells were purchased from the Chinese
Academy of Sciences (Shanghai, China) and maintained in DMEM
containing 10% FBS and 1% penicillin/streptomycin. Both cell lines
were routinely stored at 37°C in a humidified incubator with 5%
CO2.
Cell proliferation assays
To determine the effective inhibitory concentration
of RepSox on cell viability, HOS and 143B cells were seeded at
3×103 cells/well into 96-well plates and incubated at
37°C overnight. Cells were then treated with increasing
concentrations of RepSox ranging from 0 to 200 µM for 24, 48
or 96 h. The DMSO alone group was used as the negative control
group. Subsequently, 10 µl of Cell Counting Kit-8 (CCK-8)
buffer was added to each well containing 100 µl culture
medium. The plate was incubated for another 2 h at 37°C, and the
absorbance at a wavelength of 450 nm was measured using an ELX800
absorbance microplate reader (BioTek Instruments, Inc.). Cell
viability was calculated as follows: Cell viability (%) = [A
(RepSox)-A (blank)]/[A (Control)-A (blank)] ×100%; where A (RepSox)
is the absorbance of wells with cells, CCK-8 solution and RepSox; A
(blank) is the absorbance of wells with medium and CCK-8 solution
but no cells; and A (Control) is the absorbance of wells with cells
and CCK-8 solution but without RepSox. The cell growth rate was
calculated by GraphPad Prism software (version 7.0; GraphPad
Software, Inc.). Firstly, the drug concentration was converted into
a logarithmic form as the x-axis, and then data fitting was
performed through nonlinear regression, and finally the
IC50 value was obtained. For cell colony formation, HOS
and 143B cells were plated in 6-well plates at a cell density of
1,000 cells/well and then treated with different doses of RepSox
(0, 50, 100 and 200 µM) supplemented with DMEM with 10% FBS
for 2 weeks. The colonies were visualized under a light microscope
(Carl Zeiss AG) and images were captured after staining with 0.5%
crystal violet solution for 20 min at room temperature. Colonies
containing at least 50 cells were recorded for statistical
analysis.
Flow cytometry
HOS and 143B cells (5×104 cells/well)
were seeded in 6-well plates and exposed to RepSox at
concentrations of 0, 50, 100 and 200 µM for 48 h. An equal
volume of DMSO was added to the negative control group. After 48 h,
the cells were harvested with trypsin and washed twice with chilled
PBS. Then, single-cell suspensions treated with different
concentrations of RepSox were fixed overnight at 4°C with
prechilled 70% ethanol. The following day, the cells were incubated
with 500 µl PI/RNase solution for 30 min in the dark at room
temperature. Subsequently, a flow cytometer (BD FACSCanto II; BD
Biosciences) was used to analyze the samples and assess the cell
cycle results. Similarly, to investigate the effect of RepSox on
the viability of OS cells, the cells incubated with 0, 50, 100 and
200 µM RepSox were collected and washed with PBS. The cells
were then stained with an Annexin V-fluorescein isothiocyanate
(FITC)-PI cell apoptosis kit (cat. no. 556547; BD Biosciences) at
room temperature for 15 min, according to the manufacturer's
protocol, and apoptosis rates were analyzed by flow cytometry. Data
were analyzed using FlowJo software 7.6.5 (FlowJo LLC).
Soft agar colony formation assay
The OS cells were trypsinized and harvested, and
5×103 cells were mixed with a 0.3% agar solution in DMEM
containing 10% FBS. In addition, different doses of RepSox were
added at concentrations of 0, 50, 100 and 200 µM. The
suspensions were layered on top of a solidified 0.5% agarose layer
in 6-well plates. The plates were then stored at 37°C for 2 weeks,
and representative images of the cell colonies were obtained under
a light microscope. Colonies containing at least 50 cells were
recorded for statistical analysis.
Wound-healing assay
In brief, the cells (1×105 per well) were
cultured in 6-well plates with DMEM containing 10% FBS overnight at
37°C until a confluence of 90% was reached. Then the cells were
starved in serum-free medium for 24 h. A sterile pipette tip was
used to scratch the cell layer to produce an artificial wound line.
The cells were then rinsed with PBS to clear the detached cells.
The drug was diluted to the specified concentration (0, 5, 10 and
20 µM) in serum-free DMEM medium and the cells were treated
at 37°C for 24 h. An equal volume of DMSO was added to the negative
control group. The cells were monitored and images were captured
under a light microscope at 0 and 24 h.
Cell invasion and migration assays
A total of 24-well Transwell chambers with an
8-µm pore size polycarbonate membrane (Corning, Inc.) were
used to evaluate the cell migration and invasion abilities. For the
invasion assay, the Matrigel was pre-coated on the membranes of the
upper chamber at 37°C for 1 h according to the manufacturer's
protocol (BD Biosciences). For the migration and invasion assays,
100 µl suspension of cancer cells (5×104) was
added to the upper compartment with serum-free DMEM, and the lower
compartment was filled with 600 µl of DMEM supplemented with
10% FBS. A preset concentration of RepSox (0, 5, 10 and 20
µM), which was used as a source of chemoattractant, was
concurrently added to the lower compartment of the chamber. After
culturing at 37°C for 24 h, the non-invading/non-migrated cells
were wiped off with a cotton swab. The invading/migrated cells that
had invaded/migrated to the lower side were fixed at room
temperature with 4% paraformaldehyde for 20 min and stained with
0.5% crystal violet solution at room temperature for 30 min.
Finally, images of the invading/migrated cells were captured and
cells were counted under an inverted light microscope.
Western blot analysis
After treatment with the indicated concentrations of
RepSox, the cells were rinsed with precooled PBS and collected with
a cell scraper. Total protein was obtained by lysing cells with
RIPA lysis buffer (Dalian Meilun Biology Technology Co., Ltd.)
containing phosphatase inhibitor and protease inhibitor for 20 min
at 4°C, and centrifugation (12,000 × g for 15 min at 4°C). Protein
concentration determination was measured by BCA kit. Equal amounts
(10 µg/lane) of protein samples were separated by 8% or 10%
sodium dodecyl sulfate-polyacrylamide (SDS-PAGE) gels by
electrophoresis and transferred to a polyvinylidene fluoride (PVDF)
membrane (EMD Millipore). Then, the membrane was blocked with 5%
skimmed milk or 5% bovine serum albumin (BSA) (Sigma-Aldrich; Merck
KGaA) for 1 h at room temperature and incubated overnight at 4°C
with the appropriate primary antibodies (dilution 1:1,000) followed
by horseradish peroxidase (HRP)-conjugated secondary antibodies
(dilution 1:3,000; cat. nos. FDM007 and FDR007; Hangzhou Fude
Biological Technology Co., Ltd.) for 1 h at room temperature.
Subsequently, an enhanced chemiluminescence kit (FD8030; Hangzhou
Fude Biological Technology Co., Ltd.) was used for the analysis of
the protein interactions after exposure in an imaging system, and
the protein band images were quantified with ImageJ (version 1.48;
National Institutes of Health).
RNA extraction and reverse
transcription-quantitative (RT-q) PCR
The MMP-2 and MMP-9 gene expression levels in HOS
and 143B cells were detected by qPCR. Total RNA was isolated from
OS cell lines and tumor tissues by TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) after treatment with
different doses of RepSox. cDNA templates were synthesized by
reverse transcription using a PrimeScript™ RT reagent Kit (Takara
Bio, Inc.) according to the manufacturer's instructions. Then, the
cDNAs were quantified by a LightCycler® 480II PCR
instrument (Roche Diagnostics) to determine the expression of
related genes by using UltraSYBR Mixture (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The thermocycling conditions were
as follows: 95°C for 10 min, 40 cycles at 95°C for 15 sec, and 60°C
for 1 min. The sequences of the primers used were as follows: GAPDH
forward, 5′-GGAAGGTGAAGGTCGGAG TCA-3′ and reverse,
5′-GTCATTGATGGCAACAATATCC ACT-3′; MMP-9 forward,
5′-TGTACCGCTATGGTTACAC TCG-3′ and reverse,
5′-GGCAGGGACAGTTGCTTCT-3′; MMP-2 forward,
5′-TTGATGGCATCGCTCAGATC-3′ and reverse, 5′-TTGTCACGTGGCGTCACAGT-3′;
TGF-β forward, 5′-GGCCAGATCCTGTCCAAGC-3′ and reverse, 5′-GTGGGT
TTCCACCATTAGCAC-3′; cyclin E1 forward, 5′-AAGGAGC GGGACACCATGA-3′
and reverse, 5′-ACGGTCACGTTTG CCTTCC-3′; cyclin-dependent kinase
(CDK)2 forward, 5′-CCAGGAGTTACTTCTATGCCTGA-3′ and reverse, 5′-TTC
ATCCAGGGGAGGTACAAC-3′; cyclin A2 forward, 5′-CGCTGGCGGTACTGAAGTC-3′
and reverse, 5′-GAGGAACGG TGACATGCTCAT-3′; p21 forward,
5′-TGTCCGTCAGAAC CCATGC-3′ and reverse, 5′-AAAGTCGAAGTTCCATCG
CTC-3′. Relative mRNA expression was calculated as
2−ΔΔCq (19).
Xenograft tumor model
All animal experiments were approved (approval no.
20190712) by the Animal Care Ethics Committee of Sir Run Run Shaw
Hospital (Hangzhou, China). All animal studies were conducted in
accordance with the National Institutes of Health Guide for the
Care and Use of Laboratory Animals. Animals were kept under
standard laboratory conditions (temperature 22±2°C; relative
humidity 50±10%; 12-h light/dark cycle) with access to food and
water ad libitum. A xenograft model of 6-week-old male nude mice
with an average weight of 18.05 g was established by subcutaneous
injection of 143B cells on one side of the back. In our in
vivo experiments, 3×106 143B cells were made into a
suspension with sterile PBS and inoculated subcutaneously into one
side of the 6-week-old male nude mice (Shanghai Institutes for
Biological Sciences Shanghai Laboratory Animal Center) to establish
tumor formation models. One week later, when tumors grew to be
visible to the naked eye, a total of 15 nude mice were randomly
assigned to three groups. RepSox treatment was initiated until the
tumor volume reached approximately 50 mm3. Treatment (5
and 20 mg/kg) groups were administered RepSox at 5 and 20 mg/kg
every other day, respectively, while the control group was injected
with DMSO. The tumor volume (V) was monitored and calculated every
week based on the formula V (volume) = π x L (length) x W
(width)2/6. After continuous observation for 3 weeks,
all the mice were euthanized, and the tumors were resected and
weighed for further study. Animal euthanasia was performed by
cervical dislocation under 2% isoflurane anesthesia.
TUNEL assay
The TUNEL assay was performed according to the
manufacturer's instructions of a One-Step TUNEL Apoptosis Assay kit
(Beyotime Institute of Biotechnology), which was used to analyze
the level of apoptosis in cell and tissue specimens. The pretreated
cells were seeded in a 24-well plate at a density of
1×104 cells/well, and then fixed with 4% formaldehyde
for 30 min, blocked with 5% bovine serum albumin (BSA) and
incubated with 0.1% Triton X-100 at room temperature for 5 min. The
slides were washed with PBS, and 50 µl of TUNEL reaction
mixture was added to the cells, followed by incubation at 37°C in
the dark for 1 h. Nuclei were counterstained with
4,6-diamidino-2-phenylindole (DAPI) at 37°C for 10 min. Images of
the sections were captured under a fluorescence microscope, and the
positive cells were counted. The nucleus stained with DAPI was
blue. The cells exhibiting green fluorescence were considered
apoptotic cells. Analysis of TUNEL-positive cells was carried out
on five fields of view on each of the four slides in three
independent experiments.
Histological and immunohistochemical
analyses
Tissue blocks from excised tumors were fixed with
10% formalin for 48 h at room temperature and embedded in paraffin.
Then, 4-µm sections were stained with hematoxylin for 5 min
and eosin for 2 min at room temperature for histological
examinations and morphometric analysis. For immunohistochemical
staining, the paraffin sections were first deparaffinized, and then
the sections were heated in citrate buffer for antigen retrieval.
Sections were then incubated with 3% hydrogen peroxide in
phosphate-buffered saline (PBS) at 37°C for 5 min, and blocked with
3% bovine serum albumin (BSA) in PBS at 37°C for 1 h. Slides were
incubated overnight at 4°C with primary antibodies against Bax
(dilution 1:100), Bcl-2 (dilution 1:400; product no. 15071; Cell
Signaling Technology, Inc.), E-cadherin (dilution 1:400),
N-cadherin (dilution 1:125; product no. 13116; Cell Signaling
Technology, Inc.), vimentin (dilution 1:100), MMP-2 (dilution
1:100), MMP-9 (dilution 1:100) and Ki67 (dilution 1:500; product
no. 9449; Cell Signaling Technology, Inc.), followed by incubation
with biotinylated secondary antibodies (dilution 1:50; product nos.
8114 and 8125; Cell Signaling Technology, Inc.) for 1 h at room
temperature.
Statistical analysis
All the data presented in this study were obtained
from at least three independent experiments and were analyzed using
GraphPad Prism software (version 7.0; GraphPad Software, Inc.).
Values are presented as the mean ± SD of 3 independent experiments.
Statistical analyses were performed using unpaired Student's t-test
or one-way analysis of variance (ANOVA) followed by Tukey's post
hoc analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
RepSox inhibits cell proliferation and
colony formation and induces S-phase arrest
The chemical structure of RepSox is revealed in
Fig. 1A. Firstly, to evaluate the
inhibitory effect of RepSox on the viability of human OS cells,
RepSox with concentration gradients ranging from 0 to 200 µM
was used to treat HOS and 143B cells, and a CCK-8 assay was used to
detect cell viability at 24, 48 and 96 h (Fig. 1B). As revealed in Fig. 1B, RepSox significantly inhibited
the proliferation of HOS and 143B cells in a time- and
concentration-dependent manner. In the following colony formation
assays, it was also revealed that the colony size and number of HOS
and 143B cells treated with RepSox were significantly inhibited.
The results revealed a concentration dependence with statistically
significant differences in the number and size of colonies
(Fig. 1C and D). In addition, a
soft agar colony formation assay was further performed, which is a
three-dimensional environment that simulates the growth of OS in
vivo. It was revealed that as the drug concentration increased,
the inhibitory effect on the number and size of colonies tended to
become markedly more pronounced (Fig.
1E and F).
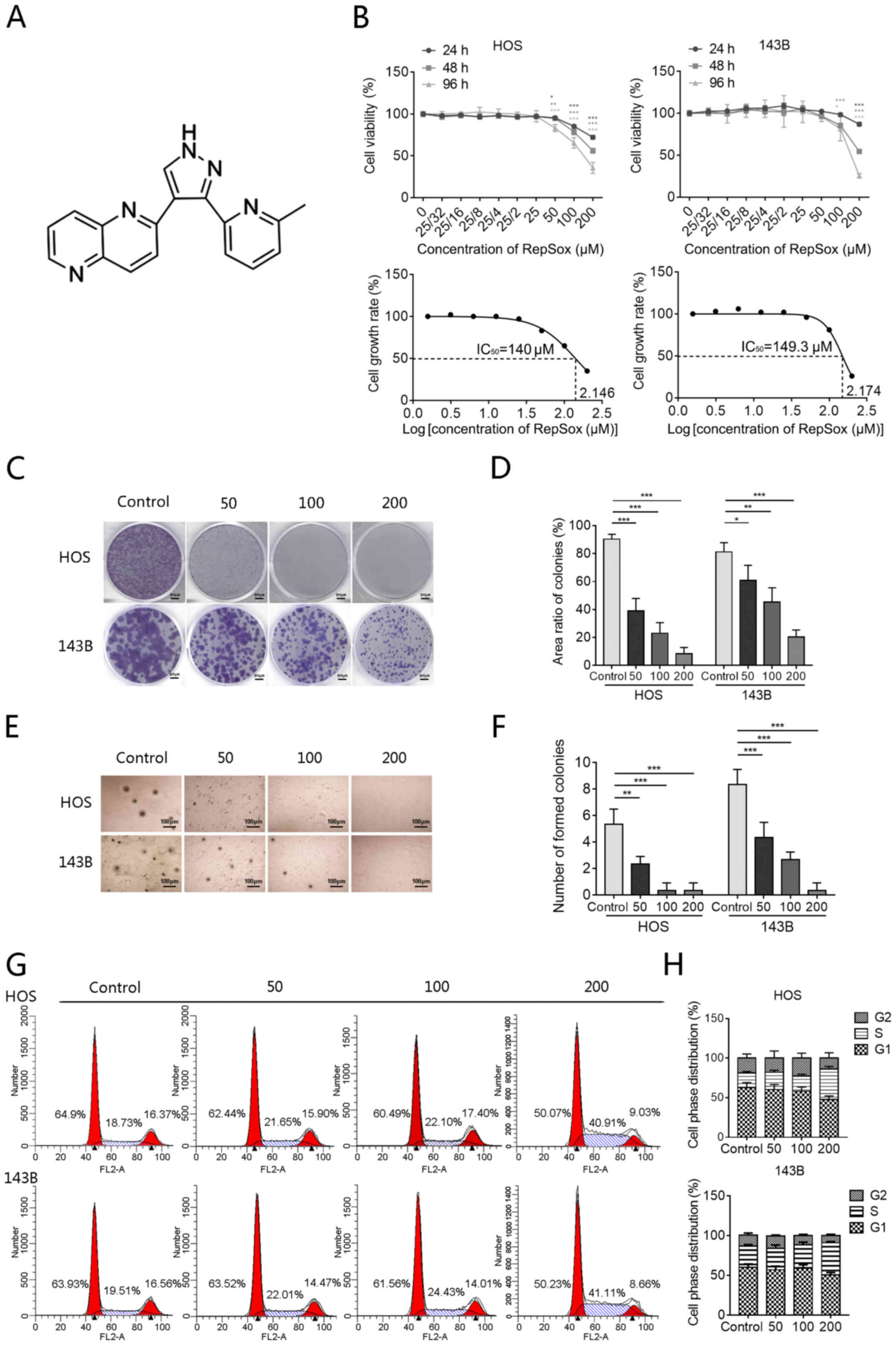 | Figure 1RepSox inhibits cell proliferation
and colony formation and induces S-phase arrest. (A) Chemical
structure of RepSox. (B) HOS and 143B cells were exposed to
different concentrations of RepSox, and Cell Counting Kit-8 assays
were used to assess cell viability at 24, 48 and 96 h. The
calculated IC50 values of RepSox in HOS and 143B cells
at 96 h were 140 and 149.3 µM, respectively. (C and D) HOS
and 143B cells were induced by different concentrations of RepSox
for 14 days and then stained with crystal violet dye. Scale bars,
500 µm. (E and F) The proliferation ability of HOS and 143B
cells was evaluated by soft agar colony formation assay, and the
number of colonies was counted. Scale bars, 100 µm. (G and
H) HOS and 143B cells were exposed to different concentrations of
RepSox for 48 h, and then cell cycle assays were performed by flow
cytometry, and the cell cycle distribution after RepSox treatment
was analyzed. Data are presented as the mean ± standard deviation
from three independent experiments. *P<0.05,
**P<0.01 and ***P<0.001 vs. the
control. IC50, half maximal inhibitory
concentration. |
To investigate whether the inhibitory effect of
RepSox on OS proliferation is related to the cell cycle, the cell
cycle distribution of HOS and 143B cells induced by RepSox was
further verified using PI/RNase staining. As demonstrated in
Fig. 1G and H, RepSox led to the
reduction of the number of cells in the G0/G1 phase and a
corresponding accumulation in the S phase in both HOS and 143B
cells. Based on the aforementioned results, it could be concluded
that RepSox can inhibit the proliferation of OS cells by inducing
S-phase arrest. To further elucidate the mechanism of the S-phase
arrest of OS cells induced by RepSox, the mRNA levels of
cycle-related genes were examined. As revealed in Fig. S1, the mRNA levels of cyclin E1
and p21 were upregulated after RepSox treatment, while the mRNA
levels of CDK2 and cyclin A2 were decreased. The aforementioned
results indicated that RepSox could inhibit cell proliferation and
induce S-phase arrest of OS cells by upregulating the expression of
cyclin E1 and p21 mRNA, and downregulating the expression of CDK2
and cyclin A2 mRNA.
RepSox promotes OS cell apoptosis
To clarify whether apoptosis is responsible for the
inhibition of proliferation in OS cells following RepSox treatment,
flow cytometry and TUNEL staining were used to detect the apoptosis
levels. To quantify apoptosis, FITC-Annexin V/PI staining was
performed to examine the proapoptotic effect of RepSox. In both HOS
and 143B cell lines, the proportion of apoptosis in the treatment
group exposed to RepSox for 48 h was significantly higher than that
in the control group, regardless of early or late apoptotic status,
and the apoptotic rate in early apoptotic cells was affected in a
dose-dependent manner (Fig. 2A and
B). TUNEL staining assays were used to detect the effect of
RepSox on DNA damage in apoptotic OS cells. Fig. 2C and D revealed that
RepSox-induced TUNEL-positive cells could be clearly observed,
whereas the proportion of TUNEL-positive cells was negligible in
the control group. Furthermore, western blotting was used to detect
apoptosis-related proteins, and the results revealed that the
expression of pro-apoptotic Bax protein increased with increasing
drug concentration, while the expression of anti-apoptotic Bcl-2
protein was decreased (Fig. 2E and
F). Collectively, these results indicated that RepSox induced
typical apoptosis in human OS cells.
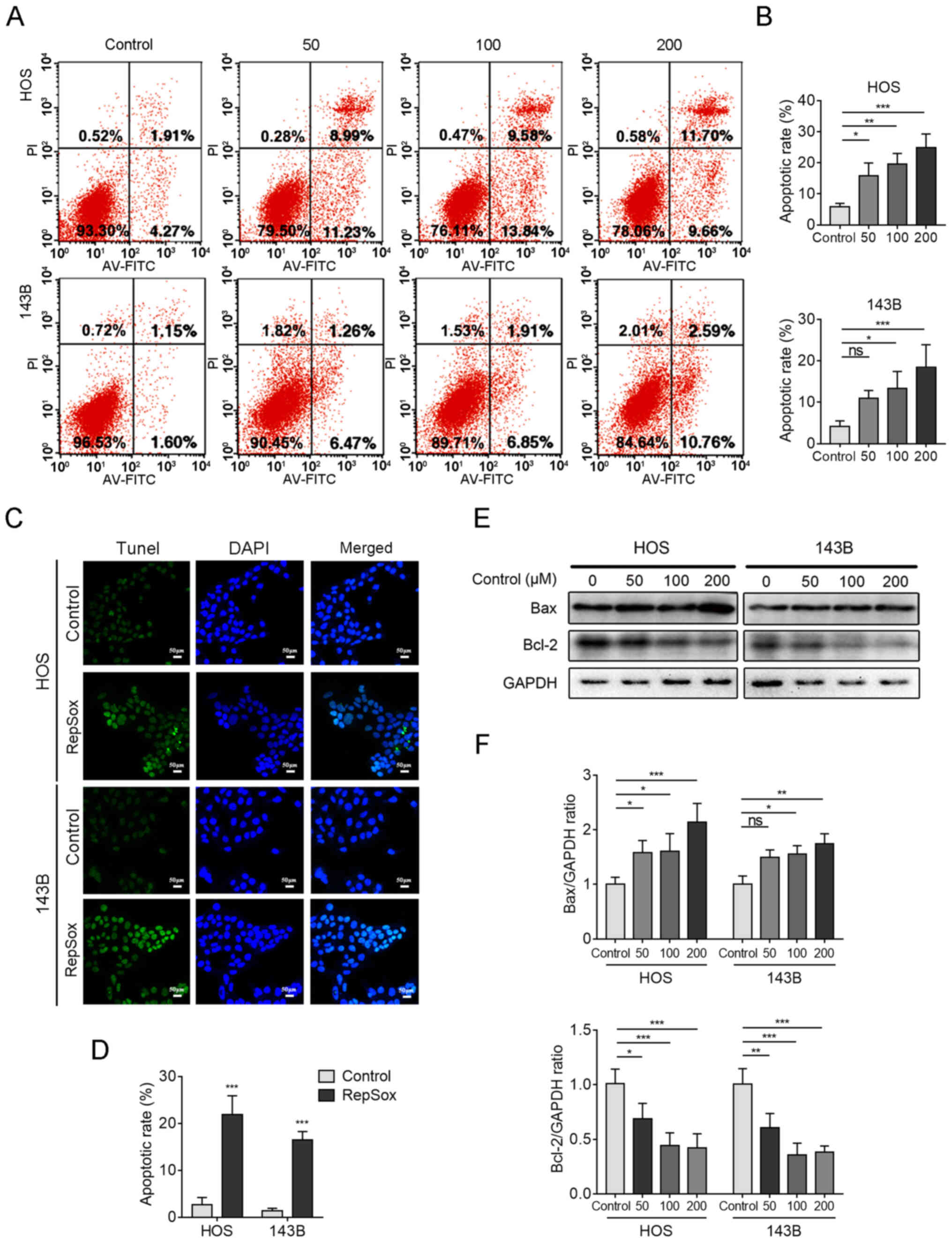 | Figure 2RepSox promotes osteosarcoma cell
apoptosis. (A) HOS and 143B cells were treated with different
concentrations of RepSox for 48 h, detected with an Annexin
V-FITC/PI cell apoptosis kit, and finally analyzed by flow
cytometry. (B) The proportion of apoptotic cells was quantified.
(C) HOS and 143B cells were treated with 200 µM RepSox,
apoptotic cells exhibited green fluorescence, and nuclei were
stained with DAPI (blue). Scale bars, 50 µm. (D) The
TUNEL-positive cells were quantified. (E) After exposure to
different concentrations of RepSox, apoptosis-related proteins in
HOS and 143B cells were assessed by western blotting. (F) The
densitometric levels of Bax and Bcl-2 were quantified and
normalized to that of GAPDH. Data are presented as the mean ±
standard deviation from three independent experiments.
*P<0.05, **P<0.01 and
***P<0.001 vs. the control. FITC/PI, fluorescein
isothiocyanate/propidine iodide; DAPI,
4′,6-diamidino-2-phenylindole; TUNEL,
terminal-deoxynucleotidyl-transferase-mediated dUTP nick end
labeling; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. |
RepSox inhibits migration in OS
cells
To further evaluate the effect of RepSox on OS cell
migratory behavior, a wound-healing assay was performed. Here, the
drug concentration was reduced below the toxicity level (from
50-200 µM to 5-20 µM) in order to rule out the bias
caused by cell proliferation inhibition. The migration distance was
obviously shorter in cells subjected to RepSox treatment for 24 h
than in untreated cells. The effect of inhibition was
dose-dependent (Fig. 3A and B).
Concurrently, longitudinal migration experiments were carried out
by using a Transwell chamber. Representative images of cell
migration and invasion are presented in Fig. 3C, and it was revealed that the
number of transmembrane cells in the RepSox treatment group was
significantly less than that in the control group. Furthermore, the
number of transmembrane cells decreased with higher concentrations
of RepSox (Fig. 3D). The
Transwell chamber was then coated with a layer of Matrigel to
detect the invasion ability of OS cells, and the obtained results
were consistent with the migration experiment (Fig. 3C and D). In addition, the results
of RT-q PCR (Fig. 3E) and western
blotting (Fig. 3F) revealed that
RepSox significantly inhibited the expression of MMP-2 and MMP-9 in
HOS and 143B cells. Next, the expression of EMT-related proteins
was further examined and it was revealed that in cells pretreated
with RepSox the protein levels of mesenchymal markers (N-cadherin
and vimentin) were significantly downregulated, but the levels of
the epithelial cell marker E-cadherin were significantly
upregulated (Fig. 3G and H). All
these results indicated that RepSox could inhibit migration in OS
cells.
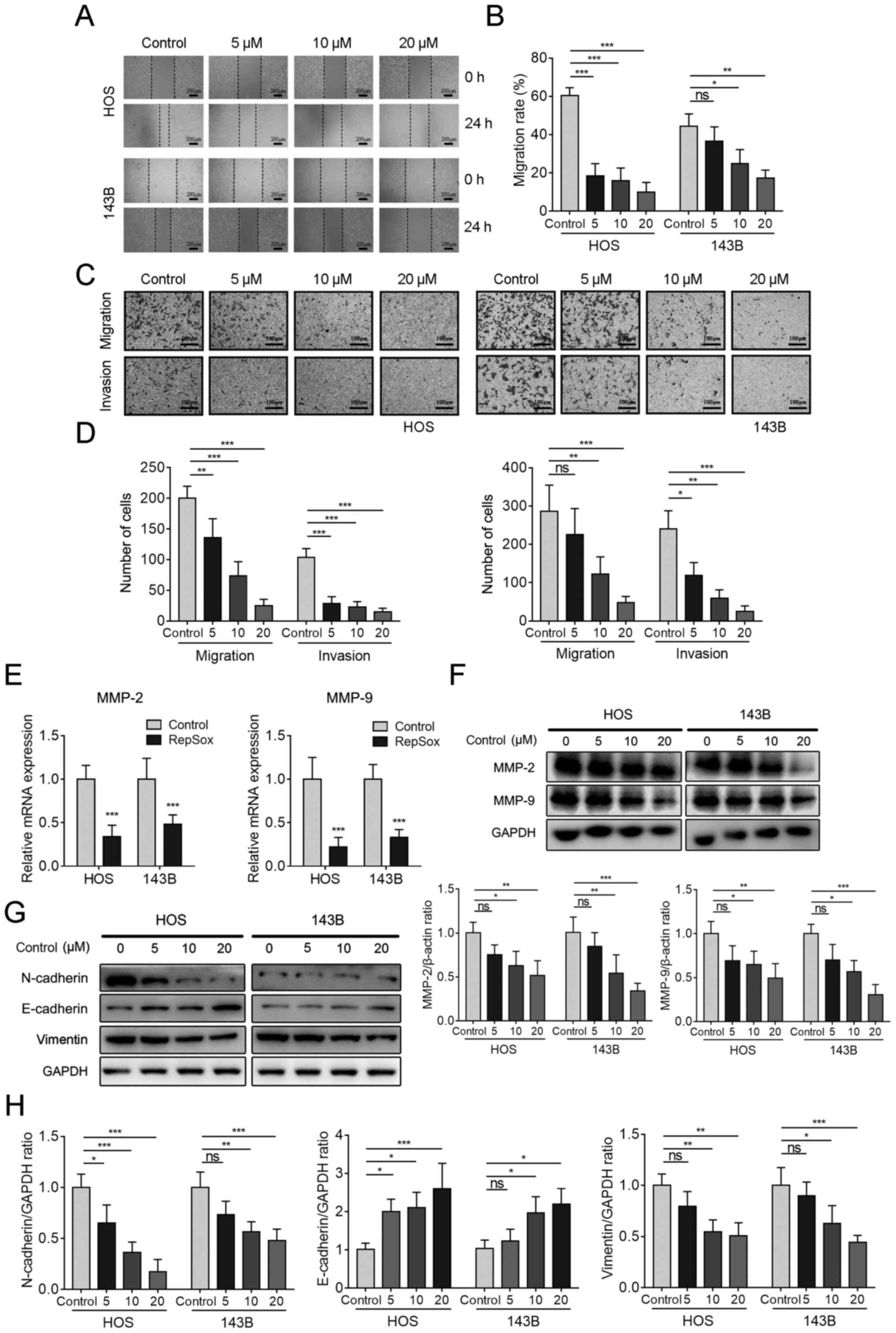 | Figure 3RepSox inhibits migration in OS
cells. (A) When the HOS and 143B cells in 6-well plates were
confluent, 200 µl pipette tips were used to scratch wounds,
after which the medium was replaced with different concentrations
of RepSox prepared in serum-free medium, and the cells were
cultured for 24 h. Scale bars, 200 µm. (B) The migration
ability was quantified. (C) Cells induced by different
concentrations of RepSox were seeded into Transwell chambers for
the cell migration assay for 24 h. In contrast to the cell
migration assay, the cell invasion assay used Matrigel-coated
Transwell chambers for 24 h. Scale bars, 100 µm. (D) Cells
that migrated and invaded were counted. (E) Reverse
transcription-quantitative PCR was used to detect the gene
expression levels of MMP-2 and MMP-9 in HOS and 143B cells after
RepSox induction for 24 h. (F) Western blotting was used to detect
the protein expression levels of MMP-2 and MMP-9 in HOS and 143B
cells after RepSox induction for 24 h. The densitometric levels of
MMP-2 and MMP-9 were quantified and normalized to GAPDH. (G)
Western blotting was conducted to assess the expression of
EMT-related proteins. (H) The protein bands of EMT-related proteins
were quantified and normalized to GAPDH. Data are presented as the
mean ± standard deviation from three independent experiments.
*P<0.05, **P<0.01 and
***P<0.001 vs. the control. MMP, matrix
metalloproteinase; EMT, epithelial-mesenchymal transition; ns, not
significance. |
RepSox exerts anti-OS effects via the
JNK/Smad3 pathway
Previous studies indicated that MAPK and Smad
signaling pathways have great importance for tumor progression
(20-25). Firstly, analysis of gene
expression revealed that treatment with 200 µM RepSox
significantly inhibited the expression of TGF-β mRNA (Fig. 4A). Next, it was verified whether
MAPKs and Smad play a crucial role in the inhibitory effects of
RepSox in OS cells. The results revealed that the phosphorylation
of JNK and Smad3 was significantly inhibited after RepSox treatment
(Fig. 4B and C), while the
phosphorylation of p38 and ERK was not significantly altered
(Fig. 4D and E). All this data
indicated that RepSox exerted an anti-OS effect via the JNK/Smad3
pathway.
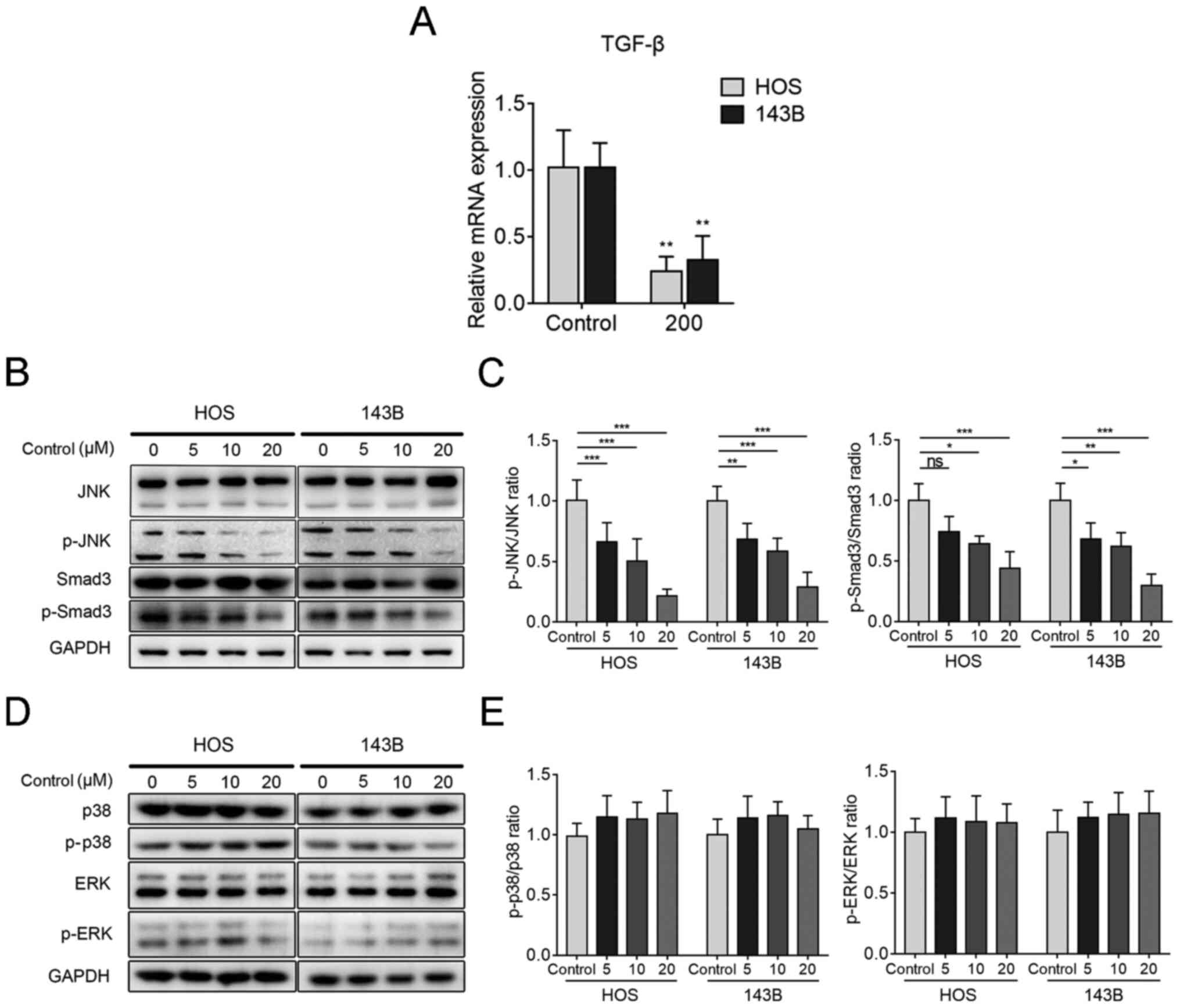 | Figure 4RepSox exerts an anti-osteosarcoma
effect via the JNK/Smad3 pathway. (A) Reverse
transcription-quantitative PCR was used to detect the gene
expression level of transforming growth factor-β in HOS and 143B
cells after RepSox induction for 24 h. (B) After treatment with
RepSox at different concentrations, western blotting was performed
to compare the expression levels of JNK, p-JNK, Smad3 and p-Smad3
proteins. (C) Western blot analysis revealed the p-JNK and p-Smad3
protein levels and then normalized them to total JNK and Smad3. (D)
HOS and 143B cells were treated as indicated in B, and western
blotting was performed to compare the expression levels of p38,
p-p38, ERK and p-ERK proteins. (E) Western blot analysis revealed
the p-p38 and p-ERK protein levels and then normalized to total p38
and ERK. Data are presented as the mean ± standard deviation from
three independent experiments. *P<0.05,
**P<0.01 and ***P<0.001 vs. the
control. JNK, c-Jun amino-terminal kinase; TGF-β, transforming
growth factor-β; p-, phosphorylated; ERK, extracellular
signal-regulated kinase; ns, no significance. |
RepSox inhibits OS growth in vivo
To clarify the anticancer effect of RepSox in
vivo, a xenograft model was established and randomly divided
into three groups, receiving intraperitoneal administration of
RepSox (5 mg/kg or 20 mg/kg every other day) or DMSO. In terms of
drug concentration selection for the in vivo experiment, on
the one hand, the CCK-8 data of our in vitro experiment were
considered, and on the other hand, some published literature
(26-28) on drug concentrations of other
drugs in OS xenograft models was consulted. After three weeks, the
tumors were removed from each nude mouse and measured (Fig. 5A and B). The RepSox-treated group
exhibited a significant decrease in both tumor volume and weight
(Fig. 5C and D). However, there
was no significant difference in body weight between the control
group and RepSox-treated groups (Fig.
5E). The results of immunohistochemistry revealed an obvious
decrease in Bcl-2, N-cadherin, vimentin, MMP-2 and MMP-9
expression, along with a reduction in the proliferation marker
Ki67. Conversely, the expression of E-cadherin and Bax was
upregulated (Fig. 5F). A TUNEL
assay was utilized to confirm the proapoptotic effect of RepSox on
tumors in vivo. It was revealed that as the RepSox
concentration increased, TUNEL-positive cells also increased in
tumor tissue compared with the control group (Fig. 5G and H). The hearts, livers,
spleens, lungs and kidneys of the mice were removed for further
histological observation. H&E-stained sections revealed intact
tissue structure in all three groups, no OS metastatic lesions and
no cytotoxic effects (Fig. 5I).
In conclusion, these results revealed that RepSox efficiently
suppressed the growth of OS and had low toxicity in nude mice.
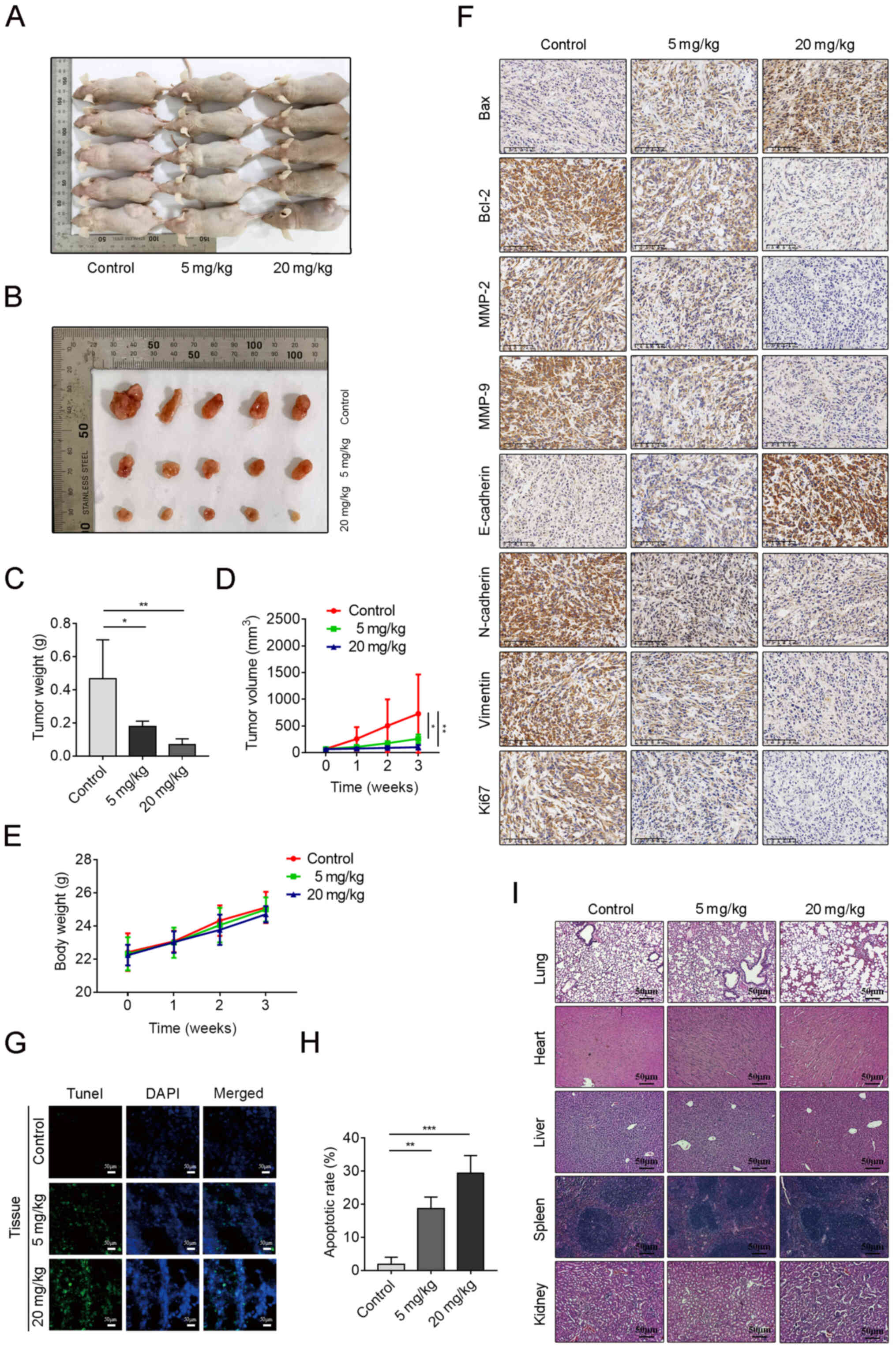 | Figure 5RepSox inhibits osteosarcoma growth
in vivo. A xenograft model in 6-week-old male nude mice was
established by subcutaneous injection of 143B cells on both sides
of the back. After one week of modeling, mice randomly divided into
three groups began to receive intraperitoneal administration of
RepSox (5 mg/kg or 20 mg/kg every other day) or DMSO (control
group). (A) Tumors in vivo are presented. (B) Tumors were
removed from the nude mice. (C) The tumors were weighed and
analyzed. (D and E) The tumor volume and body weight of mice were
measured weekly. (F) Representative images of Bax, Bcl-2, MMP-2,
MMP-9, E-cadherin, N-cadherin, vimentin and Ki67 immunostaining
obtained by a light microscope. Scale bars, 50 µm. (G) Tumor
tissue from the three groups was stained with a TUNEL kit. Scale
bars, 50 µm. (H) TUNEL-positive tissue was quantified. (I)
Hematoxylin and eosin staining of major organs is presented. Scale
bars, 50 µm. Data are presented as the mean ± standard
deviation from three independent experiments.
*P<0.05, **P<0.01 and
***P<0.001 vs. the control. MMP, matrix
metalloproteinase; TUNEL,
terminal-deoxynucleotidyl-transferase-mediated dUTP nick end
labeling; DAPI, 4′,6-diamidino-2-phenylindole. |
Discussion
OS is a malignant primary bone tumor that mainly
occurs in children and adolescents (1). Its prognosis is poor, especially in
patients with metastases at diagnosis and patients who are
resistant to chemotherapy (29,
30). In this context, it is
urgent to study the mechanism of OS development and find new
targets for OS treatment. Previous studies revealed that TGF-β
plays an important role in tumor progression and can promote the
metastasis of numerous solid tumors, such as breast, colon and
prostate cancer (8,31-33). Interestingly, TGF-β exhibits dual
effects on tumor progression, which is, inhibiting tumor
progression in premalignant tumors and promoting tumor development
in advanced tumors (34-36). However, some studies have
indicated that compared with its role in malignant epithelial
neoplasms, TGF-β in sarcoma mainly exerts a tumor-promoting effect,
especially in OS (12,37). In patients with OS, the expression
of TGF-β1 and TGF-β2 in serum is significantly higher than that in
healthy donors (11,12). Previous studies have reported that
high levels of TGF-β1 mRNA expression in tumor cells were
associated with high-grade OS and frequent metastasis to the lung
or other organs (38,39). According to the literature,
SD-208, a TGF-βRI inhibitor, can significantly reduce the incidence
of lung metastasis in OS, which is similar to the results of our
study (12). The present study
explored the inhibitory effect of RepSox on OS cells. In brief,
RepSox inhibited the proliferation and metastasis of OS cells and
induced apoptosis in vitro through the JNK/Smad3 signaling
pathway, and its inhibitory effect on tumors was also confirmed
in vivo.
In our experiments, a small molecule TGF-βRI/ALK5
inhibitor (RepSox) was selected to evaluate its effect on OS cells.
It was revealed that RepSox significantly inhibited the
proliferation of OS cells in vitro. Mechanistically, it was
revealed that this result was mainly caused by cell cycle arrest
and increased apoptosis. Therefore, a literature search was
conducted and it was revealed that TGF-β can increase the
expression of CDK inhibitors 1A (CDKN1A or p21), 2B (CDKN2B or p15)
and 1C (CNKN1C or p57) and apoptosis inducer death-associated
protein kinase (DAPK) (40-42), thereby inducing cell cycle arrest
and promoting apoptosis. Cell cycle arrest is recognized as an
important cause of proliferation inhibition. This process is
closely monitored by some checkpoints and will be induced to pause
when necessary (43,44). Cyclins and CDKs are key regulators
of the cell cycle (45). CDK2 is
activated by binding to cyclin E, while cyclin E, in complex with
CDK2, drives the transition from the late G1 phase to the S phase
(46). In addition, the
association of CDK2 and cyclin A are also essential for G1/S
progression (47). Concurrently,
as an important protein inhibitor in the cell cycle, p21 can also
bind to CDK, resulting in a block in cell cycle progression
(48). In the present study,
RepSox promoted the transition of cells to the S phase by
upregulating cyclin E1 and p21, and reduced cyclin A2 and CDK2 to
arrest the cell cycle in the S phase. In addition, c-Myc, another
key transcriptional activator of cell proliferation, has been
revealed to be inhibited (49).
As aforementioned, TGF-β plays the role of a tumor
suppressor, which is exactly the opposite of our experimental
results. A possible explanation is that cancer cells bypass these
cytostatic effects by inactivating key molecules in the TGF-β
signaling pathway and crosstalk with other oncogenic pathways
(49). Malignant tumor cells may
retain the TGF-β/Smad pathway, but they can promote tumor
progression by acquiring noncanonical PI3K/AKT, RAS/MAPK, or p53
pathway carcinogenic mutations (50). In our research, it was revealed
that the JNK/Smad3 signaling pathway was inhibited (Fig. 6), which may explain the
aforementioned phenomenon, but the specific mechanism still
requires further study.
In the subsequent wound-healing, cell migration and
invasion assays, the drug concentration was changed in order to
exclude the biased results caused by cell proliferation. The
results revealed that RepSox could also significantly inhibit the
migration and invasion of tumor cells below the cytotoxic
concentration, which fully indicated that RepSox had a quite
evident inhibitory effect on the metastasis of OS cells.
As aforementioned, TGF-β has a dual effect in
tumors, and ultimately, the function of TGF-β is related to its
ability to induce epithelial-mesenchymal transition (EMT) programs
(51). Sarcoma has a mesenchymal
origin, and its mesenchymal phenotype is maintained by the
functions of EMT transcription factors (EMT-TFs), including TWIST1,
NAIL, SLUG, ZEB1 and ZEB2, and associated with more aggressive
behaviors (52). A number of
studies indicated that overexpression of EMT-related transcription
factors is implicated in the OS pathogenesis, which may be a
significant reason for EMT (53-56). In addition, several in
vitro studies have confirmed that the ability of TGF-βs to
promote EMT programs in various OS cell lines may be linked to the
promigratory effect of TGF-βI (57-60). In addition, Smad and MAP-kinase
signaling are essential for the transcriptional induction of
numerous extracellular matrix (ECM) proteins, such as MMPs (e.g.,
MMP-2 and MMP-9) (61,62). Multiple previous studies (63-66) demonstrated that inhibition of the
phosphorylation of JNK can reduce the expression of MMP-2/MMP-9 in
OS cells. Furthermore, a study by Lamora et al (67) revealed that halofuginone decreased
the expression and activity of MMP-2 by inhibiting the TGF-β/Smad3
cascade in OS. In our study, RepSox inhibited the expression of
MMP-2 and MMP-9 in a concentration-dependent manner, reducing
transcription-induced stimulation of ECM-related proteins. In
parallel, hallmarks of mesenchymal phenotypes include the
expression of vimentin-based intermediate filaments, the exchange
of E-cadherin-based with N-cadherin-based junctions, and the
extensive synthesis of ECM proteins and matrix remodeling enzymes
(metalloproteases) that facilitate the migration and invasiveness
of mesenchymal and inflammatory cells (68). Our data revealed that the
expression of E-cadherin was increased under the effect of RepSox,
while the expression of N-cadherin and vimentin was inhibited,
indicating that RepSox enhanced the adhesion between tumor cells
and reduced the migration and invasion of tumor cells.
In in vivo experiments, several tumor
xenograft models in literature have been used, and it was revealed
that they all adopted the 143B cell line as the source of tumor
cells. In addition, in our in vitro experiments, it was
observed that 143B cells proliferated faster than HOS cells. It was
hypothesized that this may render the experimental results more
intuitive, therefore the 143B cell line was selected. Meanwhile, in
addition to the 143B cell line, other OS cell lines should also be
used for in vivo xenograft studies. However, due to
constraints on research funds and time, only the 143B cell line was
selected for in vivo experiments in the present study, which
is also a limitation of the present study.
With regard to the tumors in the in vivo
experiments that appear to have a more pronounced inhibitory effect
than in the in vitro experiments, it was hypothesized that
this may be due to the effect of migration inhibitory factor (MIF)
on the tumor microenvironment. Several studies have revealed that
treatment with anti-MIF antibodies significantly inhibited the
growth of murine colon cancer, and concurrently suppressed its
angiogenesis (69,70). Moreover, a series of in
vitro and in vivo experimental results indicated that
MIF deficiency attenuated tumor-polarized macrophage alternative
activation, immunosuppression, neoangiogenesis, and melanoma tumor
outgrowth (71). The
aforementioned results revealed the effect of MIF and its
inhibitors on the tumor microenvironment and that is also our
future direction of the present study. In addition, in vivo
pharmacokinetic and bioavailability studies on RepSox are
required.
In addition, in recent years, nanomedicine is
rapidly advancing as an emerging field. Nanomaterials are gradually
used as an effective carrier for the diagnosis and treatment of
diseases (72-76). In the chemotherapy of OS,
conventional small-molecule therapeutics exhibit low efficacies and
severe side effects, while the drug-delivery platforms based on
nanotechnology can significantly improve the antitumor efficacy and
diminish the side effects (74).
It has broad application prospects in OS chemotherapy. In addition,
the development and innovation of biocomposites also provide novel
insights for the treatment of OS (77).
In summary, our study revealed that RepSox could
induce cycle arrest and apoptosis of OS cells and inhibit EMT by
inhibiting the JNK/Smad3 pathway, which was also further
demonstrated in in vivo experiments. Therefore, RepSox may
be potentially valuable as an alternate therapeutic agent for OS
treatment.
Supplementary Data
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
DH and SF conceived and designed the experiments.
DH, JG, LZ, SL, LY and HL performed the experiments. DH, BP, WP and
ZC conducted the statistical analyses. CL helped to perform the
analyses with constructive suggestions. DH wrote the paper and SF
revised the paper. DH and JG confirm the authenticity of all the
raw data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The animal experiments were approved by the Animal
Care Ethics Committee of Sir Run Run Shaw Hospital (Hangzhou,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Friedrich P, Ortiz R, Strait K, Fuentes S,
Gamboa Y, Arambú I, Ah-Chu-Sanchez M, London W, Rodríguez-Galindo
C, Antillón-Klussmann F, et al: Central American Association of
Pediatric Hematologists Oncologists AHOPCA: Pediatric sarcoma in
Central America: Outcomes, challenges, and plans for improvement.
Cancer. 119:871–879. 2013. View Article : Google Scholar
|
|
2
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar
|
|
3
|
Bacci G, Longhi A, Versari M, Mercuri M,
Briccoli A and Picci P: Prognostic factors for osteosarcoma of the
extremity treated with neoadjuvant chemotherapy: 15-year experience
in 789 patients treated at a single institution. Cancer.
106:1154–1161. 2006. View Article : Google Scholar
|
|
4
|
Bielack SS, Kempf-Bielack B, Delling G,
Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M,
Winkelmann W, et al: Prognostic factors in high-grade osteosarcoma
of the extremities or trunk: An analysis of 1,702 patients treated
on neoadjuvant cooperative osteosarcoma study group protocols. J
Clin Oncol. 20:776–790. 2002. View Article : Google Scholar
|
|
5
|
Derynck R and Zhang YE: Smad-dependent and
Smad-independent pathways in TGF-beta family signalling. Nature.
425:577–584. 2003. View Article : Google Scholar
|
|
6
|
Zhang YE: Non-Smad signaling pathways of
the TGF-β family. Cold Spring Harb Perspect Biol. 9:92017.
View Article : Google Scholar
|
|
7
|
Batlle E and Massagué J: Transforming
growth factor-β signaling in immunity and cancer. Immunity.
50:924–940. 2019. View Article : Google Scholar
|
|
8
|
Walker RA and Dearing SJ: Transforming
growth factor beta 1 in ductal carcinoma in situ and invasive
carcinomas of the breast. Eur J Cancer. 28:641–644. 1992.
View Article : Google Scholar
|
|
9
|
Adekoya TO and Richardson RM: Cytokines
and chemokines as mediators of prostate cancer metastasis. Int J
Mol Sci. 21:212020. View Article : Google Scholar
|
|
10
|
Stolfi C, Troncone E, Marafini I and
Monteleone G: Role of TGF-beta and Smad7 in gut inflammation,
fibrosis and cancer. Biomolecules. 11:112020. View Article : Google Scholar
|
|
11
|
Xu S, Yang S, Sun G, Huang W and Zhang Y:
Transforming growth factor-beta polymorphisms and serum level in
the development of osteosarcoma. DNA Cell Biol. 33:802–806. 2014.
View Article : Google Scholar
|
|
12
|
Lamora A, Talbot J, Bougras G, Amiaud J,
Leduc M, Chesneau J, Taurelle J, Stresing V, Le Deley MC, Heymann
MF, et al: Overexpression of smad7 blocks primary tumor growth and
lung metastasis development in osteosarcoma. Clin Cancer Res.
20:5097–5112. 2014. View Article : Google Scholar
|
|
13
|
Gellibert F, Woolven J, Fouchet MH,
Mathews N, Goodland H, Lovegrove V, Laroze A, Nguyen VL, Sautet S,
Wang R, et al: Identification of 1,5-naphthyridine derivatives as a
novel series of potent and selective TGF-beta type I receptor
inhibitors. J Med Chem. 47:4494–4506. 2004. View Article : Google Scholar
|
|
14
|
Ichida JK, Blanchard J, Lam K, Son EY,
Chung JE, Egli D, Loh KM, Carter AC, Di Giorgio FP, Koszka K, et
al: A small-molecule inhibitor of tgf-Beta signaling replaces sox2
in reprogramming by inducing nanog. Cell Stem Cell. 5:491–503.
2009. View Article : Google Scholar
|
|
15
|
Mei L, Sang W, Chen Z, Zheng L, Jin K, Lou
C, Huang W and He D: Small molecule inhibitor RepSox prevented
ovariectomy-induced osteoporosis by suppressing osteoclast
differentiation and bone resorption. J Cell Physiol. 233:9724–9738.
2018. View Article : Google Scholar
|
|
16
|
Fu Y, Huang C, Xu X, Gu H, Ye Y, Jiang C,
Qiu Z and Xie X: Direct reprogramming of mouse fibroblasts into
cardiomyocytes with chemical cocktails. Cell Res. 25:1013–1024.
2015. View Article : Google Scholar
|
|
17
|
Jajosky AN, Coad JE, Vos JA, Martin KH,
Senft JR, Wenger SL and Gibson LF: RepSox slows decay of CD34+
acute myeloid leukemia cells and decreases T cell immunoglobulin
mucin-3 expression. Stem Cells Transl Med. 3:836–848. 2014.
View Article : Google Scholar
|
|
18
|
Ide M, Jinnin M, Tomizawa Y, Wang Z,
Kajihara I, Fukushima S, Hashizume Y, Asano Y and Ihn H:
Transforming growth factor β-inhibitor Repsox downregulates
collagen expression of scleroderma dermal fibroblasts and prevents
bleomycin-induced mice skin fibrosis. Exp Dermatol. 26:1139–1143.
2017. View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
20
|
Jiang X, Shan J, Dai N, Zhong Z, Qing Y,
Yang Y, Zhang S, Li C, Sui J, Ren T, et al: Apurinic/apyrimidinic
endonuclease 1 regulates angiogenesis in a transforming growth
factor β-dependent manner in human osteosarcoma. Cancer Sci.
106:1394–1401. 2015. View Article : Google Scholar
|
|
21
|
Lu KH, Su SC, Lin CW, Hsieh YH, Lin YC,
Chien MH, Reiter RJ and Yang SF: Melatonin attenuates osteosarcoma
cell invasion by suppression of C-C motif chemokine ligand 24
through inhibition of the c-Jun N-terminal kinase pathway. J Pineal
Res. 65:e125072018. View Article : Google Scholar
|
|
22
|
Sun Y, Xia P, Zhang H, Liu B and Shi Y:
P53 is required for Doxorubicin-induced apoptosis via the TGF-beta
signaling pathway in osteosarcoma-derived cells. Am J Cancer Res.
6:114–125. 2015.
|
|
23
|
Wang H, Zhang T, Sun W, Wang Z, Zuo D,
Zhou Z, Li S, Xu J, Yin F, Hua Y, et al: Erianin induces G2/M-phase
arrest, apoptosis, and autophagy via the ROS/JNK signaling pathway
in human osteosarcoma cells in vitro and in vivo. Cell Death Dis.
7:e22472016. View Article : Google Scholar
|
|
24
|
Wang S, Li H, Chen S, Wang Z, Yao Y, Chen
T, Ye Z and Lin P: Andrographolide induces apoptosis in human
osteosarcoma cells via the ROS/JNK pathway. Int J Oncol.
56:1417–1428. 2020.
|
|
25
|
Wang Y, Deng X, Yu C, Zhao G, Zhou J,
Zhang G, Li M, Jiang D, Quan Z and Zhang Y: Synergistic inhibitory
effects of capsaicin combined with cisplatin on human osteosarcoma
in culture and in xenografts. J Exp Clin Cancer Res. 37:2512018.
View Article : Google Scholar
|
|
26
|
Jie Z, Xie Z, Zhao X, Sun X, Yu H, Pan X,
Shen S, Qin A, Fang X and Fan S: Glabridin inhibits osteosarcoma
migration and invasion via blocking the p38- and JNK-mediated
CREB-AP1 complexes formation. J Cell Physiol. 234:4167–4178. 2019.
View Article : Google Scholar
|
|
27
|
Lin RC, Yang SF, Chiou HL, Hsieh SC, Wen
SH, Lu KH and Hsieh YH: Licochalcone A-induced apoptosis through
the activation of p38MAPK pathway mediated mitochondrial pathways
of apoptosis in human osteosarcoma cells in vitro and in vivo.
Cells. 8:82019. View Article : Google Scholar
|
|
28
|
Lu KH, Chen PN, Hsieh YH, Lin CY, Cheng
FY, Chiu PC, Chu SC and Hsieh YS: 3-Hydroxyflavone inhibits human
osteosarcoma U2OS and 143B cells metastasis by affecting EMT and
repressing u-PA/MMP-2 via FAK-Src to MEK/ERK and RhoA/MLC2 pathways
and reduces 143B tumor growth in vivo. Food Chem Toxicol.
97:177–186. 2016. View Article : Google Scholar
|
|
29
|
Goorin AM, Harris MB, Bernstein M,
Ferguson W, Devidas M, Siegal GP, Gebhardt MC, Schwartz CL, Link M
and Grier HE: Phase II/III trial of etoposide and high-dose
ifosfamide in newly diagnosed metastatic osteosarcoma: A pediatric
oncology group trial. J Clin Oncol. 20:426–433. 2002. View Article : Google Scholar
|
|
30
|
Kempf-Bielack B, Bielack SS, Jürgens H,
Branscheid D, Berdel WE, Exner GU, Göbel U, Helmke K, Jundt G,
Kabisch H, et al: Osteosarcoma relapse after combined modality
therapy: An analysis of unselected patients in the Cooperative
Osteosarcoma Study Group (COSS). J Clin Oncol. 23:559–568. 2005.
View Article : Google Scholar
|
|
31
|
Bierie B and Moses HL: Tumour
microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer.
Nat Rev Cancer. 6:506–520. 2006. View Article : Google Scholar
|
|
32
|
Friedman E, Gold LI, Klimstra D, Zeng ZS,
Winawer S and Cohen A: High levels of transforming growth factor
beta 1 correlate with disease progression in human colon cancer.
Cancer Epidemiol Biomarkers Prev. 4:549–554. 1995.
|
|
33
|
Wikström P, Stattin P, Franck-Lissbrant I,
Damber JE and Bergh A: Transforming growth factor beta1 is
associated with angiogenesis, metastasis, and poor clinical outcome
in prostate cancer. Prostate. 37:19–29. 1998. View Article : Google Scholar
|
|
34
|
Roberts AB and Wakefield LM: The two faces
of transforming growth factor beta in carcinogenesis. Proc Natl
Acad Sci USA. 100:8621–8623. 2003. View Article : Google Scholar
|
|
35
|
Derynck R, Akhurst RJ and Balmain A:
TGF-beta signaling in tumor suppression and cancer progression. Nat
Genet. 29:117–129. 2001. View Article : Google Scholar
|
|
36
|
Costanza B, Umelo IA, Bellier J,
Castronovo V and Turtoi A: Stromal modulators of TGF-β in cancer. J
Clin Med. 6:62017. View Article : Google Scholar
|
|
37
|
Matsuyama S, Iwadate M, Kondo M, Saitoh M,
Hanyu A, Shimizu K, Aburatani H, Mishima HK, Imamura T, Miyazono K,
et al: SB-431542 and Gleevec inhibit transforming growth
factor-beta-induced proliferation of human osteosarcoma cells.
Cancer Res. 63:7791–7798. 2003.
|
|
38
|
Franchi A, Arganini L, Baroni G, Calzolari
A, Capanna R, Campanacci D, Caldora P, Masi L, Brandi ML and Zampi
G: Expression of transforming growth factor beta isoforms in
osteosarcoma variants: Association of TGF beta 1 with high-grade
osteosarcomas. J Pathol. 185:284–289. 1998. View Article : Google Scholar
|
|
39
|
Mohseny AB, Cai Y, Kuijjer M, Xiao W, van
den Akker B, de Andrea CE, Jacobs R, ten Dijke P, Hogendoorn PC and
Cleton-Jansen AM: The activities of Smad and Gli mediated
signalling pathways in high-grade conventional osteosarcoma. Eur J
Cancer. 48:3429–3438. 2012. View Article : Google Scholar
|
|
40
|
Jang CW, Chen CH, Chen CC, Chen JY, Su YH
and Chen RH: TGF-beta induces apoptosis through Smad-mediated
expression of DAP-kinase. Nat Cell Biol. 4:51–58. 2002. View Article : Google Scholar
|
|
41
|
Hannon GJ and Beach D: p15INK4B is a
potential effector of TGF-beta-induced cell cycle arrest. Nature.
371:257–261. 1994. View Article : Google Scholar
|
|
42
|
Reynisdóttir I, Polyak K, Iavarone A and
Massagué J: Kip/Cip and Ink4 Cdk inhibitors cooperate to induce
cell cycle arrest in response to TGF-beta. Genes Dev. 9:1831–1845.
1995. View Article : Google Scholar
|
|
43
|
Ghelli Luserna di Rora'AIacobucci I and
Martinelli G: The cell cycle checkpoint inhibitors in the treatment
of leukemias. J Hematol Oncol. 10:772017. View Article : Google Scholar
|
|
44
|
Wang JL, Quan Q, Ji R, Guo XY, Zhang JM,
Li X and Liu YG: Isorhamnetin suppresses PANC-1 pancreatic cancer
cell proliferation through S phase arrest. Biomed Pharmacother.
108:925–933. 2018. View Article : Google Scholar
|
|
45
|
Szmyd R, Niska-Blakie J, Diril MK, Renck
Nunes P, Tzelepis K, Lacroix A, van Hul N, Deng LW, Matos J,
Dreesen O, et al: Premature activation of Cdk1 leads to mitotic
events in S phase and embryonic lethality. Oncogene. 38:998–1018.
2019. View Article : Google Scholar
|
|
46
|
Koff A, Giordano A, Desai D, Yamashita K,
Harper JW, Elledge S, Nishimoto T, Morgan DO, Franza BR and Roberts
JM: Formation and activation of a cyclin E-cdk2 complex during the
G1 phase of the human cell cycle. Science. 257:1689–1694. 1992.
View Article : Google Scholar
|
|
47
|
Girard F, Strausfeld U, Fernandez A and
Lamb NJ: Cyclin A is required for the onset of DNA replication in
mammalian fibroblasts. Cell. 67:1169–1179. 1991. View Article : Google Scholar
|
|
48
|
Xiong Y, Hannon GJ, Zhang H, Casso D,
Kobayashi R and Beach D: p21 is a universal inhibitor of cyclin
kinases. Nature. 366:701–704. 1993. View Article : Google Scholar
|
|
49
|
Yeh HW, Lee SS, Chang CY, Lang YD and Jou
YS: A new switch for TGFβ in cancer. Cancer Res. 79:3797–3805.
2019. View Article : Google Scholar
|
|
50
|
Massagué J: TGFbeta in Cancer. Cell.
134:215–230. 2008. View Article : Google Scholar
|
|
51
|
Morrison CD, Parvani JG and Schiemann WP:
The relevance of the TGF-β Paradox to EMT-MET programs. Cancer
Lett. 341:30–40. 2013. View Article : Google Scholar
|
|
52
|
Yu X, Yustein JT and Xu J: Research models
and mesenchymal/epithelial plasticity of osteosarcoma. Cell Biosci.
11:942021. View Article : Google Scholar
|
|
53
|
Yang G, Yuan J and Li K: EMT transcription
factors: Implication in osteosarcoma. Med Oncol. 30:6972013.
View Article : Google Scholar
|
|
54
|
Sharili AS, Allen S, Smith K, Hargreaves
J, Price J and McGonnell I: Expression of Snail2 in long bone
osteosarcomas correlates with tumour malignancy. Tumour Biol.
32:515–526. 2011. View Article : Google Scholar
|
|
55
|
Wensman H, Göransson H, Leuchowius KJ,
Strömberg S, Pontén F, Isaksson A, Rutteman GR, Heldin NE, Pejler G
and Hellmén E: Extensive expression of craniofacial related
homeobox genes in canine mammary sarcomas. Breast Cancer Res Treat.
118:333–343. 2009. View Article : Google Scholar
|
|
56
|
Wu J, Liao Q, He H, Zhong D and Yin K:
TWIST interacts with β-catenin signaling on osteosarcoma cell
survival against cisplatin. Mol Carcinog. 53:440–446. 2014.
View Article : Google Scholar
|
|
57
|
Chen J, Song Y, Yang J, Gong L, Zhao P,
Zhang Y and Su H: The up-regulation of cysteine-rich protein 61
induced by transforming growth factor beta enhances osteosarcoma
cell migration. Mol Cell Biochem. 384:269–277. 2013. View Article : Google Scholar
|
|
58
|
Huang Y, Yang Y, Gao R, Yang X, Yan X,
Wang C, Jiang S and Yu L: RLIM interacts with Smurf2 and promotes
TGF-β induced U2OS cell migration. Biochem Biophys Res Commun.
414:181–185. 2011. View Article : Google Scholar
|
|
59
|
Kunita A, Kashima TG, Ohazama A,
Grigoriadis AE and Fukayama M: Podoplanin is regulated by AP-1 and
promotes platelet aggregation and cell migration in osteosarcoma.
Am J Pathol. 179:1041–1049. 2011. View Article : Google Scholar
|
|
60
|
Sung JY, Park SY, Kim JH, Kang HG, Yoon
JH, Na YS, Kim YN and Park BK: Interferon consensus
sequence-binding protein (ICSBP) promotes epithelial-to-mesenchymal
transition (EMT)-like phenomena, cell-motility, and invasion via
TGF-β signaling in U2OS cells. Cell Death Dis. 5:e12242014.
View Article : Google Scholar
|
|
61
|
Borok Z: Role for alpha3 integrin in EMT
and pulmonary fibrosis. J Clin Invest. 119:7–10. 2009.
|
|
62
|
Javelaud D and Mauviel A: Crosstalk
mechanisms between the mitogen-activated protein kinase pathways
and Smad signaling downstream of TGF-beta: Implications for
carcinogenesis. Oncogene. 24:5742–5750. 2005. View Article : Google Scholar
|
|
63
|
Chueh FS, Chen YY, Huang AC, Ho HC, Liao
CL, Yang JS, Kuo CL and Chung JG: Bufalin-inhibited migration and
invasion in human osteosarcoma U-2 OS cells is carried out by
suppression of the matrix metalloproteinase-2, ERK, and JNK
signaling pathways. Environ Toxicol. 29:21–29. 2014. View Article : Google Scholar
|
|
64
|
Fromigué O, Hamidouche Z and Marie PJ:
Blockade of the RhoA-JNK-c-Jun-MMP2 cascade by atorvastatin reduces
osteosarcoma cell invasion. J Biol Chem. 283:30549–30556. 2008.
View Article : Google Scholar
|
|
65
|
Jung O and Lee SY: Synergistic anticancer
effects of timosaponin AIII and ginsenosides in MG63 human
osteosarcoma cells. J Ginseng Res. 43:488–495. 2019. View Article : Google Scholar
|
|
66
|
Liao CL, Lai KC, Huang AC, Yang JS, Lin
JJ, Wu SH, Gibson Wood W, Lin JG and Chung JG: Gallic acid inhibits
migration and invasion in human osteosarcoma U-2 OS cells through
suppressing the matrix metalloproteinase-2/-9, protein kinase B
(PKB) and PKC signaling pathways. Food Chem Toxicol. 50:1734–1740.
2012. View Article : Google Scholar
|
|
67
|
Lamora A, Mullard M, Amiaud J, Brion R,
Heymann D, Redini F and Verrecchia F: Anticancer activity of
halofuginone in a preclinical model of osteosarcoma: Inhibition of
tumor growth and lung metastases. Oncotarget. 6:14413–14427. 2015.
View Article : Google Scholar
|
|
68
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar
|
|
69
|
Nishihira J, Ishibashi T, Fukushima T, Sun
B, Sato Y and Todo S: Macrophage migration inhibitory factor (MIF):
Its potential role in tumor growth and tumor-associated
angiogenesis. Ann NY Acad Sci. 995:171–182. 2003. View Article : Google Scholar
|
|
70
|
Ogawa H, Nishihira J, Sato Y, Kondo M,
Takahashi N, Oshima T and Todo S: An antibody for macrophage
migration inhibitory factor suppresses tumour growth and inhibits
tumour-associated angiogenesis. Cytokine. 12:309–314. 2000.
View Article : Google Scholar
|
|
71
|
Yaddanapudi K, Putty K, Rendon BE, Lamont
GJ, Faughn JD, Satoskar A, Lasnik A, Eaton JW and Mitchell RA:
Control of tumor-associated macrophage alternative activation by
macrophage migration inhibitory factor. J Immunol. 190:2984–2993.
2013. View Article : Google Scholar
|
|
72
|
Li Y, Li X, Lu Y, Chaurasiya B, Mi G, Shi
D, Chen D, Webster TJ, Tu J and Shen Y: Co-delivery of Poria cocos
extract and doxorubicin as an 'all-in-one' nanocarrier to combat
breast cancer multidrug resistance during chemotherapy.
Nanomedicine. 23:1020952020. View Article : Google Scholar
|
|
73
|
Xu J, Wang H, Hu Y, Zhang YS, Wen L, Yin
F, Wang Z, Zhang Y, Li S, Miao Y, et al: Inhibition of CaMKIIα
activity enhances antitumor effect of fullerene C60 nanocrystals by
suppression of autophagic degradation. Adv Sci (Weinh).
6:18012332019. View Article : Google Scholar
|
|
74
|
Zhang Y, Wang F, Li M, Yu Z, Qi R, Ding J,
Zhang Z and Chen X: Self-stabilized hyaluronate nanogel for
intracellular codelivery of doxorubicin and cisplatin to
osteosarcoma. Adv Sci (Weinh). 5:17008212018. View Article : Google Scholar
|
|
75
|
Li D, Xu W, Li P, Ding J, Cheng Z, Chen L,
Yan L and Chen X: Self-targeted polysaccharide prodrug suppresses
orthotopic hepatoma. Mol Pharm. 13:4231–4235. 2016. View Article : Google Scholar
|
|
76
|
Wang J, Li Z, Wang Z, Yu Y, Li D, Li B and
Ding J: Nanomaterials for combinational radio-immuno oncotherapy.
Adv Funct Mater. 30:19106762020. View Article : Google Scholar
|
|
77
|
Zhao D, Zhu T, Li J, Cui L, Zhang Z,
Zhuang X and Ding J: Poly(lactic-co-glycolic acid)-based composite
bone-substitute materials. Bioact Mater. 6:346–360. 2020.
View Article : Google Scholar
|