Introduction
Oral cancer (OC) is highly malignant, and patient
prognosis remains poor even after standard treatments. OC is a
leading cause of cancer-related death, and a total of 354,864 new
cases of OC were diagnosed worldwide in 2018 (1,2). The
high prevalence of OC has been associated with the use of tobacco,
including smokeless tobacco, and heavy alcohol consumption,
particularly in developing countries in Asia (2–4). Oral
squamous cell carcinoma accounts for approximately 90% of all OC
cases (5). Either surgery or
radiotherapy is recommended as standard therapies for early stage
OC. In highly advanced stages, concurrent systemic chemotherapy
with radiotherapy is suggested with surgical removal of residual
tumors if feasible. During the past few decades, the 5-year overall
survival of OC has remained unfavorable due to the low sensitivity
to therapies and metastatic potential that are thought to be caused
by genetic aberrations. The 5-year survival rate of OC is up to 80%
in the early stage of disease and only 20–30% in advanced stages,
which is dependent on various factors, including the primary site
in the oral cavity, comorbidity, and selection of treatment
(6,7). Overall survival of OC patients has
improved by 15% over the last 50 years, but only 5% over the last
20 years (8). Recent developments
in new treatments, including molecular-targeted therapy and
immunotherapy, have improved the survival of OC patients.
Cetuximab, an inhibitor of epidermal growth factor receptor (EGFR),
is reportedly efficacious against advanced squamous cell carcinoma
of the head and neck (9). Treatment
with nivolumab, an anti-programmed cell death-1 (PD-1) antibody
(Ab), following cetuximab has improved the overall survival of
patients with advanced head and neck cancers (10). Nonetheless, the efficacy of these
treatments against OC remains limited. Therefore, more effective
molecular therapies targeting cancer-specific molecules with less
adverse events in combination with personalized medicine using
several types of cancer biomarkers are urgently needed.
Gene expression profile analysis and subsequent
tissue microarray analysis of a variety of solid tumor tissues have
been employed to identify potential molecular targets for cancer
diagnostics and therapeutics. Dozens of oncoantigens, which are
essential for disease progression of various solid cancers, in
addition to various molecules involved in cell cycle progression
and/or cell survival have been identified (11–37).
As a potential biomarker and molecular target for the treatment of
OC, Holliday junction recognition protein (HJURP) is highly
expressed in the majority of OCs, while expression is comparatively
low in normal tissues. As reported in our previous study, HJURP
contributes to the immortality of cancer cells through the DNA
double-strand break repair pathway by interacting with MSH5 and
NBS1 (24). HJURP is a part of a
centromeric protein with four domains that is required for
centromere protein A (CENP-A) nucleosome assembly at the
centromeres to ensure accurate chromosomal segregation during cell
division (38,39). High expression of HJURP was found to
be associated with a poorer clinical outcome of patients with
non-small cell lung cancer (24),
hepatocellular carcinoma (40),
gliomas (41), and ovarian cancer
(42), and was proposed as a
predictive marker of responses to radiotherapy of breast cancer
patients (43). However, the
precise role in OC and the clinical potential of HJURP as a
molecular target remain unclear. Therefore, the aim of the present
study was to investigate the role of HJURP in the malignant nature
of OC and its potential as a diagnostic and prognostic tissue
biomarker and a therapeutic target for OC.
Materials and methods
Cell lines and clinical tissue
samples
Human OC cells (CAL 27, Ca9-22, FaDu, HSC2, HSC3,
HSC4, and SCC-9) were cultured in medium supplemented with 10%
fetal bovine serum (FBS; Gibco, Thermo Fisher Scientific Inc.) and
1% penicillin/streptomycin (Wako Pure Chemical Industries) at 37°C
under a humidified atmosphere of 5% CO2/95% air. Human
primary oral mucosal keratinocytes (HOMKs) were commercially
purchased and cultured in medium supplemented with EpiLife defined
growth supplement (Gibco, Thermo Fisher Scientific Inc.). The
features of all cells are summarized in Table I. The requirement for ethics
approval for the use of commercially available primary human cells
such as HOMK cells in this study was waived by the ethics
committee. For real-time quantitative polymerase chain reaction
(qPCR) experiments, 14 frozen oral squamous cell cancer tissues (4
female, 10 male patients; median age, 60 years; age range, 45–74
years; all cases were Caucasians) were purchased from ProteoGenex,
Inc., and commercially available normal tongue tissue polyA RNA was
obtained from Clontech Laboratories, Inc. Moreover, 152 existing
formalin-fixed paraffin-embedded OC tissues and adjacent normal
oral tissues obtained from patients (66 females and 86 males;
median age, 69 years; age range, 28–92 years; all cases analyzed in
this study were Asians) who underwent curative surgery with
adjuvant chemotherapy or neoadjuvant chemotherapy at Kumamoto
University between 2004 and 2012 were used for immunohistochemical
analysis on tissue microarrays. The Union for International Cancer
Control TNM classification was used to determine the clinical stage
of the OC samples. The study protocol and the use of existing
clinical materials in this study were approved by the relevant
Ethics Committees [Kumamoto University; Shiga University of Medical
Science (no. G2009-163)] based on the national ethical guidelines
for human subjects. It was confirmed that this study was fully
ethically compliant and the informed consent was waived due to the
retrospective nature of the study and in accordance with the
national ethical guidelines.
 | Table I.Human OC cell lines and HOMKs. |
Table I.
Human OC cell lines and HOMKs.
| Cell line | Histology | Resource
distributor | Catalog no. |
|---|
| CAL 27 | Squamous cell
carcinoma of tongue | ATCC | CRL-2095 |
| Ca9-22 | Gingival squamous
cell carcinoma | RIKEN BRC | RCB1976 |
| FaDu | Squamous cell
carcinoma of pharynx | ATCC | HTB-43 |
| HSC2 | Squamous cell
carcinoma of mouth | RIKEN BRC | RCB1945 |
| HSC3 | Squamous cell
carcinoma of tongue | RIKEN BRC | RCB1975 |
| HSC4 | Squamous cell
carcinoma of tongue | RIKEN BRC | RCB1902 |
| SCC-9 | Squamous cell
carcinoma of tongue | ATCC | CRL-1629 |
| HOMK | Human oral mucosa
keratinocytes | Cell Research Corp.
Pte Ltd | hOMK100 |
qPCR
Total RNA was isolated from cultured cells and
clinical tissues using the Maxwell® 16 LEV simplyRNA
Cells Kit (Promega Corp.) in accordance with the manufacturer's
protocol. For reverse transcription, cDNA was synthesized from
total RNA using PrimeScript™ RT Master Mix (Takara Bio Inc.).
Samples were incubated at 37°C for 15 min and 85°C for 5 sec. The
qPCR experiments were performed with TaqMan Fast Universal PCR
Master Mix (Thermo Fisher Scientific Inc.) and a QuantStudio™ 3
Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.) in accordance with the manufacturers' protocols. All
experiments were performed in triplicate. The primers Hs01565312_m1
and Hs01060665_g1 (Applied Biosystems; Thermo Fisher Scientific,
Inc.) were used for amplification of HJURP and ACTB
(as an internal control), respectively. The thermal cycling
conditions consisted of an initial denaturation step at 95°C for 20
sec followed by 40 cycles at 95°C for 1 sec and 60°C for 20 sec.
Comparative HJURP mRNA expression was calculated by the
2−ΔΔCq method (44) with
ACTB mRNA expression as a reference.
Western blot analysis
The cells were washed with cold phosphate-buffered
saline (PBS) (−) and lysed with radioimmunoprecipitation assay
buffer with a protease inhibitor cocktail (Thermo Fisher Scientific
Inc.). After homogenization, the cell lysates were cooled on ice
for 30 min and then centrifuged at 15,000 rpm for 15 min to
separate the supernatant from cellular debris. The amount of total
protein was quantified with a detergent compatible protein assay
kit (Bio-Rad Laboratories, Inc.) and then mixed with sample buffer,
boiled at 100°C for 5 min, and incubated at room temperature for 5
min. Proteins were separated by electrophoresis using 10%
Mini-PROTEAN® TGX™ Precast Gels (Bio-Rad Laboratories,
Inc.) and then transferred to Trans-Blot® Turbo™ 0.2 µm
polyvinylidene fluoride membranes (Bio-Rad Laboratories, Inc.),
which were blocked with Block Ace solution (DS Pharma Biomedical)
and incubated overnight at 4°C with a rabbit polyclonal Abs against
HJURP (dilution, 1:500; catalog no. HPA008436; Sigma-Aldrich; Merck
KGaA), rabbit monoclonal Abs against p21 (1:1,000; catalog no.
2947; Cell Signaling Technology, Inc.) and Lamin B1 (1:1,000;
catalog no. 13435; Cell Signaling Technology, Inc.), a mouse
monoclonal Ab against Myc-Tag (1:1,000; catalog no. 2276; Cell
Signaling Technology, Inc.), and a rabbit polyclonal Ab against
β-actin (1:2,000; catalog no. 4970; Cell Signaling Technology,
Inc.). After washing with Tris-buffered saline containing Tween-20
(Cell Signaling Technology, Inc.), the membranes were incubated
with anti-rabbit (1:3,000; catalog no. NA934V; GE Healthcare) or
anti-mouse (1:2,000; catalog no. NA931V; GE Healthcare) horseradish
peroxidase-conjugated secondary Abs for 1 h at room temperature,
and visualized using enhanced chemiluminescence reagent with a
Fusion Solo S image analyzer (Vilber Lourmat).
Immunocytochemical analysis
Cultured cells were grown on Lab-Tek II chamber
slides (Nalge Nunc International), washed with cold PBS (−), fixed
with 4% paraformaldehyde for 15 min, and permeabilized with 0.2%
Triton X-100 in PBS (−) for 2 min at room temperature. Non-specific
binding was blocked by 3% bovine serum albumin (BSA; Wako Pure
Chemical Industries) in PBS for 30 min and then incubated with a
rabbit polyclonal Abs against HJURP (1:100; HPA008436;
Sigma-Aldrich; Merck KGaA) in PBS (−) supplemented with 1% BSA
overnight at 4°C in a wet box. After washing with PBS (−), Alexa
Fluor 488-conjugated anti-rabbit Abs (1:800; catalog no. A11008;
Life Technologies, Inc.) were applied for 90 min at room
temperature in the wet box with protection from light. Afterward,
the cells were mounted on glass slides using
VECTASHIELD® Antifade Mounting Medium with DAPI
(4′,6-diamidino-2-phenylindole; Vector Laboratories, Inc.) and
visualized with a confocal laser scanning microscope at ×63
magnification (Leica TCS SP8 X; Leica Microsystems).
Immunohistochemical analysis and
tissue microarray
To verify the biological and clinicopathological
significance of HJURP in clinical OC tissues, HJURP protein
expression was examined using tissue microarrays. Tumor tissue
microarrays were created from formalin-fixed paraffin-embedded
primary OC tissues resected from 152 patients (59 who underwent
curative surgery with neoadjuvant chemotherapy and 93 who underwent
curative surgery with adjuvant chemotherapy). For construction of
the tissue microarrays, the tissue specimens were cut into
sections, which were stained with hematoxylin and eosin to identify
appropriate tumor areas for sampling. Three to five tissue cores
with a diameter of 0.6 mm were taken from selected areas of each
tumor donor block using a tissue microarrayer (Beecher Instruments
Inc.) and placed into a recipient paraffin block. A core of normal
oral epithelial tissue was obtained from each specimen and
5-µm-thick sections of the tissue microarray blocks were used for
immunohistochemical analysis.
Tissue microarray slides were deparaffinized in
xylene and rehydrated in graded concentrations of ethanol. Then,
antigen retrieval was conducted by heating the samples in a
microwave oven in Target Retrieval Solution at pH 6.0 (Dako).
Endogenous peroxidase was blocked using hydrogen peroxide and the
slides were incubated in Protein Block Serum-Free solution (Dako)
for 30 min, followed by incubation with rabbit polyclonal Abs
against HJURP (1:500; HPA008436; Sigma-Aldrich; Merck KGaA)
overnight at 4°C. After washing, the slides were incubated with
EnVision + System-HRP Labeled polymer anti-rabbit secondary Abs
(Dako) for 30 min, followed by 3,3′-diaminobenzidine (Dako) as the
substrate chromogen and hematoxylin (Dako) as a nuclear
counterstain. Images of the immunostained samples were acquired
with a NanoZoomer® whole slide scanner (Hamamatsu
Photonics K.K.) and positivity of the HJURP protein was
semi-quantitatively analyzed by three independent investigators
without prior knowledge of the clinicopathological data. Staining
of more than 10% of the tumor nuclei was considered positive for
HJURP expression, while staining of less than 10% of the tumor
nuclei was considered negative as previously described (24). A specimen was considered positive by
consensus of all three investigators. Since most positive cases
showed homogenous nuclear staining in tumor tissues, the cutoff
value of the staining index of HJURP in positive tissues was not
used in this study.
RNA interference assay
HSC4 and Ca9-22 cells (1×106) were plated
on 10-cm dishes and transfected with either small-interfering RNAs
(siRNAs) against HJURP or control siRNAs using Lipofectamine 2000
reagent (Invitrogen; Thermo Fisher Scientific Inc.) in accordance
with the manufacturer's recommendations, as previously described
(24). The target sequences of the
siRNAs were as follows: si-HJURP-#1,
5′-GUCAGUUGCUUGGGCCUUA-3′; si-HJURP-#2,
5′-CAAGCAUCAUCUCCACCAA-3′; Control siRNA-1 (LUC),
5′-CGUACGCGGAAUACUUCGATT-3′; and Control siRNA-2 (EGFP),
5′-GAAGCAGCACGACUUCUUCTT-3′ (Sigma-Aldrich; Merck KGaA). Knockdown
of HJURP expression by siRNAs was confirmed by western blot
analysis using Abs against HJURP. It was also confirmed that si-LUC
and si-EGFP are suitable controls with no off-target effect on the
various types of cancer cells including OC as previously described
(11–37).
Cell viability assay
HSC4 or Ca9-22 cells (1×104) transfected
with siRNAs against HJURP or control siRNAs were plated in the
wells of a 6-well plate with growth medium containing 10% FBS
(Thermo Fisher Scientific Inc.). The viability of the cells was
determined on post-transfection day 7 with a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
dye reduction assay using Cell Counting Kit-8 solution (Dojindo
Laboratories).
Colony formation assay
HSC4 and Ca9-22 cells (1×105) transfected
with siRNAs against HJURP or control siRNAs were cultured on 10-cm
dishes. After 7 days of incubation, the cells were washed three
times with PBS (−) and fixed with 4% paraformaldehyde
phosphate-buffered solution (Wako Pure Chemical Industries) for 1
h. After drying at room temperature, the cells were stained with
Giemsa staining solution (Wako Pure Chemical Industries) and images
were captured using a PIXUS-MP990 multifunction device (Canon).
Flow cytometry
HSC4 and Ca9-22 cells (1×106) transfected
with siRNAs against HJURP or control siRNAs were used for cell
cycle analysis using a CycleTEST™ PLUS DNA Reagent Kit (BD
Biosciences), while quantitative analysis of senescence-associated
β-galactosidase (SA-β-gal)-positive cells was conducted using a
Cellular Senescence Detection Kit-SPiDER-βGal (catalog no. SG03;
Dojindo Laboratories) in accordance with the manufacturers'
instructions. After transfection, the cells were harvested,
filtered through a 70-µm nylon mesh, and kept on ice in the dark.
The cells (1×104) were analyzed within 3 h using a
FACSVerse flow cytometer (BD Biosciences).
Live cell imaging
HSC4 and Ca9-22 cells transfected with siRNAs
against HJURP or control siRNAs were cultured on 35-mm glass
dishes containing growth medium supplemented with 10% FBS.
Time-lapse images were acquired every 60 min following HJURP
knockdown using the EVOS FL Auto Cell Imaging System (Thermo Fisher
Scientific Inc.) to reveal cellular dynamics.
SA-β-gal staining and detection of
senescence-associated proteins
HSC4 or Ca9-22 cells (1×104) transfected
with siRNAs were cultured on 35-mm culture dishes, washed with PBS
(−), and stained with a Senescence β-galactosidase Staining Kit
(Cell Signaling Technology). The presence of SA-β-gal activity was
determined by incubating cells with X-Gal solution overnight at
37°C in a dry incubator without CO2. Stained cells were
evaluated under a light microscope. HSC4 and Ca9-22 cells
(1×103) transfected with siRNAs were used for
quantitation of SA-β-gal activity with a Cellular Senescence Plate
Assay Kit-SPiDER-βGal (catalog no. SG05; Dojindo Laboratories) and
a Cell Count Normalization Kit (catalog no. C544; Dojindo
Laboratories) for normalization in accordance with the
manufacturer's instructions. Flow cytometric quantitative analysis
of cells positive for SA-β-gal was performed using a Cellular
Senescence Detection Kit-SPiDER-βGal (catalog no. SG03; Dojindo
Laboratories) as mentioned above. All assays were performed in
triplicate. Expression levels of senescence-associated secretory
phenotype (SASP) markers were detected using lysates of HSC4 and
Ca9-22 cells with a human cytokine Ab array according to the
manufacturer's protocol (Human Angiogenesis Antibody Array; catalog
no. ab193655; Abcam).
Dominant-negative peptide assay
Short amino acid sequences derived from the TLTY box
of the CENP-A binding domain of HJURP (TLTYETPQ 54–61; ref.
45), which is a direct binding
site for CENP-A, were linked to a membrane transducing 11
poly-arginine sequence (11R; ref. 32). The cell permeable peptide
11R-HJURPTLTY54-61 (RRRRRRRRRRR-GGG-TLTYETPQ) and a
scramble peptide (RRRRRRRRRRR-GGG-PTQTYLET) as a control
(Sigma-Aldrich) with >95% purity were synthesized. HSC4 and
Ca9-22 cells (1×104) were incubated with the peptides at
different concentrations (20–200 µM) for 3 days to examine the
growth suppressive effects of a cell permeable peptide in OC cells.
The viability of treated cells was evaluated using the MTT
assay.
To confirm the effect of the synthesized peptides on
the interaction between HJURP and CENP-A in OC cells,
immunoprecipitation experiments were performed using OC cells
treated with or without synthesized peptides and polyclonal Abs
against HJURP (Sigma-Aldrich; Merck KGaA) for immunoprecipitation
and mouse monoclonal Abs against CENP-A (1:1,000; catalog no.
MA1-20832; Invitrogen; Thermo Fisher Scientific Inc.) for western
blot analysis. For the immunoprecipitation experiments, protein
samples were incubated with polyclonal Abs against HJURP (1:100;
HPA008436; Sigma-Aldrich; Merck KGaA) overnight at 4°C. After 2 h
of incubation with 20 µl of Pierce Protein A/G Plus Agarose beads
(catalog no. 20423, Thermo Fisher Scientific Inc.), proteins bound
to the beads were collected by centrifugation at 3,000 rpm for 1
min at 4°C and washed with lysis buffer. Then, the beads were
resuspended in Laemmli sample buffer and boiled for 5 min for
western blot analysis, which was performed using monoclonal primary
Abs (Invitrogen; Thermo Fisher Scientific Inc.) and anti-mouse
secondary Abs (1:2,000; GE Healthcare) against CENP-A.
Statistical analysis
All statistical analyses were conducted using
StatView 5.0 statistical software (SAS Institute, Inc.) and IBM
SPSS Statistics for Windows, version 25.0. (IBM Corp.). Differences
between groups of cell-based assays were compared using the
Student's t-test (unpaired data), and multiple comparisons were
conducted with one-way ANOVA followed by Tukey's post hoc test.
Fisher's exact test was used to assess the correlation of HJURP
expression levels determined by tissue microarray analysis with
relevant clinicopathological variables, such as patient age, sex,
primary tumor region, pT (pathologic tumor) classification, and pN
(pathologic lymph node) classification. Tumor-specific survival
curves were drawn from the date of surgery to the time of death
related to OC or the last follow-up observation. Kaplan-Meier
curves were calculated for each relevant variable and HJURP
expression. Differences in survival times among patient subgroups
were analyzed using the log-rank test. Univariate and multivariate
analyses were performed with Cox proportional hazards models to
identify prognostic factors of OC patients. First, associations
between death and possible prognostic factors, including positive
HJURP expression, age, sex, primary region, pT classification, pN
classification, and treatment (curative surgery with adjuvant
chemotherapy vs. curative surgery with neoadjuvant chemotherapy)
were analyzed. Second, multivariate Cox analysis was applied in
stepwise procedures that always forced positive HJURP expression
into the model along with each significant variable. As significant
prognostic factors were continually added to the model, independent
factors with a P-value <0.05 were considered statistically
significant.
Database analysis
The relationship between HJURP expression and
survival of head and neck cancer patients was evaluated with
reference to the ProgGene database (http://genomics.jefferson.edu/proggene/) and GEPIA
(Gene Expression Profiling Interactive Analysis) database
(http://gepia2.cancer-pku.cn/#index).
Signaling pathways related to HJURP were screened against the
ONCOMINE database (https://www.oncomine.org/resource/login.html) and GSEA
(Gene Set Enrichment Analysis) database (https://www.gsea-msigdb.org/gsea/msigdb/search.jsp).
Transient expression of HJURP
Myc-DDK-tagged HJURP-expression plasmids (catalog
no. RC201283) and its control pCMV6-Entry plasmids (catalog no.
PS100001) were purchased from OriGene Technologies, Inc. HSC2 and
SCC9 cells were transfected with these vectors using FuGENE
transfection reagent (Promega Corp.) according to the
manufacturer's instructions. Cell viability was measured by MTT
assay using Cell Counting Kit-8 solution (Dojindo Laboratories) and
colony formation assay with Giemsa staining (Wako Pure Chemical
Industries).
Results
Expression of HJURP in OC cell lines
and tissues
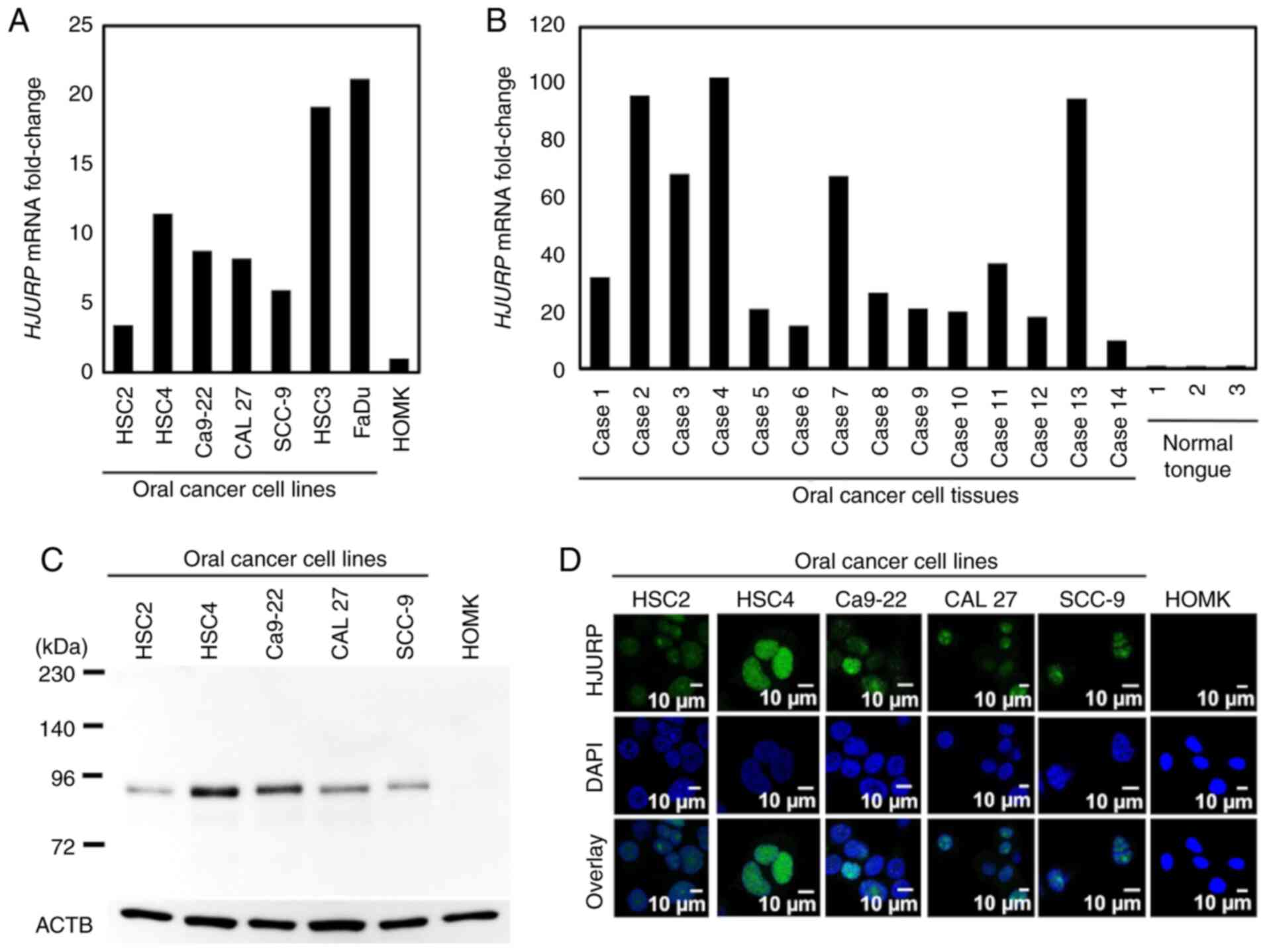
qPCR revealed HJURP mRNA expression in all OC
cell lines and tissues, but very low expression in the HOMKs and
normal tongue tissues (Fig. 1A and
B). Western blot analysis showed high HJURP protein expression
in all OC cell lines as compared with that observed in the HOMKs
(Fig. 1C). Immunocytochemical
staining showed that HJURP protein was mainly localized in the
nucleus of cancer cells, but expression was limited in the HOMKs
(Fig. 1D).
HJURP is associated with the poor
prognosis of OC patients
To develop new anticancer drugs with minimum risk of
adverse effects and highly cancer specific biomarkers, we validated
HJURP as a potential therapeutic target that is frequently
overexpressed in OC cells, but scarcely expressed in normal vital
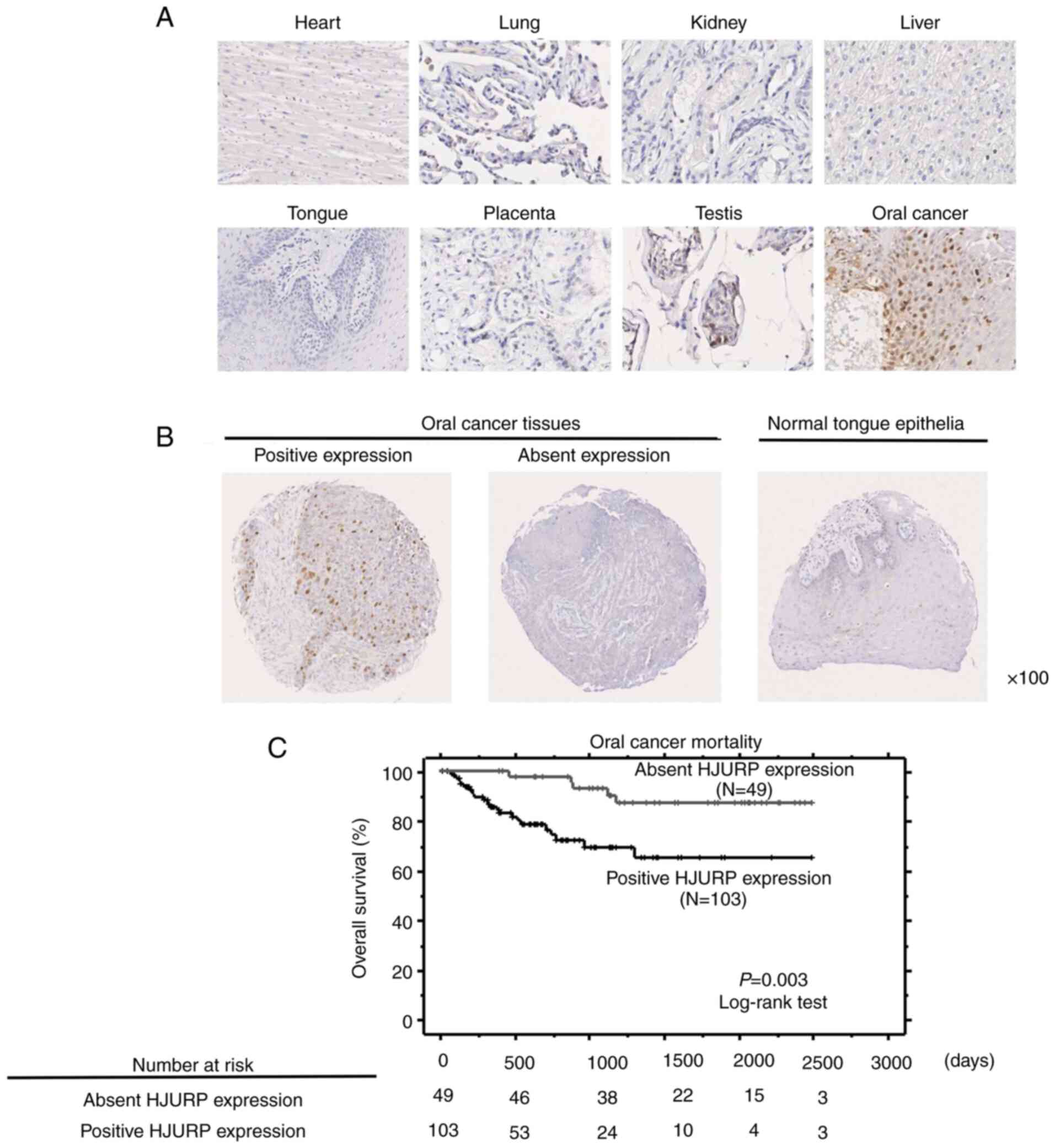
organs. HJURP expression was examined by immunohistochemical
analysis in normal tissues (heart, lung, kidney, liver, and tongue
as representative vital organs; placenta, and testis as references)
and OC tissues. The HJURP protein was mainly observed in the nuclei
of OC cells, with weak expression in testis cells and limited
detection in the remaining normal tissues (Fig. 2A). Furthermore, tissue microarrays
of the tissue samples from OC patients who underwent curative
surgery confirmed HJURP protein expression in 103 (67.8%) of 152 OC
specimens, but not in the normal tongue epithelial tissues
(Fig. 2B). Evaluation of the
association of HJURP expression with clinicopathological parameters
showed that HJURP expression was significantly correlated to age
(higher in patients ≥65 years, P=0.0216 by Fisher's exact test;
Table II). The results of
Kaplan-Meier analysis revealed that HJURP protein expression was
significantly correlated with a poorer prognosis of OC patients
(P=0.003, by log-rank test; Fig.
2C). Univariate analysis was conducted to investigate the
correlation between possible prognostic factors, including HJURP
expression status (absent vs. positive), age (<65 vs. ≥65
years), sex (female vs. male), tumor region (tongue vs. other
regions), pT classification (T1-2 vs. T3-4), pN classification (N0
vs. N1-2), and treatment (curative surgery with adjuvant
chemotherapy vs. curative surgery with neoadjuvant chemotherapy).
The results showed that positive HJURP expression and advanced pN
stage (N1-2) were significantly associated with a poorer prognosis
of the OC patients (P=0.0057 and 0.0014, respectively, Table III). Furthermore, multivariate
analysis revealed that positive HJURP expression and advanced pN
stage were independent prognostic factors (P=0.0093 and 0.0031,
respectively, Table III).
 | Table II.Association of HJURP protein
expression in OC tissues with patient characteristics. |
Table II.
Association of HJURP protein
expression in OC tissues with patient characteristics.
|
Characteristics | Total no. of
patients (N=152) | Positive HJURP
expression (N=103) | Absent HJURP
expression (N=49) | P-value (positive
vs. absent) |
|---|
| Sex |
|
|
|
|
|
Male | 86 | 61 | 25 | 0.3835 |
|
Female | 66 | 42 | 24 |
|
| Age (years) |
|
|
|
|
|
<65 | 60 | 34 | 26 | 0.0216a |
|
≥65 | 92 | 69 | 23 |
|
| Region |
|
|
|
|
|
Tongue | 76 | 49 | 27 | 0.4878 |
|
Othersb | 76 | 54 | 22 |
|
| pT
classification |
|
|
|
|
|
T1-2 | 93 | 61 | 32 | 0.5935 |
|
T3-4 | 59 | 42 | 17 |
|
| pN
classification |
|
|
|
|
| N0 | 109 | 69 | 40 | 0.0824 |
|
N1-2 | 43 | 34 | 9 |
|
 | Table III.Cox's proportional hazards model
analysis of prognostic factors in patients with OC. |
Table III.
Cox's proportional hazards model
analysis of prognostic factors in patients with OC.
| Variables | HR | 95% CI |
Unfavorable/Favorable | P-value |
|---|
| Univariate
analysis |
|
|
|
|
| HJURP
expression | 3.983 | 1.495-10.609 |
Positive/Absent | 0.0057a |
| Age
(years) | 2.051 | 0.901-4.668 | ≥65/<65 | 0.0869 |
|
Sex | 2.090 | 0.988-4.422 | Female/Male | 0.0539 |
|
Region | 1.658 | 0.782-3.517 | Othersb/Tongue | 0.1872 |
|
T-factor | 1.610 | 0.766-3.388 | T3-4/T1-2 | 0.2091 |
|
N-factor | 3.345 | 1.592-7.028 | N1-2/N0 | 0.0014a |
|
Treatment | 1.368 | 0.647-2.894 |
Neoadjuvant/Adjuvant chemotherapy | 0.412 |
| Multivariate
analysis |
|
|
|
|
| HJURP
expression | 3.704 | 1.381-9.933 |
Positive/Absent | 0.0093a |
|
N-factor | 3.083 | 1.463-6.498 | N1-2/N0 | 0.0031a |
To validate the potential of HJURP as a prognostic
biomarker, the prognostic value of HJURP gene expression was
investigated using the ProgGene database. The results showed that
HJURP expression was significantly associated with poor
prognosis of patients with head and neck cancers, including OC
(dataset no. E-MTAB-1328; P=0.0286). Furthermore, GEPIA for
analysis of the RNA sequencing data from The Cancer Genome Atlas
and the Genotype-Tissue Expression project revealed that
HJURP overexpression was significantly correlated with a
shorter survival period of patients with head and neck cancers
(dataset TCGA-HNSC; P=0.032). These data independently support the
immunohistochemical data.
HJURP knockdown inhibits the growth of
OC cells
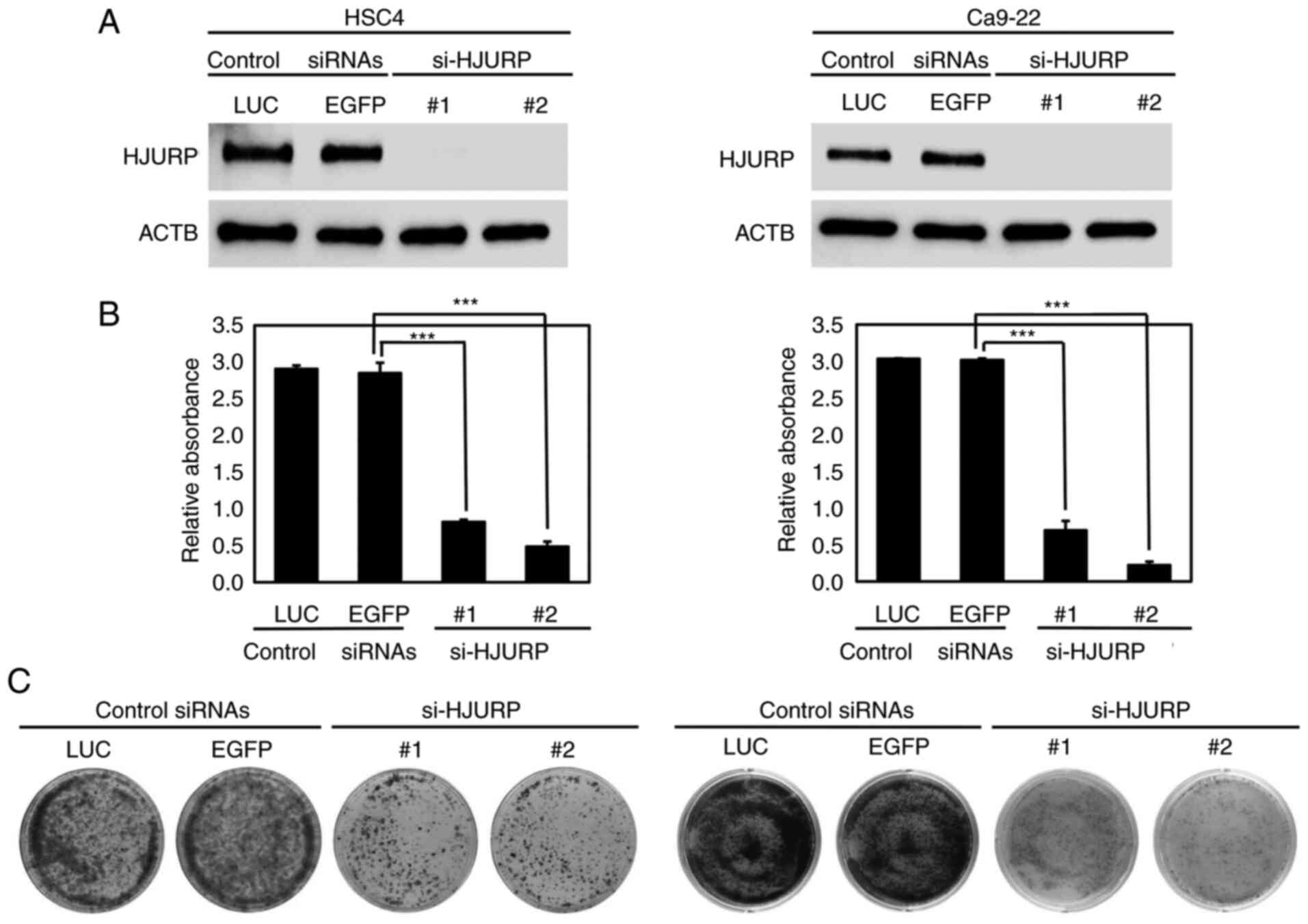
To determine whether HJURP is involved in the growth
of OC cells, HSC4 and Ca9-22 cells were transfected with siRNAs
against HJURP (si-HJURP-#1 and si-HJURP-#2), along with two
different control siRNAs (si-LUC and si-EGFP) with no off-target
effect on the cells as reported by our previous studies (11–37).
HJURP expression was reduced in the OC cells by si-HJURP
transfection as compared with the control siRNAs (Fig. 3A). Suppression of HJURP expression
significantly inhibited the viability of HSC4 and Ca9-22 cells
(P<0.001; Fig. 3B).
Additionally, the results of the colony formation assays revealed
that silencing of HJURP expression decreased the colony number of
HSC4 and Ca9-22 cells, as compared to the cell transfected with the
control siRNAs (Fig. 3C).
HJURP knockdown inhibits progression
of the OC cell cycle
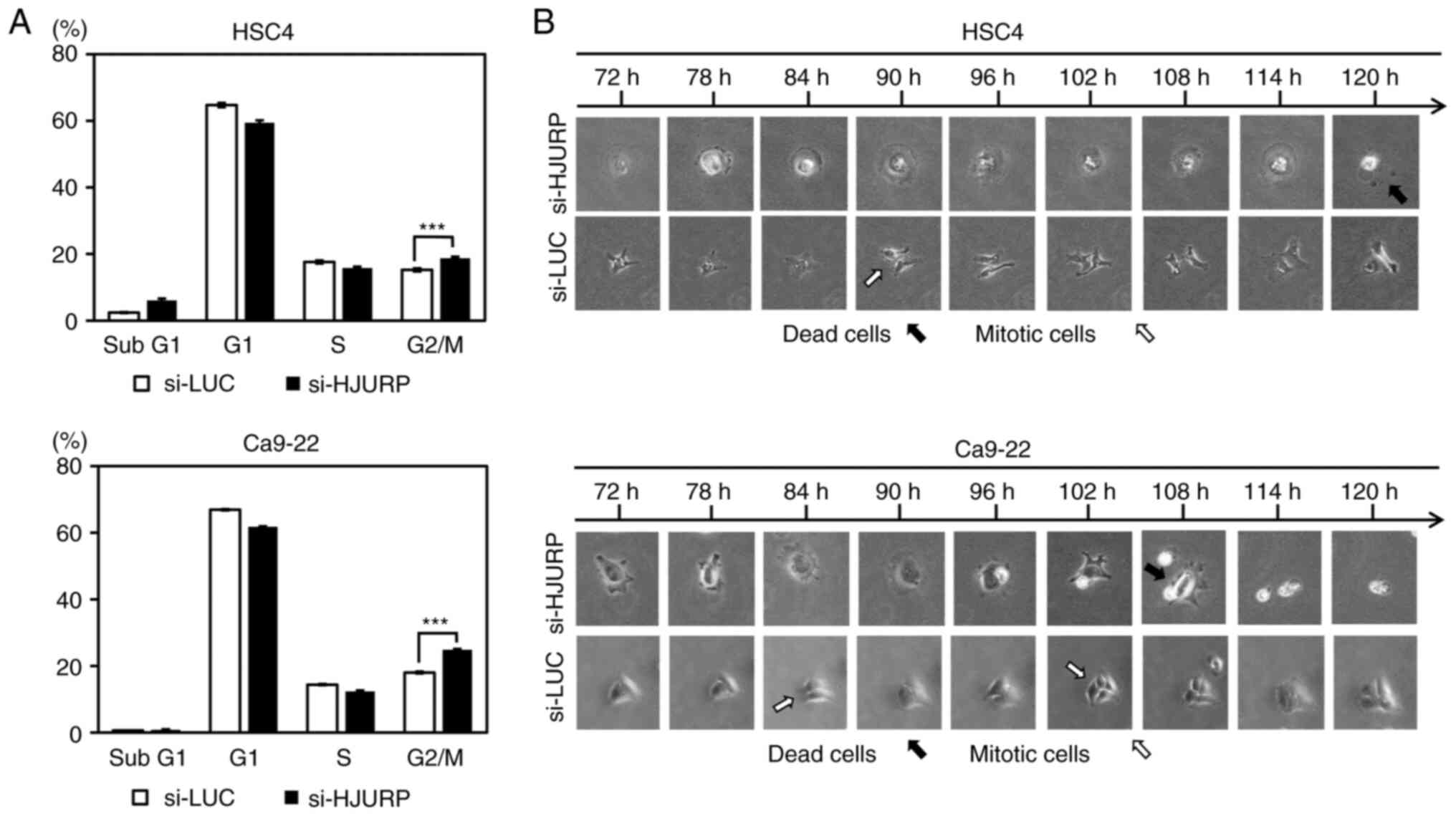
Flow cytometry was performed to clarify the
functional role of HJURP in the growth of OC cells (HSC4 and
Ca9-22) with suppressed HJURP expression by transfection of siRNAs.
The proportion of cells in the G2/M phase was significantly
increased by transfection with si-HJURP as compared to control
siRNA (si-LUC) (Figs. 4A and
S1). In addition, cell dynamics
were monitored using live imaging of HSC4 and Ca9-22 cells
transfected with si-HJURP or si-LUC (Fig. 4B). Time-lapse imaging detected
regular division of cells transfected with si-LUC, while very few
cells transfected with si-HJURP had divided, but rather cell death
was observed.
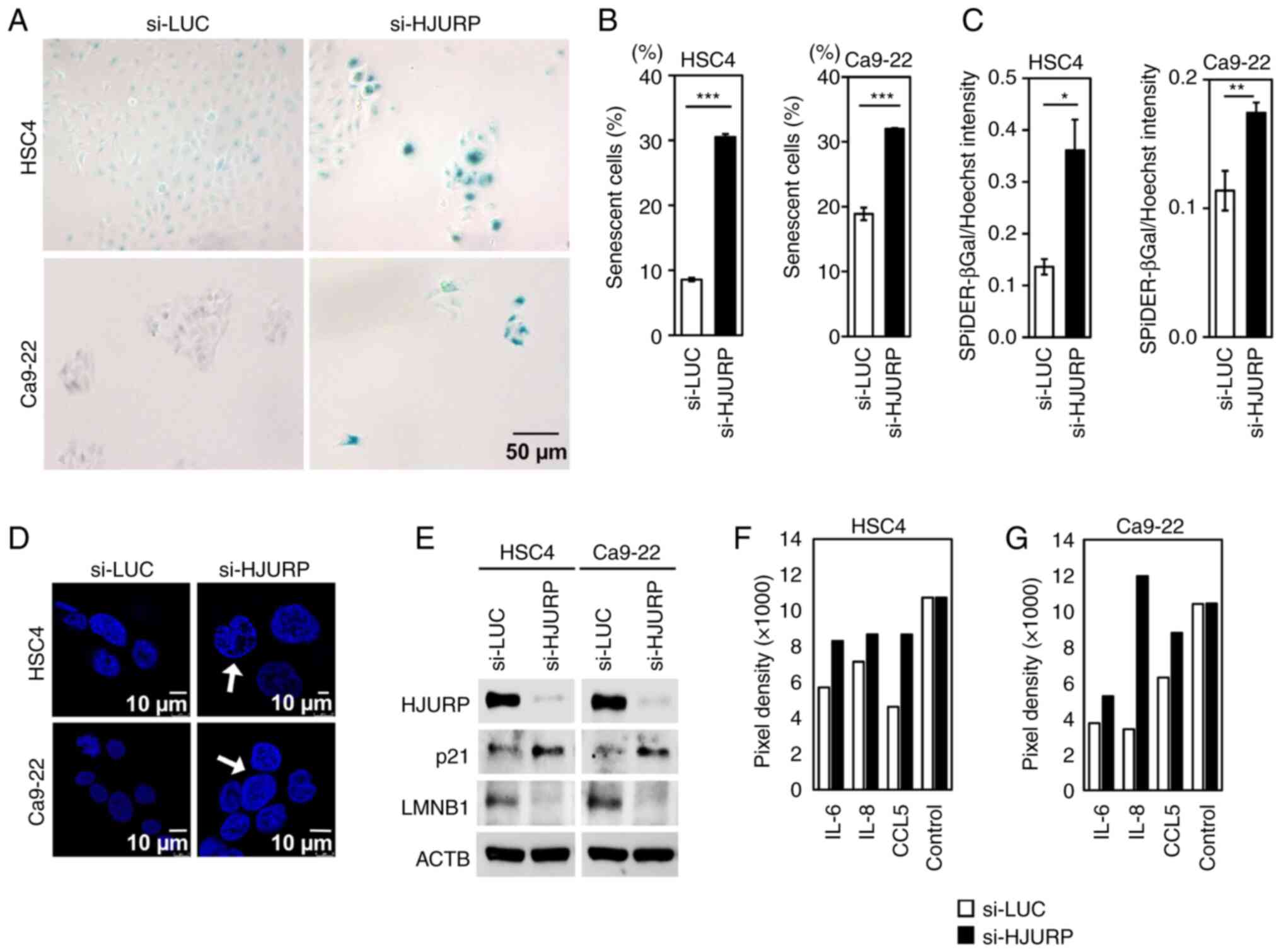
HJURP knockdown induces senescence of
OC cells
Since HJURP is reported to play a role in the
regulation of senescence of lung cancer cells (24), senescence-associated β-galactosidase
(SA-β-Gal) staining of OC cells (HSC4 and Ca9-22) transfected with
siRNAs against HJURP was performed. The results showed that HJURP
knockdown increased the number of β-galactosidase-positive cells
with enhanced SA-β-gal activity (Fig.
5A-C) and nuclear enlargement, which is a marker of
senescence-associated heterochromatic foci (SAHF) (Fig. 5D). The expression of
senescence-associated proteins in OC cells transfected with
si-HJURP or si-LUC was further investigated by western blot
analysis and the use of a human cytokine Ab array. After HJURP
silencing, the expression levels of cyclin-dependent kinase
inhibitor 1A (p21/CDKN1A) and SASP markers (IL-6, IL-8 and CCL5 in
Fig. 5F and G) were increased,
whereas lamin B1 expression was reduced in the OC cells (Fig. 5E-G). These data suggest that HJURP
plays a pivotal functional role in the senescence of OC cells.
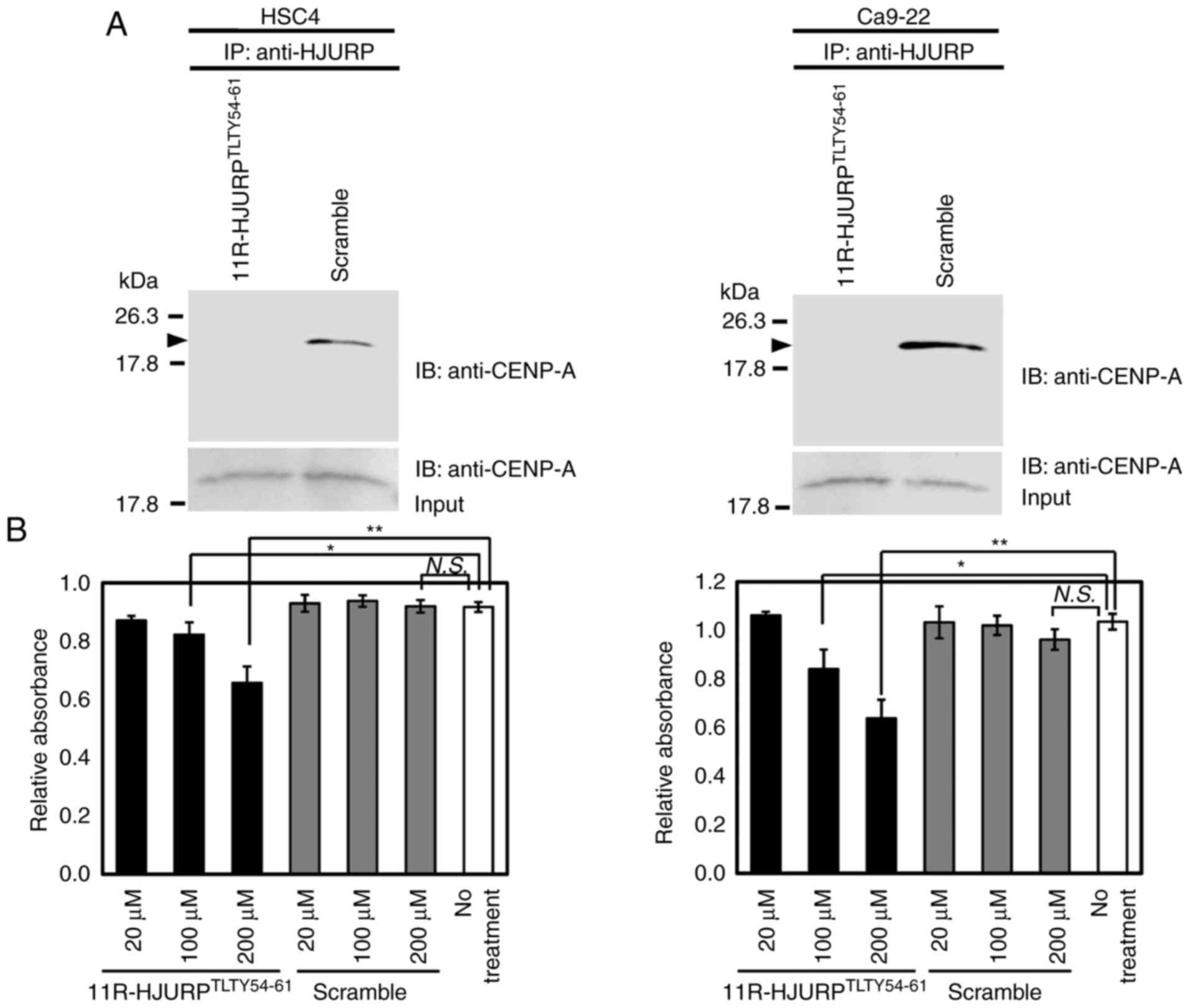
Growth inhibition of OC cells by
dominant-negative peptides of HJURP
HJURP is known to play roles in cell cycle
progression and chromosomal dynamics by binding to CENP-A (38,39,45).
In addition, the GSEA database revealed that HJURP was likely
involved in various pathways, including cell cycle progression and
chromosome maintenance, as well as deposition of new
CENP-A-containing nucleosomes at the centromeres, which are
compatible with the results of cell cycle analysis and live cell
imaging of OC cells transfected with siRNAs against HJURP. The
results of western blot analysis, immunocytochemistry, and the
ONCOMINE database (data not shown) also confirmed the co-expression
of HJURP with CENP-A in OC cells. Immunoprecipitation and
subsequent western blot analysis further confirmed the binding of
endogenous HJURP protein to endogenous CENP-A in OC cell lines
(data not shown). Based on these data, the functional association
of HJURP with CENP-A was investigated as a molecular therapeutic
target.
To investigate the functional significance of
inhibition of the interaction between HJURP and CENP-A on the
growth of OC cells, HSC4 and Ca9-22 cells were treated with the
cell permeable 11R-HJURPTLTY54-61 peptide, which
contains a direct binding site for CENP-A or a scramble peptide.
The results confirmed that 11R-HJURPTLTY54-61 inhibited
the interactions between HJURP and CENP-A probably through
dominant-negative effects (Fig. 6A)
and decreased the viability of OC cells in a dose-dependent manner
(Fig. 6B). These data suggest that
inhibition of the formation of functional complexes of HJURP and
CENP-A is a potential therapeutic strategy.
Promotion of cell growth by enforced
HJURP expression
To further confirm the cell growth promoting effect
of HJURP, we transfected plasmids expressing HJURP or mock plasmids
into HSC2 and SCC9 cells, which weakly expressed endogenous HJURP.
Transfection of HJURP expression vector increased the viability of
both cells compared with the mock plasmid as detected by MTT and
colony formation assays (Fig. S2).
The result demonstrated that overexpression of HJURP contributed to
the enhanced cell viability of the OC cells.
Discussion
Molecular-targeted therapies have brought about a
new era of treatment for various highly malignant cancers,
including oral cancer (OC). Currently, monoclonal antibodies (Abs)
against epidermal growth factor receptor (EGFR) (cetuximab) and
immune checkpoint inhibitors (nivolumab and pembrolizumab) are
available for treatment of OC. However, the efficacy is reportedly
limited and various adverse events have been observed (9,10,46).
Therefore, identifying new molecular targets for the development of
novel therapeutics and biomarkers for precision medicine are
urgently required to improve the prognosis and quality of life of
OC patients. Potential target molecules with higher and frequent
expression in cancer cells, but low expression in normal cells, are
needed for new treatments. In the present study, Holliday junction
recognition protein (HJURP) was investigated as a potential target
of the growth and survival of OC cells.
HJURP was found to be highly expressed in the
majority of OC cell lines and clinical tissues, but expression was
very low in normal tongue tissues and oral epithelial cells. Gene
expression data demonstrated that HJURP expression was
relatively low in normal organs (BioGPS database; http://biogps.org/#goto=welcome), suggesting that
HJURP is a potential diagnostic and therapeutic target. Comparative
genomic hybridization and genome sequencing data (https://cancer.sanger.ac.uk/cosmic) were
referenced to assess the mechanism of HJURP gene aberrations
in OC. The results showed that only 6 (1.4%) of 424 OC cases
carried HJURP missense mutations. According to the
cBioportal database for Cancer Genomics (http://www.cbioportal.org/), missense mutations,
deletions, and genetic amplification of HJURP were not detected in
all 40 OC cases, suggesting that HJURP overexpression might be
caused by epigenetic mechanisms in oral carcinogenesis.
The tissue microarray analysis revealed that HJURP
is a potential prognostic biomarker for OC, as independently
validated by the ProgGene and GEPIA databases. Upregulation of
HJURP in OC tissues could provide a clinical prognostic indicator
that warrants intensive follow-up of patients and/or additional
treatments after surgery. The present study showed that HJURP
knockdown by siRNAs or inhibition of direct binding of HJURP to
CENP-A by cell permeable peptides significantly inhibited the
growth of OC cells probably through dysregulation of the cell cycle
and/or cellular senescence. TMA analysis showed that HJURP
expression is not related to tumor size. The data may reflect that
HJURP knockdown is mainly targeting cell cycle regulation and/or
cell survival of OC cells. Time-lapse microscopy also revealed that
OC cells could not divide, and subsequently and promptly started to
die. G2/M arrest and subsequent and prompt cell death after HJURP
knockdown may explain the reason why the difference in the G2/M
phase seems to be small, when comparing with the significant
decrease in cell viability and colony numbers of OC cells. Our
previous report suggested that inhibition of HJURP in lung cancer
cells by siRNAs leads to the excess of chromosomal instability,
G2/M arrest, as well as cellular senescence (24). Proteins such as CENP-A, MIS18A,
MIS18B, and MIS18BP1 complex, and cyclin-dependent kinases interact
with HJURP and regulate cancer cell cycle progression (38,39,45).
Among these interacting proteins, we observed co-expression of
HJURP with CENP-A protein in OC tissues, and the level of CENP-A
protein was significantly reduced in HJURP-depleted OC cells,
suggesting that reduction of CENP-A and/or dysregulation of unknown
oncogenic pathways after HJURP knockdown might contribute to OC
cell death. Therefore, targeting the interaction between HJURP and
CENP-A as well as HJURP expression is likely to be one of the
effective strategies for the development of new therapeutics for
OC.
Transfection of HJURP expression vector is likely to
increase the viability of OC cells that weakly express HJURP
compared with mock plasmid; however, this needs to be confirmed by
using HJURP-negative cells and conditional expression assays in a
future study. Since the precise molecular mechanism underlying
HJURP activation and its oncogenic role have not yet been fully
elucidated, further detailed analyses of unknown oncogenic
functions of HJURP as well as the mechanism about how HJURP
influences the progression and senescence of OC cells through its
downstream signals are warranted.
Taken together, these results revealed that HJURP is
an oncoprotein that regulates OC cell growth probably by
dysregulating cell cycle progression, cellular senescence, and
various unknown oncogenic pathways. Since HJURP has multiple
oncogenic functions and may have various binding partners as a
molecular chaperon, further mechanical study of HJURP in
tumorigenesis using in vivo models and screening of more
effective approaches targeting its pathway are eagerly awaited.
HJURP and its binding to CENP-A are potential molecular targets for
the development of new treatments and could be useful prognostic
biomarkers of OC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported in part by a Grant-in-Aid for Scientific
Research (B), Grant-in-Aid for Challenging Research (Exploratory),
and Grant-in-Aid for Scientific Research on Innovative Areas from
the Japan Society for the Promotion of Science (JSPS KAKENHI grant
nos. 15H04761, 19H03559, 21K19444, and 16H06277). Y.D. is a member
of Shiga Cancer Treatment Project supported by Shiga Prefecture
(Japan) and the International Joint Research Project (FY2016-2021)
of the Institute of Medical Sciences (The University of Tokyo).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
BT, AT, and YD conceived the research concept and
designed the study. BT, AT, and YD developed the study methodology.
YY, MS, and YD acquired the data, managed the patients, and
provided the facilities. BT, AT, MZ, and YD analyzed and
interpreted the data (e.g., statistical analysis, biostatistics,
and computational analysis) and also confirm the accuracy of the
data. BT, AT, and YD wrote, reviewed, and/or revised the
manuscript. BT, AT, and YD provided administrative, technical,
and/or material support (i.e., reporting or organizing data,
constructing databases). YD supervised the study. All authors read
and approved the final version of the manuscript for
publication.
Ethics approval and consent to
participate
The present study and the use of clinical materials
were approved by the Ethics Committees [Kumamoto University; Shiga
University of Medical Science (no. G2009-163)]. It was confirmed
that this study was fully ethically compliant and the informed
consent was waived due to the retrospective nature of the study and
in accordance with the national ethical guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors have no competing interests to
declare.
Glossary
Abbreviations
Abbreviations:
|
DAPI
|
4′,6-diamidino-2-phenylindole
|
|
HJURP
|
Holliday junction recognition
protein
|
|
FBS
|
fetal bovine serum
|
|
HOMKs
|
human oral mucosal keratinocytes
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
OC
|
oral cancer
|
|
p21/CDKN1A
|
cyclin-dependent kinase inhibitor
1A
|
|
SA-β-Gal
|
senescence-associated
β-galactosidase
|
|
SAHF
|
senescence-associated heterochromatic
foci
|
References
|
1
|
Ferlay J, Colombet M, Soerjomataram I,
Mathers C, Parkin DM, Piñeros M, Znaor A and Bray F: Estimating the
global cancer incidence and mortality in 2018: GLOBOCAN sources and
methods. Int J Cancer. 144:1941–1953. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gupta B and Johnson NW: Systematic review
and meta-analysis of association of smokeless tobacco and of betel
quid without tobacco with incidence of oral cancer in South Asia
and the Pacific. PLoS One. 9:e1133852014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Franceschi S, Talamini R, Barra S, Barón
AE, Negri E, Bidoli E, Serraino D and La Vecchia C: Smoking and
drinking in relation to cancers of the oral cavity, pharynx,
larynx, and esophagus in northern Italy. Cancer Res. 50:6502–6507.
1990.PubMed/NCBI
|
|
5
|
Cooper JS, Porter K, Mallin K, Hoffman HT,
Weber RS, Ang KK, Gay EG and Langer CJ: National cancer database
report on cancer of the head and neck: 10-year update. Head Neck.
31:748–758. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dumache R, Rogobete AF, Andreescu N and
Puiu M: Genetic and epigenetic biomarkers of molecular alterations
in oral carcinogenesis. Clin Lab. 61:1373–1381. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Genden EM, Ferlito A, Silver CE, Takes RP,
Suárez C, Owen RP, Haigentz M, Stoeckli SJ, Shaha AR, Rapidis AD,
et al: Contemporary management of cancer of the oral cavity. Eur
Arch Otorhinolaryngol. 267:1001–1017. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chinn SB and Myers JN: Oral cavity
carcinoma: Current management, controversies, and future
directions. J Clin Oncol. 33:3269–3276. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bonner JA, Harari PM, Giralt J, Azarnia N,
Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, et al:
Radiotherapy plus cetuximab for squamous-cell carcinoma of the head
and neck. N Engl J Med. 354:567–578. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ferris RL, Licitra L, Fayette J, Even C,
Blumenschein G Jr, Harrington KJ, Guigay J, Vokes EE, Saba NF,
Haddad R, et al: Nivolumab in patients with recurrent or metastatic
squamous cell carcinoma of the head and neck: Efficacy and safety
in CheckMate 141 by prior cetuximab use. Clin Cancer Res.
25:5221–5230. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Daigo Y and Nakamura Y: From cancer
genomics to thoracic oncology: Discovery of new biomarkers and
therapeutic targets for lung and esophageal carcinoma. Gen Thorac
Cardiovasc Surg. 56:43–53. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Daigo Y, Takano A, Teramoto K, Chung S and
Nakamura Y: A systematic approach to the development of novel
therapeutics for lung cancer using genomic analyses. Clin Pharmacol
Ther. 94:218–223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ishikawa N, Daigo Y, Takano A, Taniwaki M,
Kato T, Hayama S, Murakami H, Takeshima Y, Inai K, Nishimura H, et
al: Increases of amphiregulin and transforming growth factor-alpha
in serum as predictors of poor response to gefitinib among patients
with advanced non-small cell lung cancers. Cancer Res.
65:9176–9184. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ishikawa N, Daigo Y, Yasui W, Inai K,
Nishimura H, Tsuchiya E, Kohno N and Nakamura Y: ADAM8 as a novel
serological and histochemical marker for lung cancer. Clin Cancer
Res. 10:8363–8370. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kakiuchi S, Daigo Y, Ishikawa N, Furukawa
C, Tsunoda T, Yano S, Nakagawa K, Tsuruo T, Kohno N, Fukuoka M, et
al: Prediction of sensitivity of advanced non-small cell lung
cancers to gefitinib (Iressa, ZD1839). Hum Mol Genet. 13:3029–3043.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kato T, Daigo Y, Hayama S, Ishikawa N,
Yamabuki T, Ito T, Miyamoto M, Kondo S and Nakamura Y: A novel
human tRNA-dihydrouridine synthase involved in pulmonary
carcinogenesis. Cancer Res. 65:5638–5646. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kikuchi T, Daigo Y, Katagiri T, Tsunoda T,
Okada K, Kakiuchi S, Zembutsu H, Furukawa Y, Kawamura M, Kobayashi
K, et al: Expression profiles of non-small cell lung cancers on
cDNA microarrays: Identification of genes for prediction of
lymph-node metastasis and sensitivity to anti-cancer drugs.
Oncogene. 22:2192–2205. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Suzuki C, Daigo Y, Ishikawa N, Kato T,
Hayama S, Ito T, Tsuchiya E and Nakamura Y: ANLN plays a critical
role in human lung carcinogenesis through the activation of RHOA
and by involvement in the phosphoinositide 3-kinase/AKT pathway.
Cancer Res. 65:11314–11325. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kakiuchi S, Daigo Y, Tsunoda T, Yano S,
Sone S and Nakamura Y: Genome-wide analysis of organ-preferential
metastasis of human small cell lung cancer in mice. Mol Cancer Res.
1:485–499. 2003.PubMed/NCBI
|
|
20
|
Taniwaki M, Daigo Y, Ishikawa N, Takano A,
Tsunoda T, Yasui W, Inai K, Kohno N and Nakamura Y: Gene expression
profiles of small-cell lung cancers: Molecular signatures of lung
cancer. Int J Oncol. 29:567–575. 2006.PubMed/NCBI
|
|
21
|
Oshita H, Nishino R, Takano A, Fujitomo T,
Aragaki M, Kato T, Akiyama H, Tsuchiya E, Kohno N, Nakamura Y and
Daigo Y: RASEF is a novel diagnostic biomarker and a therapeutic
target for lung cancer. Mol Cancer Res. 11:937–951. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hayama S, Daigo Y, Yamabuki T, Hirata D,
Kato T, Miyamoto M, Ito T, Tsuchiya E, Kondo S and Nakamura Y:
Phosphorylation and activation of cell division cycle associated 8
by aurora kinase B plays a significant role in human lung
carcinogenesis. Cancer Res. 67:4113–4122. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ishikawa N, Daigo Y, Takano A, Taniwaki M,
Kato T, Tanaka S, Yasui W, Takeshima Y, Inai K, Nishimura H, et al:
Characterization of SEZ6L2 cell-surface protein as a novel
prognostic marker for lung cancer. Cancer Sci. 97:737–745. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kato T, Sato N, Hayama S, Yamabuki T, Ito
T, Miyamoto M, Kondo S, Nakamura Y and Daigo Y: Activation of
Holliday junction recognizing protein involved in the chromosomal
stability and immortality of cancer cells. Cancer Res.
67:8544–8553. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Suzuki C, Takahashi K, Hayama S, Ishikawa
N, Kato T, Ito T, Tsuchiya E, Nakamura Y and Daigo Y:
Identification of Myc-associated protein with JmjC domain as a
novel therapeutic target oncogene for lung cancer. Mol Cancer Ther.
6:542–551. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takahashi K, Furukawa C, Takano A,
Ishikawa N, Kato T, Hayama S, Suzuki C, Yasui W, Inai K, Sone S, et
al: The neuromedin U-growth hormone secretagogue receptor
1b/neurotensin receptor 1 oncogenic signaling pathway as a
therapeutic target for lung cancer. Cancer Res. 66:9408–9419. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Taniwaki M, Takano A, Ishikawa N, Yasui W,
Inai K, Nishimura H, Tsuchiya E, Kohno N, Nakamura Y and Daigo Y:
Activation of KIF4A as a prognostic biomarker and therapeutic
target for lung cancer. Clin Cancer Res. 13:6624–6631. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yamabuki T, Takano A, Hayama S, Ishikawa
N, Kato T, Miyamoto M, Ito T, Ito H, Miyagi Y, Nakayama H, et al:
Dikkopf-1 as a novel serologic and prognostic biomarker for lung
and esophageal carcinomas. Cancer Res. 67:2517–2525. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fujitomo T, Daigo Y, Matsuda K, Ueda K and
Nakamura Y: Identification of a nuclear protein, LRRC42, involved
in lung carcinogenesis. Int J Oncol. 45:147–156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Koinuma J, Akiyama H, Fujita M, Hosokawa
M, Tsuchiya E, Kondo S, Nakamura Y and Daigo Y: Characterization of
an Opa interacting protein 5 involved in lung and esophageal
carcinogenesis. Cancer Sci. 103:577–586. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nguyen MH, Koinuma J, Ueda K, Ito T,
Tsuchiya E, Nakamura Y and Daigo Y: Phosphorylation and activation
of cell division cycle associated 5 by mitogen-activated protein
kinase play a crucial role in human lung carcinogenesis. Cancer
Res. 70:5337–5347. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hayama S, Daigo Y, Kato T, Ishikawa N,
Yamabuki T, Miyamoto M, Ito T, Tsuchiya E, Kondo S and Nakamura Y:
Activation of CDCA1-KNTC2, members of centromere protein complex,
involved in pulmonary carcinogenesis. Cancer Res. 66:10339–10348.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Takano A, Ishikawa N, Nishino R, Masuda K,
Yasui W, Inai K, Nishimura H, Ito H, Nakayama H, Miyagi Y, et al:
Identification of nectin-4 oncoprotein as a diagnostic and
therapeutic target for lung cancer. Cancer Res. 69:6694–6703. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kobayashi Y, Takano A, Miyagi Y, Tsuchiya
E, Sonoda H, Shimizu T, Okabe H, Tani T, Fujiyama Y and Daigo Y:
Cell division cycle-associated protein 1 overexpression is
essential for the malignant potential of colorectal cancers. Int J
Oncol. 44:69–77. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Thang PM, Takano A, Yoshitake Y, Shinohara
M, Murakami Y and Daigo Y: Cell division cycle associated 1 as a
novel prognostic biomarker and therapeutic target for oral cancer.
Int J Oncol. 49:1385–1393. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Daigo K, Takano A, Thang PM, Yoshitake Y,
Shinohara M, Tohnai I, Murakami Y, Maegawa J and Daigo Y:
Characterization of KIF11 as a novel prognostic biomarker and
therapeutic target for oral cancer. Int J Oncol. 52:155–165.
2018.PubMed/NCBI
|
|
37
|
Nakamura M, Takano A, Thang PM, Tsevegjav
B, Zhu M, Yokose T, Yamashita T, Miyagi Y and Daigo Y:
Characterization of KIF20A as a prognostic biomarker and
therapeutic target for different subtypes of breast cancer. Int J
Oncol. 57:277–288. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Foltz DR, Jansen LE, Bailey AO, Yates JR
III, Bassett EA, Wood S, Black BE and Cleveland DW:
Centromere-specific assembly of CENP-a nucleosomes is mediated by
HJURP. Cell. 137:472–484. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dunleavy EM, Roche D, Tagami H, Lacoste N,
Ray-Gallet D, Nakamura Y, Daigo Y, Nakatani Y and
Almouzni-Pettinotti G: HJURP is a cell-cycle-dependent maintenance
and deposition factor of CENP-A at centromeres. Cell. 137:485–497.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hu B, Wang Q, Wang Y, Chen J, Li P and Han
M: Holliday junction-recognizing protein promotes cell
proliferation and correlates with unfavorable clinical outcome of
hepatocellular carcinoma. Onco Targets Ther. 10:2601–2607. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Valente V, Serafim RB, de Oliveira LC,
Adorni FS, Torrieri R, Tirapelli DP, Espreafico EM, Oba-Shinjo SM,
Marie SK, Paçó-Larson ML, et al: Modulation of HJURP (Holliday
Junction-Recognizing Protein) levels is correlated with
glioblastoma cells survival. PLoS One. 8:e622002013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li L, Li X, Meng Q, Khan AQ and Chen X:
Increased expression of Holliday junction-recognizing protein
(HJURP) as an independent prognostic biomarker in advanced-stage
serous ovarian carcinoma. Med Sci Monit. 24:3050–3055. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hu Z, Huang G, Sadanandam A, Gu S, Lenburg
ME, Pai M, Bayani N, Blakely EA, Gray JW and Mao JH: The expression
level of HJURP has an independent prognostic impact and predicts
the sensitivity to radiotherapy in breast cancer. Breast Cancer
Res. 12:R182010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shuaib M, Ouararhni K, Dimitrov S and
Hamiche A: HJURP binds CENP-A via a highly conserved N-terminal
domain and mediates its deposition at centromeres. Proc Natl Acad
Sci USA. 107:1349–1354. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cohen EEW, Soulières D, Le Tourneau C,
Dinis J, Licitra L, Ahn MJ, Soria A, Machiels JP, Mach N, Mehra R,
et al: Pembrolizumab versus methotrexate, docetaxel, or cetuximab
for recurrent or metastatic head-and-neck squamous cell carcinoma
(KEYNOTE-040): A randomised, open-label, phase 3 study. Lancet.
393:156–167. 2019. View Article : Google Scholar : PubMed/NCBI
|