Introduction
Oral squamous cell carcinoma (OSCC) is the most
common type of cancer of the head and neck region, occurring in the
oral mucosa with a low 5-year survival rate (1,2).
The progression of OSCC is dependent on the interplay between
cancer cells, the surrounding host-derived stromal cells (SCs),
such as cancer-associated fibroblasts (CAFs), endothelial cells,
extracellular matrix non-cellular and tumor-associated macrophages
(TAMs) composing the tumor microenvironment (TME). Inflammation
promotes nearly all stages of OSCC carcinogenesis and progression
(3). Recent studies have
indicated that macrophages and relevant cytokines are involved in
inflammation (4,5). TAM-associated cancer inflammation
regulates tumor growth and progression via various mechanisms,
depending on the type and frequency of released tumor-derived
factors (6,7). Of note, the infiltration of TAMs and
their functional activation are key prognostic biomarkers of OSCC
lymphangiogenesis, metastatic dissemination and overall survival
(8,9).
TAMs are derived from circulating monocytes in the
peripheral blood and play a crucial role in cancer progression
(10). Macrophages are divided
into the M1 (classically activated) and M2 (alternatively
activated) phenotypes following polarization (11). M1 macrophages exert antitumor
effects by promoting inflammation and immune reactions, whereas M2
macrophages can promote tumor progression through various pathways,
such as immunosuppression (12).
In OSCC, TAMs primarily exhibit characteristics and functions
related to M2 pro-tumor macrophages, and in OSCC, monocyte
differentiation into M2 type macrophages can be promoted, which can
regulate the progression of OSCC by crosstalk with cancer cells
(13,14). Although it has been well
established that TAMs play an essential role in tumor immune
suppression and progression, the potential regulatory mechanisms of
these TAMs with tumor-promoting functions remain unclear (15).
Although it has been reported that OSCC cells
recruit TAMs by inducing monocyte differentiation (13), the effects of the cancer stroma on
TAM infiltration have not yet been fully elucidated. According to
the invasive ability, OSCC is divided into either endophytic (ED)
or exophytic (EX) type OSCC. ED-type OSCC can invade and
occasionally metastasize. Conversely, EX-type OSCC, such as
verrucous OSCC, presents an outward growth, does not invade the
subepithelial connective tissue and does not metastasize (16-18). The authors have previously
reported that EX-type OSCC-associated SCs [verrucous SCC-associated
SCs (VSCC-SCs)] and ED-type OSCC associated SCs (SCC-SCs) exert
differential effects on the differentiation, proliferation,
invasion and migration of HSC-2 and HSC-3 cells (19,20). In these previous studies, the
morphology and viability of VSCC-SCs and SCC-SCs were assayed using
Giemsa, immunofluorescence (IF) staining and MTS assays,
respectively, which indicated that the VSCC-SCs and SCC-SCs were
heterogeneous cell populations and the viability of the VSCC-SCs
was higher than that of the SCC-SCs. Different detailed marker
profiles between VSCC-SCs and SCC-SCs were also analyzed using
microarray data (19,20). However, the effects of VSCC-SCs
and SCC-SCs on the infiltration of TAMs into the TME of OSCC remain
poorly understood.
The present study thus aimed to investigate the
effects of the cancer stroma derived from different subtypes of
OSCC on the infiltration of TAMs into the TME of OSCC. To achieve
this, the human oral cancer cell line, HSC-3, was selected as the
cell model due to it being a moderately or poorly differentiated
oral cancer cell line, this cell line also has a prominent invasive
ability and can form the stroma more easily and as such, is widely
used in studies on TAMs (21,22). In addition, murine RAW264.7 cells
were selected as a macrophage cell model; human dermal fibroblasts
(HDFs) were selected as a negative control of cancer SCs, mainly
since these are a type of normal fibroblasts, which are not
affected by cancer cells (23).
VSCC-SCs and SCC-SCs were extracted from patients with OSCC to
examine their effects on the infiltration/polarization of TAMs into
the TME of OSCC and to elucidate the relevant regulatory
mechanisms. The findings presented herein highlight a potential
regulatory mechanism underlying the effects of cancer-associated
stroma on the infiltration of TAMs into the TME of OSCC.
Materials and methods
Cells and cell culture
HSC-3 (JCRB0623), HDFs (CC-2511) and RAW264.7 cells
(RCB0535) were purchased from the Japanese Collection of Research
Bioresources Cell Bank (JCRB), Lonza and the RIKEN BioResource
Center Cell Bank, respectively. VSCC-SCs and SCC-SCs were extracted
from surgical tissues at the Department of Oral and Maxillofacial
Surgery at Okayama University (Okayama, Japan). VSCC tissues were
obtained from 1 patient with VSCC and SCC tissues were obtained
from 1 patient with SCC (mean age, 81 years; sex of both patients,
female) to separate the SCs and generate cell culture. Sections of
fresh OSCC tissue (1 mm3) were washed several times with
α-modified Eagle's medium (α-MEM; Thermo Fisher Scientific, Inc.)
containing antibiotic-antimycotic (Thermo Fisher Scientific, Inc.)
and then minced. The tissues were then treated with α-MEM
containing 1 mg/ml collagenase II (Invitrogen; Thermo Fisher
Scientific, Inc.) and dispase (Invitrogen; Thermo Fisher
Scientific, Inc.) for 2 h at 37°C with agitation (22.36 × g). The
released cells were centrifuged for 5 min at 111.8 × g at room
temperature, suspended in α-MEM containing 10% FBS (Biowest),
filtered through a cell strainer (100 µm; Falcon; Corning
Life Sciences), plated in a tissue culture flask and incubated at
37°C in a humidified atmosphere of 5% CO2 and 95% air.
After 1 week, the stromal cells were separated using Accutase
(Invitrogen; Thermo Fisher Scientific, Inc.) based on the different
adhesive properties of epithelial and stromal cells (19,20). The HSC-3 and RAW264.7 cells, as
well as the VSCC-SCs, SCC-SCs and HDFs were maintained in α-MEM
(Thermo Fisher Scientific, Inc.) supplemented with 10% FBS and 1%
antimycotic-antibiotic (Thermo Fisher Scientific, Inc.) at 37°C in
a humidified atmosphere of 5% CO2 and 95% air. The
present study was approved by the Ethics Committee of Okayama
University (project identification code: 1703-042-001). Written
informed consent was obtained from all the patients.
Indirect co-culture
The VSCC-SCs, SCC-SCs, HDFs, and RAW264.7 and HSC-3
cells were collected using Accutase (Invitrogen; Thermo Fisher
Scientific, Inc.) and EDTA (Thermo Fisher Scientific, Inc.),
respectively, at 90% confluency. The VSCC-SCs, SCC-SCs and HDFs
(15×104 cells) were mixed with HSC-3 cells
(5×104 cells) at a 3:1 ratio in 500 µl α-MEM with
10% FBS and added to a 0.4-µm Transwell filter in a 24-well
plate (Greiner Bio-One). The RAW264.7 cells were seeded into the
bottom chamber in 500 µl α-MEM with 10% FBS at a density of
15×104 cells/500 µl.
Giemsa and IF staining
Following indirect co-culture for 48 h, the RAW264.7
cells in the bottom chamber were collected and seeded into 6-well
plates with coverslips (Matsunami Glass, 22×22 mm). Following
incubation for 48 h at 37°C in a humidified atmosphere of 5%
CO2 and 95% air, coverslips were used for Giemsa and IF
staining. Giemsa staining was conducted using a Giemsa staining kit
(Diff-Quick, Nanjing Jiancheng Bioengineering Institute). The
slides were first washed with distilled water and fixed with
Diff-Quik Fixative provided with the Giemsa staining kit for 2 min
at room temperature. Subsequently, the slides were stained with
Diff-Quik Solution I and Diff-Quik Solution II provided with the
Giemsa staining kit for 2 min at room temperature. The stained
cells were photographed using a bright-field microscope (BX51;
Olympus Corporation). A total of five images (×20 magnification)
were acquired for each sample to determine the percentage of
activated macrophages and the percentage of multinucleated giant
cells in the different groups. The independent experiment was
repeated in triplicate and the data were analyzed using ImageJ
software (V 1.53K; National Institutes of Health).
For IF, the slides were washed three times with TBS
(5 min each). The cells were then fixed with 4% paraformaldehyde
for 10 min and blocked with blocking solution (DS Pharma Biomedical
Co., Ltd.) for 20 min. The primary antibodies rat-anti F4/80 (1:10;
cat. no. CI:A3-1, Bio-Rad Laboratories, Inc.) and rabbit-anti CD163
(1: 200; cat. no. ab182422, Abcam) were added followed by
incubation for 1 h at room temperature. After washing with TBS
three times, the secondary antibodies anti-rat IgG Alexa Fluor 488
(1:200; cat. no. A48262, Thermo Fisher Scientific, Inc.) and
anti-rabbit IgG Alexa Fluor 568 (1:200; cat. no. A10042, Thermo
Fisher Scientific, Inc.) were added followed by incubation for 1 h
at room temperature in the dark. After washing with TBS and
distilled water three times, the samples were stained with 0.2 g/ml
DAPI (Dojindo Molecular Technologies, Inc.). The stained cells were
photographed using an All-in-One BZ-X700 fluorescence microscope
(×40 magnification, Keyence Corporation), and independent
experiments were repeated in triplicate.
MTS assay
Following indirect co-culture for 48 h, RAW264.7
cells in the bottom chamber were collected and seeded into 96-well
plates at a density of 2×103 cells/well. Subsequently,
20 µl MTS reagent (Cell Titer 96 Aqueous One Solution Cell
Proliferation assay, Promega Corporation) were added to each well
followed by incubation for 4 h at 37°C in a humidified atmosphere
of 5% CO2 and 95% air for 1, 2 and 3 days. The
absorbance of each well was measured at 490 nm using an ELISA
reader (SH-1000 Lab, Corona Electric Co., Ltd.). Independent
experiments were repeated in triplicate.
Transwell (migration) assay and Giemsa
staining
The VSCC-SCs, SCC-SCs, HDFs, and RAW264.7 and HSC-3
cells were collected using Accutase (Invitrogen; Thermo Fisher
Scientific, Inc.) and EDTA (Thermo Fisher Scientific, Inc.) at 90%
confluency. The VSCC-SCs, SCC-SCs and HDFs (15×104
cells) were mixed with HSC-3 (5×104 cells) cells at a
3:1 ratio in 500 µl α-MEM with 10% FBS and added to the
bottom chamber. The RAW264.7 cells were seeded into the upper
chamber of 8-µm Transwell filters without Matrigel in
24-well plates (Corning, Falcon cell culture inserts; BD
Biosciences) in α-MEM without FBS at a density of 2×104
cells/500 µl. Following incubation for 1 day at 37°C in a
humidified atmosphere of 5% CO2 and 95% air, the upper
chambers were stained using the Giemsa staining kit (Diff-Quick,
Nanjing Jiancheng Bioengineering Institute). The chambers were
first washed with distilled water and fixed with Diff-Quik Fixative
provided with the Giemsa staining kit for 2 min at room
temperature. Subsequently, the slides were stained with Diff-Quik
Solution I and Diff-Quik Solution II provided with the Giemsa
staining kit for 2 min at room temperature. The stained cells were
photographed using a bright-field microscope (BX51; Olympus
Corporation). A total of five images (×20 magnification) were
acquired for each sample to assay the cell migration number.
Independent experiments were repeated in triplicate and the data
were analyzed using ImageJ software (V 1.53K; National Institutes
of Health).
Experimental animals
All animal experiments were conducted in accordance
with the relevant guidelines and regulations approved by the
Institutional Committee at Okayama University (OKU-2017406). The
anesthesia protocol was performed according to the Laboratory
Animal Anesthesia 3rd edition (24,25). Following intraperitoneal
anesthesia with ketamine hydrochloride (75 mg/kg body weight) and
medetomidine hydrochloride (0.5 mg/kg body weight), the mice were
determined to be appropriately anesthetized by whether they would
return to a prone position when they were placed on their backs.
Subsequently, 200 µl mixed cells, including HSC-3 cells
(1×106 cells, 100 µl) and SCs (VSCC-SCs, SCC-SCs
and HDFs, 3×106 cells, 100 µl) were injected into
the subcutaneous tissue in the central region of the top of the
head of 20 healthy female BALB-c nu-nu mice (age, 4 weeks; mean
weight, 15 g; Shimizu Laboratory Supplies Co., Ltd.) gradually and
slowly, as previously described (26). All mice were reared in an animal
room at 25°C with 50-60% humidity under a 12-h light/dark cycle and
were provided with free access to food and water. The animal health
and behavior were examined by the staff at the animal center
once/per day. Any difficulties in eating and water intake, any
symptoms of agony (self-harm, abnormal posture, respiratory
distress, crying), the long-term appearance of abnormalities with
no signs of recovery (diarrhea, bleeding, dirt on the genital
area), if the animal's distress was judged to be intolerable, such
as heavy weight loss (≥20% within a few days) or in the case that
the transplanted cancer cells grew to a size of ≥3 cm, were used as
the humanitarian endpoints of the study and the experiment was
immediately terminated and the animal was euthanized. After 4
weeks, all mice were sacrificed by isoflurane excess inhalation
anesthesia (concentration >5%). Cardiac arrest was then verified
by pulse palpation followed by the dislocation of the cervical
spine of the mice. The experimental animals were divided into the
HSC-3, HSC-3 + VSCC-SCs, HSC-3 + SCC-SCs and HSC-3 + HDFs groups
and each group contained 5 mice with tumor formation (n=5 per
group). Of the 5 mice per group, 1 mouse in each group exhibited
poor tumor formation; therefore, their data were removed and the
data from the remaining 4 mice were used for analysis.
Immunohistochemistry (IHC)
Following antigen retrieval in a microwave for 1 or
8 min in 0.01 M tri-sodium citrate buffer (pH 6) and 0.01 M Dako
Target Retrieval Solution (pH 9; cat. no. S2367; Agilent
Technologies, Inc.), 5-µm-thick sections were blocked with
10% normal serum for 20 min at room temperature and incubated with
primary antibodies, including rabbit anti-CD34 (1:500; cat. no.
ab81289, Abcam), anti-CD45 (1:200; cat. no. ab10558, Abcam),
anti-CD11b (1:1,000; cat. no. ab133357, Abcam) and anti-CD163
(1:200; cat. no. ab182422, Abcam) overnight at 4°C. After washing
three times with TBS, all sections were incubated with
avidin-biotin complexes (PK-6101, rabbit ABC kit; Vector
Laboratories, Inc.) for 1 h at room temperature. Following
visualization using DAB/H2O2 mixed solution
(Histofine DAB substrate; Nichirei Corporation), the skin invasion
front and bottom invasion front of the tissues were photographed
using a bright field microscope (BX51; Olympus Corporation). A
total of ten images (×40 magnification) were acquired for each
mouse to assay the expression of CD34, CD45, CD11b and CD163 using
IHC scoring with ImageJ software (V 1.53K; National Institutes of
Health). The IHC score=the score of percentage of positive cells x
the score of intensity. The score of percentage of positive cells
was classified as follows: 0 (<1%), 1 (1-24%), 2 (25-49%), 3
(50%-74%) and 4 (75-100%). The score of intensity were classified
as follows: 0, no staining; 1, light yellow (weak staining); 2,
brown (moderate strong staining); and 3, dark brown (strong
staining) (27). The microvessel
density (MVD) in the central area was assayed by calculating the
CD34(+) vessel structure in each image (positive area/image).
Microarray and bioinformatics
analyses
Microarray and bioinformatics methods were used to
identify the potential genes involved in the differential effects
of VSCC-SCs and SCC-SCs on the infiltration of TAMs into the TME of
OSCC. GeneSpring GX 14.9 (Agilent Technologies, Inc.) software was
used to determine the differentially expressed genes (DEGs) in
SCC-SCs compared with those in VSCC-SCs. A |LogFC|>1 was
considered the cut-off value (the dataset was uploaded onto the
Gene Expression Omnibus database, https://wwwncbi.nlm.nih.gov/geo/query/acc.cgi?&acc=GSE164374).
The biological process of upregulated DEGs was examined by Gene
Ontology (GO) enrichment analysis using Cytoscape 3.7.2 (https://cytoscape.org/); an adjusted P<0.05 was
considered as the cut-off value. The hub genes in vessel
formation-associated biological processes and TAM-associated
biological processes were identified using a protein-to-protein
interaction network (PPI) with STRING (http://string-db.org/) and Cytoscape 3.7.2 (cytohubba)
software. A combined score >0.4 was considered as the cut-off
value and the hub genes were selected according to the degree. The
common hub genes between vessel formation-associated biological
processes and TAM-associated biological processes were identified
using Venn (a software plug-in within SangerBox; http://sangerbox.com). These common hub genes could
not be detected in the animal models as the VSCC-SCs and SCC-SCs
disappeared gradually after the injection. Given that the
microarray data already represented the actual mRNA expression
level in VSCC-SCs and SCC-SCs, the protein expression level of
these common hub genes was not further confirmed.
Statistical analysis
Statistical analysis was conducted using GraphPad
Prism 9 (GraphPad Software, Inc.). The cell experiments were
repeated in triplicate and the animal experiments were repeated
using 5 independent mice. The parametric data are presented as the
mean ± SD and were analyzed by ordinary one-way ANOVA followed by
Tukey's post hoc test. The non-parametric data are presented as the
median and interquartile range (IQR) and were analyzed using the
Kruskal-Wallis test followed by Dunn's test. A value of P<0.05
was considered to indicate a statistically significant
difference.
Results
Crosstalk between SCC-SCs and HSC-3 cells
exerts a more prominent promoting effect on the activation and
fusion of macrophages than VSCC-SCs in vitro
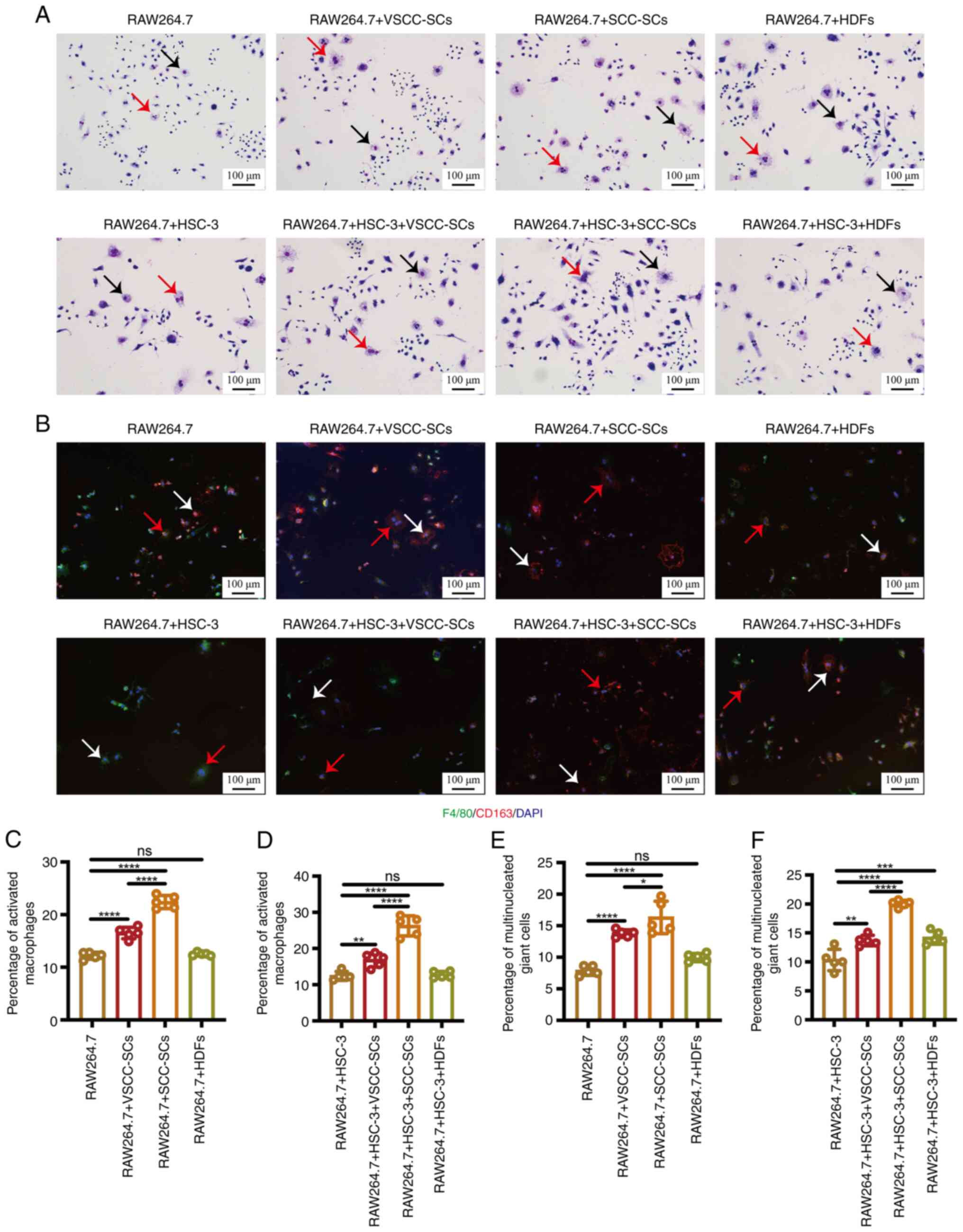
Following indirect co-culture, Giemsa staining was
used to examine the morphology of the macrophages in the different
groups to determine the effects of VSCC-SCs and SCC-SCs on the
activation and fusion of macrophages (Fig. 1A). The percentage of activated
macrophages in the RAW264.7 + SCC-SCs group was the highest,
followed by the RAW264.7 + VSCC-SCs group. Only minimal differences
were observed between the RAW264.7 and RAW264.7 + HDFs groups
(Fig. 1C). Following crosstalk
with HSC-3 cells in vitro, the percentage of activated
macrophages in the RAW264.7 + HSC-3 + SCC-SCs group was slightly
higher than that in the RAW264.7 + HSC-3 + VSCC-SCs and markedly
higher than the RAW264.7 + HSC-3 and RAW264.7 + HSC-3 + HDFs
groups. Only minimal differences were observed between the RAW264.7
+ HSC-3 and RAW264.7 + HSC-3 + HDFs groups (Fig. 1D). The percentage of
multinucleated giant cells in the RAW264.7 + SCC-SCs group was the
highest, followed by the RAW264.7 + VSCC-SCs group. Only a minimal
difference was observed between the RAW264.7 group and RAW264.7 +
HDFs group. (Fig. 1E). Following
crosstalk with HSC-3 cells in vitro, the percentage of
multinucleated giant cells in the RAW264.7 + HSC-3 + SCC-SCs was
higher than that in the RAW264.7 + HSC-3 + VSCC-SCs and RW264.7 +
HSC-3 + HDFs groups, and was markedly higher than that in the
RAW264.7 + HSC-3 group (Fig. 1F).
Furthermore, IF staining was used to examine the expression of
F4/80 and CD163 in the different groups to determine the
characteristics of activated macrophages and multinucleated giant
cells. These two cell types were mainly F4/80(+) and CD163(+),
indicating that the characteristics of these cells were similar to
those of M2 type macrophages (Fig.
1B). These data suggest that both VSCC-SCs and SCC-SCs promoted
the activation and fusion of macrophages following crosstalk with
HSC-3 cells in vitro and that the SCC-SCs exerted a more
prominent promoting effect than the VSCC-SCs, while the HDFs had a
minimal effect.
Crosstalk between SCC-SCs and HSC-3 cells
exerts a more prominent promoting effect on the proliferation of
macrophages than VSCC-SCs in vitro
Following indirect co-culture, MTS assay was used to
examine the proliferative ability of different groups to assay the
effects of VSCC-SCs, SCC-SCs and HDFs on the proliferation of
macrophages in vitro. On day 1, the optical density (OD)
value in the RAW264.7 + HDFs group was slightly higher than that in
the RAW264.7 + SCC-SCs and RAW264.7 + VSCC-SCs groups, and markedly
higher than that in the RAW264.7 group. There was a minimal
difference between the RAW264.7 + VSCC-SCs and RAW264.7 + SCC-SCs
groups (Fig. 2A). Following
crosstalk with the HSC-3 cells in vitro, the OD value in the
RAW264.7 + HSC-3 + HDFs group was the highest, followed by that in
the RAW264.7 + HSC-3 + SCC-SCs group. The OD value of the RAW264.7
+ HSC-3 group was slightly higher than that of the RAW264.7 +
VSCC-SCs group (Fig. 2B). On day
2, the OD value in the RAW264.7 + HDFs and RAW264.7 groups was
slightly higher than that in the RAW264.7 + VSCC-SCs group, and
markedly higher than that in the RAW264.7 + SCC-SCs group. Only a
minimal difference was observed between the RAW264.7 and RAW264.7 +
HDFs groups (Fig. 2C). Following
crosstalk with HSC-3 cells in vitro, the OD value in the
RAW264.7 + HSC-3 + SCC-SCs group was the highest, followed by the
RAW264.7 + HSC-3 + HDFs group. The OD value in the RAW264.7 + HSC-3
+ VSCC-SCs group was slightly higher than that in the RAW264.7 +
HSC-3 group (Fig. 2D). On day 3,
the OD value in the RAW264.7 and RAW264.7 + HDFs groups was
slightly higher than that in the RAW264.7 + VSCC-SCs group and
markedly higher than that in the RAW264.7 + SCC-SCs group. There
was only a minimal difference between the RAW264.7 and RAW264.7 +
HDFs groups (Fig. 2E). Following
crosstalk with HSC-3 cells, the OD value in the RAW264.7 + HSC-3 +
SCC-SCs group was the highest, followed by the RAW264.7 + HSC-3 +
HDFs group. The OD value of the RAW264.7 + HSC-3 + VSCC-SCs group
was slightly higher than that of the RAW264.7 + HSC-3 group
(Fig. 2F). These data suggested
that both the VSCC-SCs and SCC-SCs inhibited the proliferation of
macrophages in vitro, and that the SCC-SCs exerted a more
prominent inhibitory effect than the VSCC-SCs. Following crosstalk
with HSC-3 cells, both the VSCC-SCs and SCC-SCs promoted the
proliferation of macrophages in vitro, and the SCC-SCs
exerted a more prominent promoting effect than the VSCC-SCs.
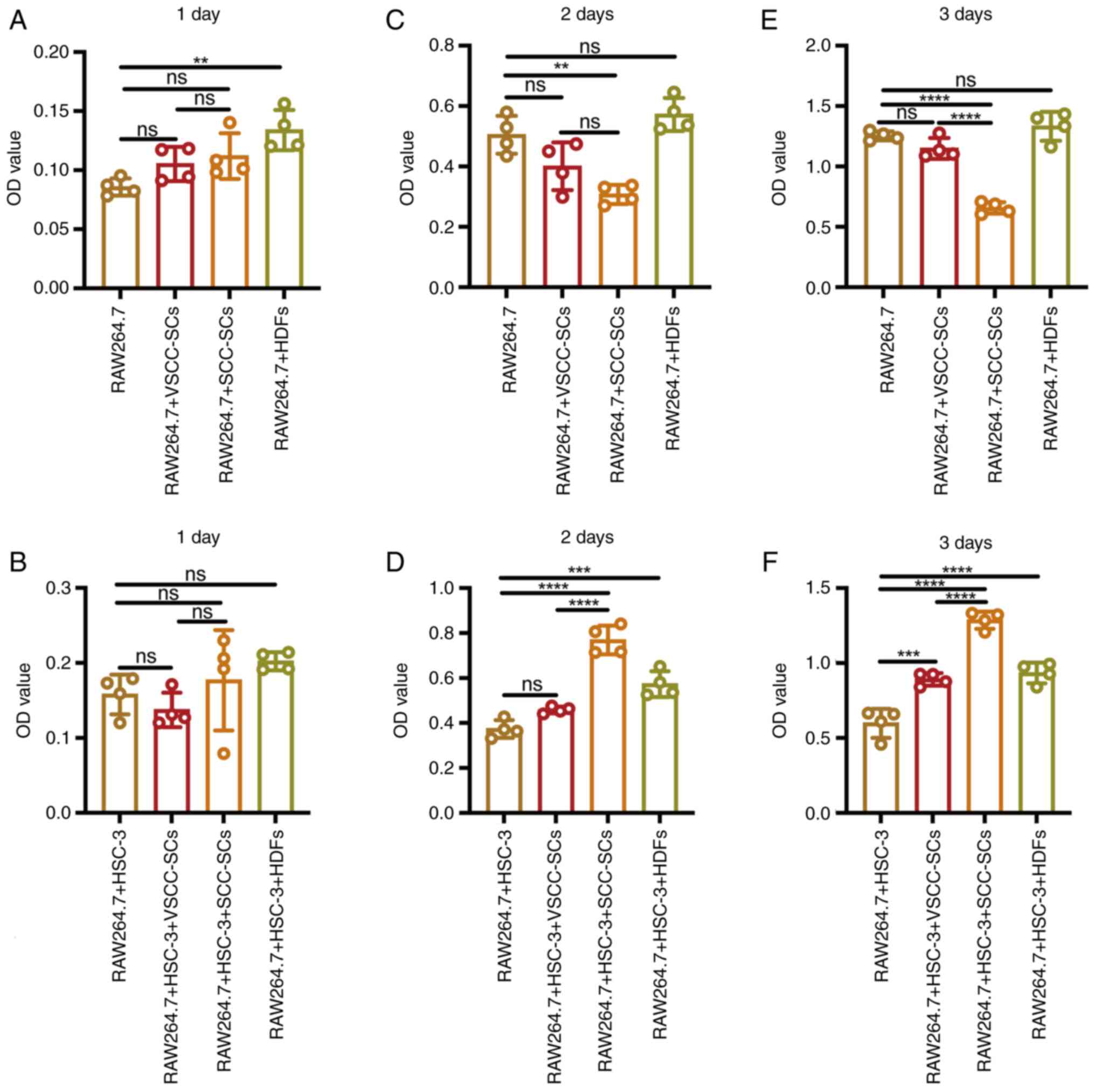 | Figure 2Effects of VSCC-SCs, SCC-SCs and HDFs
on the proliferation of macrophages following crosstalk with HSC-3
cells in vitro. (A, C and E) MTS assay was used to examine
the proliferative ability in the different groups to assay the
effects of VSCC-SCs, SCC-SCs and HDFs on the proliferation of
macrophages in vitro on day (A) 1, (C) day 2and (E) day 3.
(B, D and F) MTS assay was used to examine the proliferative
ability in the different groups to assay the effects of VSCC-SCs,
SCC-SCs and HDFs on the proliferation of macrophages following
crosstalk with HSC-3 cells in vitro on (B) day 1, (D) day 2
and (F) day 3. Data are presented as the mean ± SD of one
independent experiment and the independent experiments were
repeated in triplicate. Statistical analysis was performed using
one-way ANOVA followed by Tukey's post hoc test.
**P<0.01, ***P<0.001 and
****P<0.0001. ns, not significant (P>0.05);
VSCC-SCs, verrucous squamous cell carcinoma-associated stromal
cells; SCC-SCs, squamous cell carcinoma-associated stromal cells;
HDFs, human dermal fibroblasts. |
Both VSCC-SCs and SCC-SCs promote the
migration of macrophages via crosstalk with HSC-3 cells in
vitro
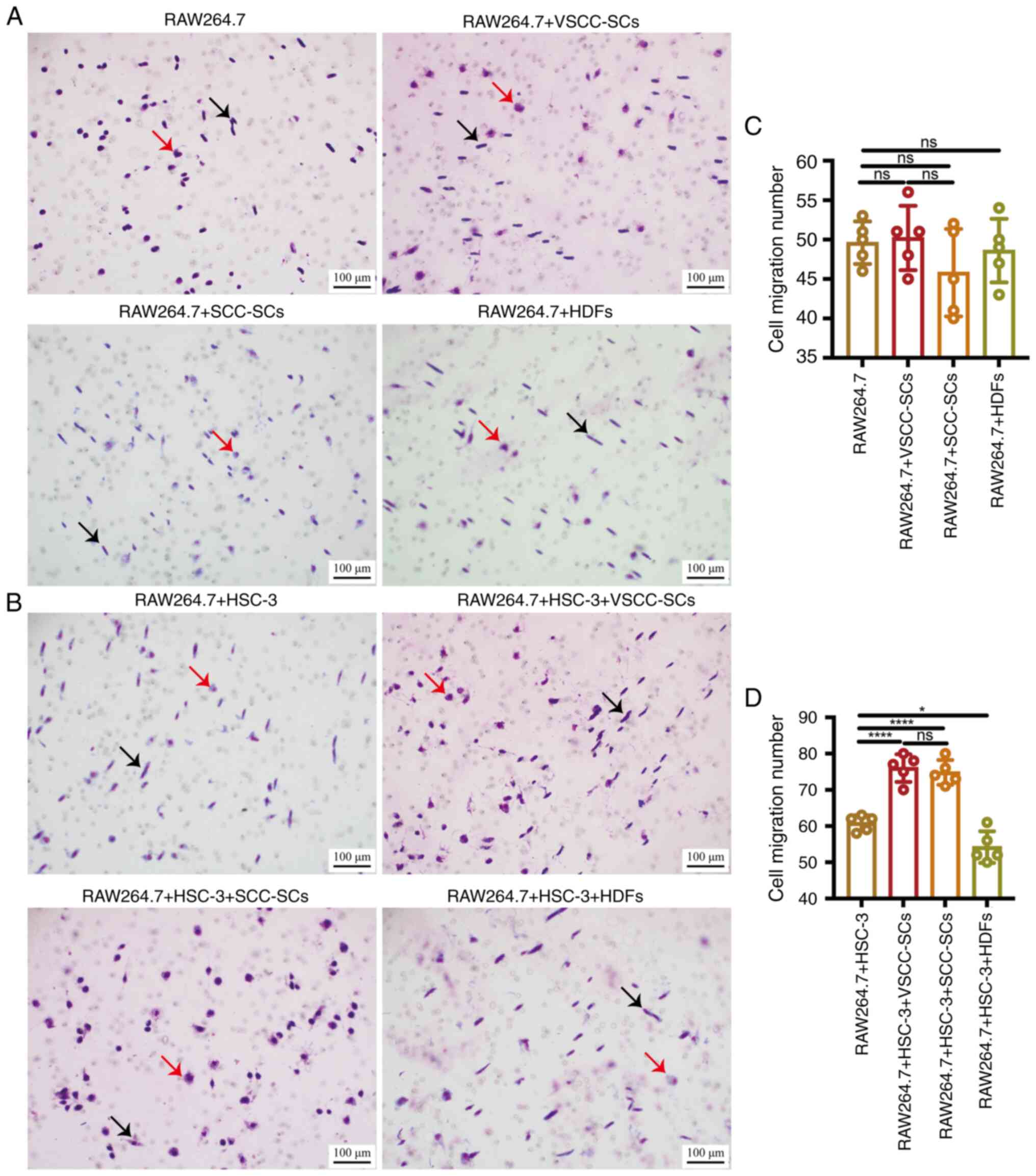
Transwell (migration) and Giemsa staining were used
to examine the effects of VSCC-SCs and SCC-SCs on the migration of
macrophages in vitro. The cell migration number in the
RAW264.7 + SCC-SCs group was slightly lower than that in the other
groups, and there was only a minimal difference between the other
three groups. All the four groups mainly contained spindle-shaped
macrophages and several round-shaped macrophages could also be
observed (Fig. 3A and C). The
cell migration number in the RAW264.7 + HSC-3 + SCC-SCs and
RAW264.7 + HSC-3 + VSCC-SCs groups was slightly higher than that in
the RAW264.7 + HSC-3 group and markedly higher than that in the
RAW264.7 + HSC-3 + HDFs group, and there was a minimal difference
between the RAW264.7 + HSC-3 + SCC-SCs and RAW264.7 + HSC-3 +
VSCC-SCs groups (Fig. 3B and D).
The RAW264.7 + HSC-3 and RAW264.7 + HSC-3 + HDFs groups mainly
contained spindle-shaped macrophages and several round-shaped
macrophages could also be observed, while the RAW264.7 + HSC-3 +
VSCC-SCs group primarily contained round-shaped macrophages and
spindle-shaped macrophages. The RAW264.7 + HSC-3 + SCC-SCs group
mainly contained round-shaped macrophages (Fig. 3B). Therefore, both the VSCC-SCs
and SCC-SCs promoted the migration and changes in the shape of
macrophages following crosstalk with HSC-3 cells in vitro,
and the SCC-SCs exerted a more prominent effect on changes in the
shape of macrophages than the VSCC-SCs following crosstalk with
HSC-3 cells in vitro.
Crosstalk between SCC-SCs and HSC-3 cells
exerts a more prominent promoting effect on the MVD in the TME of
OSCC than VSCC-SCs in vivo
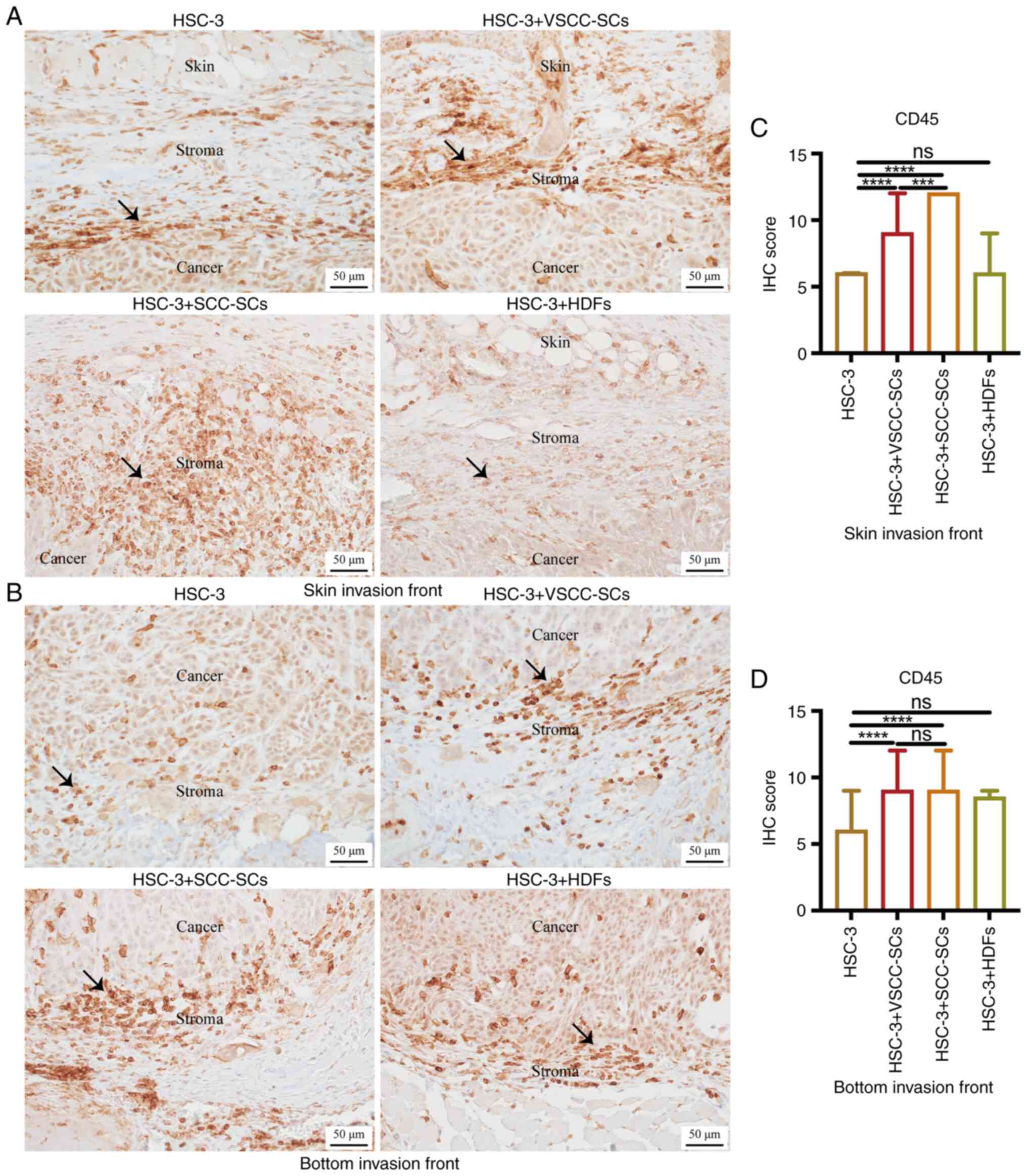
Given that the macrophages were derived from blood,
IHC staining was used to examine CD34 expression to determine the
effects of VSCC-SCs, SCC-SCs and HDFs on the MVD in the TME of
OSCC. In the skin invasion front, the IHC score in the HSC-3 +
SCC-SCs group was higher than that in the HSC-3 + VSCC-SCs group,
and markedly higher than that in the HSC-3 and HSC-3 + HDFs groups.
The IHC score in the HSC-3 + HDFs group was higher than that in the
only HSC-3 group (Fig. 4A and B).
In the central area, the positive area/image in the HSC-3 + SCC-SCs
group was higher than that in HSC-3 + VSCC-SCs group, and markedly
higher than that in the HSC-3 and HSC-3 + HDFs groups. There was a
minimal difference between the HSC-3 and HSC-3 + HDFs groups. In
addition, the size of the vessels in the HSC-3 + SCC-SCs group was
slightly larger than that in the HSC-3 + VSCC-SCs group and
markedly larger than that in the HSC-3 and HSC-3 + HDFs groups
based on the length of MVD (Fig. 4C
and D). At the bottom invasion front, the IHC score in the
HSC-3 + SCC-SCs group was higher than that in the HSC-3 + VSCC-SCs
group and markedly higher than that in HSC-3 and HSC-3 + HDFs
groups. The IHC score in the HSC-3 + HDFs group was slightly higher
than that in the only HSC-3 group (Fig. 4E and F). Thus, these data
demonstrated that both the VSCC-SCs and SCC-SCs promoted the MVD in
the TME of OSCC following crosstalk with HSC-3, and the SCC-SCs
exerted a more prominent promoting effect than the VSCC-SCs, while
the HDFs had a minimal promote effect in the skin and bottom
invasion fronts.
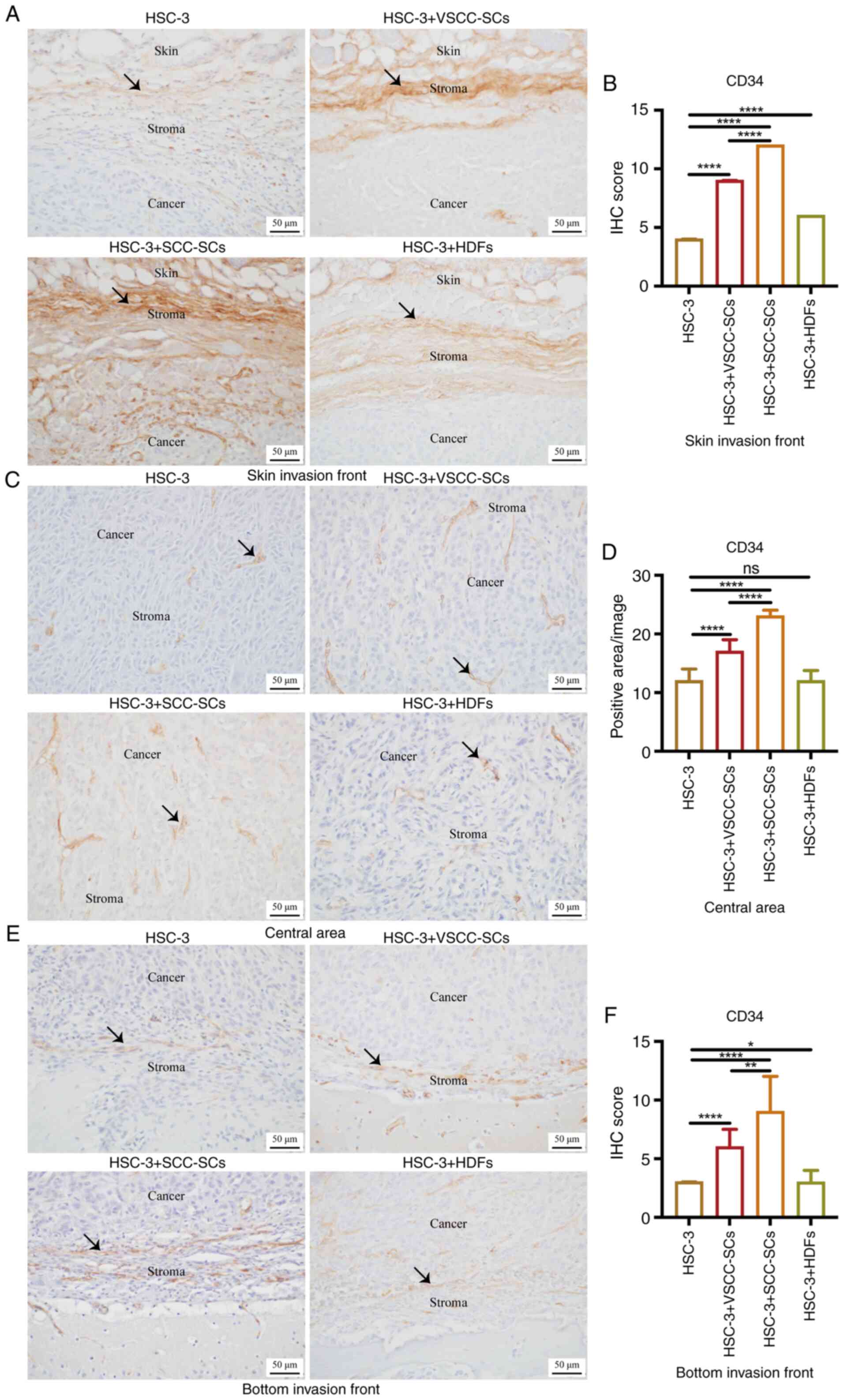 | Figure 4Effects of VSCC-SCs, SCC-SCs and HDFs
on the MVD in the tumor microenvironment of OSCC following
crosstalk with HSC-3 cells in vivo. (A, C and E)
Immunohistochemical staining was used to examine the expression of
CD34 in the (A) skin invasion front, (C) central area and (E)
bottom invasion front. Black arrows indicate vessel structure. (B,
D and F) Quantification of CD34 expression in the (B) skin invasion
front, (D) central area and (F) bottom invasion front in the
different groups. Data are presented as the median and IQR, n=4.
Statistical analysis was performed using the Kruskal-Wallis test
followed by Dunn's test.*P<0.05,
**P<0.01 and ****P<0.0001. ns, not
significant (P>0.05); VSCC-SCs, verrucous squamous cell
carcinoma-associated stromal cells; SCC-SCs, squamous cell
carcinoma-associated stromal cells; HDFs, human dermal
fibroblasts. |
Crosstalk between SCC-SCs and HSC-3 cells
exerts a more prominent promoting effect on the infiltration of
CD45(+) monocytes into the TME of OSCC than VSCC-SCs in vivo
Given that the macrophages were derived from
monocytes from peripheral blood, IHC staining was used to examine
CD45 expression to determine the effects of VSCC-SCs, SCC-SCs and
HDFs on the infiltration of CD45(+) monocytes into the TME of OSCC.
In the skin invasion front, the IHC score of CD45 in HSC-3 +
SCC-SCs group was higher than that in HSC-3 + VSCC-SCs group, and
markedly higher than that in the HSC-3 and HSC-3 + HDFs groups.
There was a minimal difference between the HSC-3 and HSC-3 + HDFs
groups (Fig. 5A and C). In the
bottom invasion front, the IHC score of CD45 in the HSC-3 +
VSCC-SCs and HSC-3 + SCC-SCs group was slightly higher than that in
the HSC-3 + HDFs group, and markedly higher than that in the HSC-3
group. There was a minimal difference between the HSC-3 + VSCC-SCs
and HSC-3 + SCC-SCs groups (Fig. 5B
and D). Therefore, both the VSCC-SCs and SCC-SCs promoted the
infiltration of CD45(+) monocytes into the TME of OSCC, and the
SCC-SCs exerted a more prominent promoting effect than the
VSCC-SCs, while the HDFs exerted a minimal effect.
Crosstalk between SCC-SCs and HSC-3 cells
exerts a more prominent promoting effect on the infiltration of
CD11b(+) pre-macrophages and macrophages (M0 type macrophages) into
the TME of OSCC than VSCC-SCs in vivo
Given that pre-macrophages [bone marrow-derived
cells (BMDCs)] and macrophages are CD11b(+), IHC staining was used
to observe CD11b expression to determine the effects of VSCC-SCs,
SCC-SCs and HDFs on the infiltration of CD11b(+) M0 type
macrophages into the TME of OSCC. In the skin invasion front, the
IHC score of CD11b in the HSC-3 + SCC-SCs group was the highest,
followed by that in the HSC-3 + VSCC-SCs group. There was a minimal
difference between the HSC-3 and HSC-3 + HDFs groups (Fig. 6A and C). At the bottom invasion
front, the IHC score of CD11b in the HSC-3 + SCC-SCs group was
higher than that in the HSC-3 + VSCC-SCs group, and markedly higher
than that in the HSC-3 and HSC-3 + HDFs groups. There was a minimal
difference between the HSC-3 and HSC-3 + HDFs groups (Fig. 6B and D). Therefore, both the
VSCC-SCs and SCC-SCs promoted the infiltration of CD11b(+) M0 type
macrophages into the TME of OSCC, and SCC-SCs exerted a more
prominent promoting effect than the VSCC-SCs, while the HDFs
exerted a minimal effect.
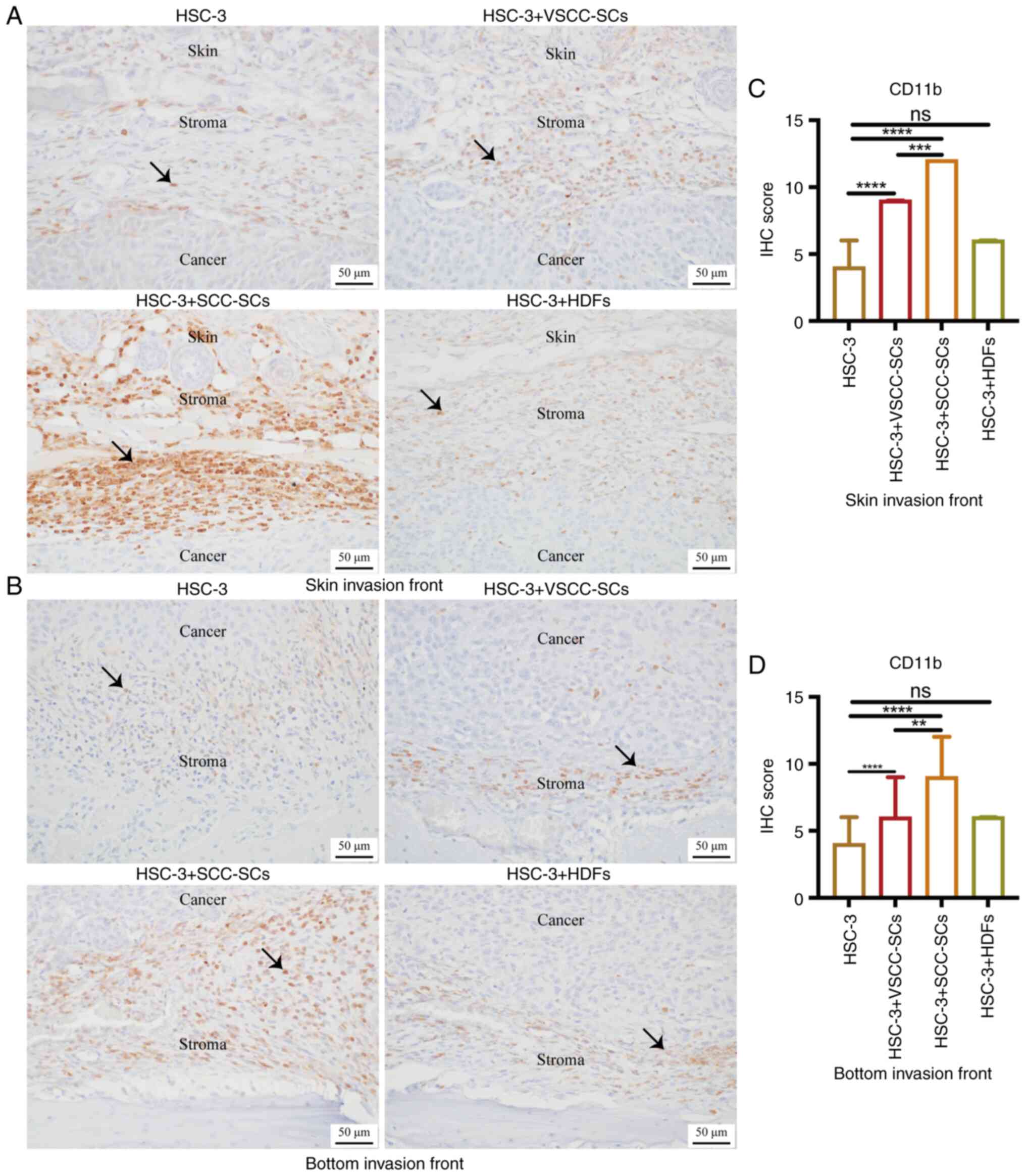 | Figure 6Effects of VSCC-SCs, SCC-SCs and HDFs
on the infiltration of CD11b(+) M0 type macrophages into the tumor
microenvironment of OSCC following crosstalk with HSC-3 cells in
vivo. (A and B) Immunohistochemical staining was used to
examine the CD11b expression level to assay the effects of
VSCC-SCs, SCC-SCs and HDFs on the infiltration of CD11b(+) M0 type
macrophages in the (A) skin and (B) bottom invasion fronts of OSCC,
respectively following crosstalk with HSC-3 cells in vivo.
Black arrows indicated the CD11b(+) M0 type macrophages. (C and D)
Quantification of CD11b expression level in the (C) skin and (D)
bottom invasion fronts in the different groups, respectively. Data
are presented as the median and IQR, n=4. Statistical analysis was
performed using the Kruskal-Wallis test followed by Dunn's
test.**P<0.01, ***P<0.001 and
****P<0.0001. ns, not significant (P>0.05); OSCC,
oral squamous cell carcinoma; VSCC-SCs, verrucous squamous cell
carcinoma-associated stromal cells; SCC-SCs, squamous cell
carcinoma-associated stromal cells; HDFs, human dermal
fibroblasts. |
Crosstalk between SCC-SCs and HSC-3 cells
exerts a more prominent promoting effect on the infiltration of
CD163(+) M2 type TAMs into the TME of OSCC than VSCC-SCs in
vivo
IHC staining was used to examine CD163 expression to
assay the effects of VSCC-SCs, SCC-SCs and HDFs on the infiltration
of CD163(+) M2 type TAMs into the TME of OSCC. In the skin invasion
front, the IHC score of CD163 in the HSC-3 + SCC-SCs group was the
highest, followed by that in the HSC-3 + VSCC-SCs group. There was
a minimal difference between the HSC-3 and HSC-3 + HDFs groups
(Fig. 7A and C). At the bottom
invasion front, there were negative results in these four groups
(Fig. 7B). Therefore, both the
VSCC-SCs and SCC-SCs promoted the infiltration of CD163(+) M2 type
TAMs into the TME of OSCC via crosstalk with HSC-3, and the SCC-SCs
exerted a more prominent promoting effect than the VSCC-SCs, while
the HDFs exerted a minimal effect.
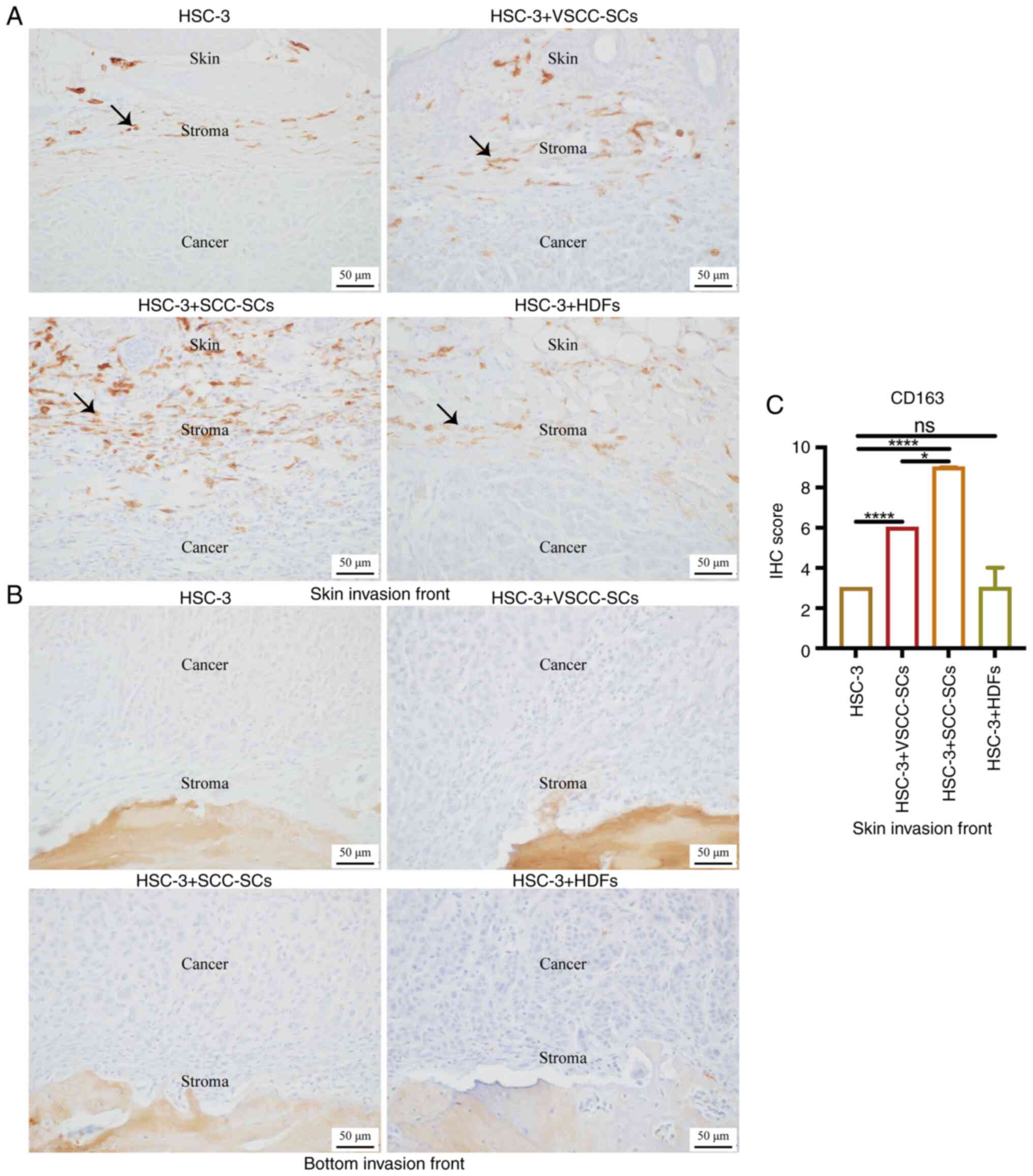 | Figure 7Effects of VSCC-SCs, SCC-SCs and HDFs
on the CD163(+) M2 type TAMs into the tumor microenvironment of
OSCC following crosstalk with HSC-3 cells in vivo. A and B
IHC staining was used to test the CD163 expression level to assay
the effects of VSCC-SCs, SCC-SCs and HDFs on the infiltration of
CD163(+) M2 type TAMs into the (A) skin and (B) bottom invasion
fronts of OSCC, respectively following crosstalk with HSC-3 cells
in vivo. Black arrows indicate CD163(+) M2 type macrophages.
(C) Quantification of CD163 expression level in the skin invasion
front in different groups. Data are presented as the median and
IQR, n=4. Statistical analysis was performed using the
Kruskal-Wallis test followed by Dunn's test.*P<0.05
and ****P<0.0001. ns, not significant (P>0.05);
OSCC, oral squamous cell carcinoma; VSCC-SCs, verrucous squamous
cell carcinoma-associated stromal cells; SCC-SCs, squamous cell
carcinoma-associated stromal cells; HDFs, human dermal fibroblasts;
TAMs, tumor-associated macrophages. |
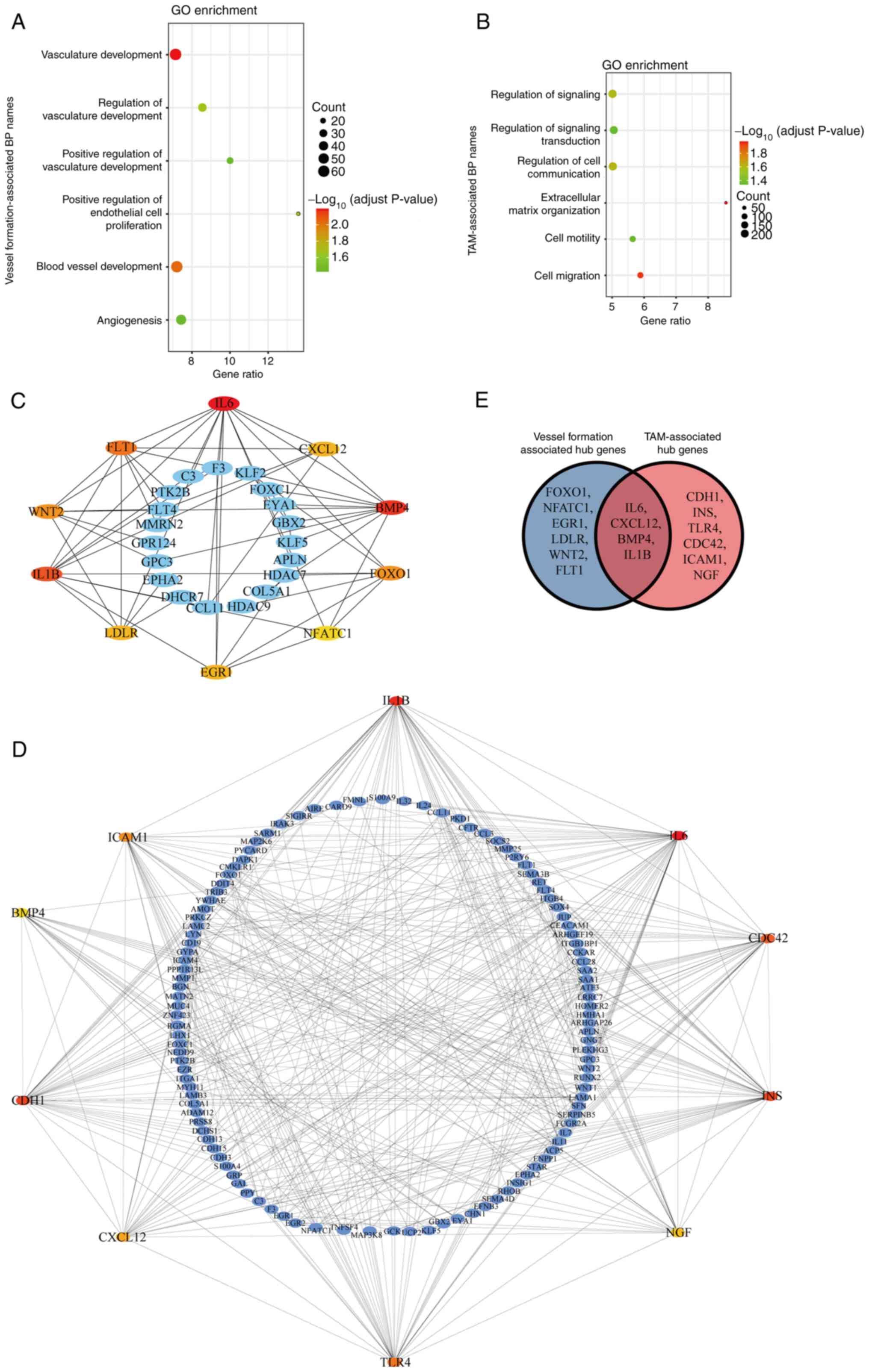
IL1B, BMP4, IL6 and CXCL12 may be
involved in the differential effects of VSCC-SCs and SCC-SCs on the
infiltration of TAMs into the TME of OSCC
The DEGs in the SCC-SCs were compared with those in
VSCC-SCs using microarray analysis. The biological process of
upregulated DEGs in SCC-SCs was analyzed using GO enrichment
analysis, which indicated that these up-DEGs are primarily enriched
in vessel formation-associated biological processes, such as
vasculature development and blood vessel development (Fig. 8A), as well as TAM-associated
biological processes, such as cell migration and cell motility
(Fig. 8B). Furthermore, the hub
genes in the vessel formation-associated biological processes and
TAM-associated biological processes were analyzed by PPI, which
suggested that interleukin (IL)6, C-X-C motif chemokine ligand 12
(CXCL12), bone morphogenetic protein 4 (BMP4), forkhead box O1
(FOXO1), nuclear factor of activated T-cells 1 (NFATC1), early
growth response protein 1 (EGR1), low density lipoprotein receptor
(LDLR), IL1B, Wnt family member 2 (WNT2) and Fms related receptor
tyrosine kinase 1 (FLT1) were hub genes in vessel
formation-associated biological processes (Fig. 8C), and IL1B, BMP4, intercellular
adhesion molecule 1 (ICAM1), IL6, cell division cycle 42 (CDC42),
insulin (INS), nerve growth factor (NGF), Toll-like receptor 4
(TLR4), CXCL12 and cadherin 1 (CDH1) were hub genes in
TAM-associated biological processes (Fig. 8D). The common hub genes between
them were identified using Venn diagrams, which suggested that IL6,
CXCL12, IL1B and BMP4 were common hub genes in both vessel
formation-associated biological processes and TAM-associated
biological processes (Fig. 8E).
Thus, IL1B, BMP4, IL6 and CXCL12 may be involved in the
differential effects of VSCC-SCs and SCC-SCs on the infiltration of
TAMs into the TME of OSCC.
Discussion
TAMs, as the main component of the TME, can regulate
the angiogenesis, invasion and migration of cancers by secreting
relevant factors. In addition, TAMs are involved in cancer
immunotherapy, such as checkpoint inhibitor therapy, adoptive cell
transfusion, and cancer vaccine therapy. For the targeting of TAMs,
there are three different strategies: The elimination of TAMs, the
inhibition of monocyte recruitment and the reprogramming of TAMs.
Therefore, TAMs particularly the M2 type, are closely associated
with the initiation and progression of cancer (28,29). In the majority of cancer models,
TAMs are derived mainly from the differentiation of monocytes in
peripheral blood (30,31). M2 type TAMs are closely associated
with MVD in pancreatic ductal adenocarcinoma, and can be detected
by CD31 and CD34 (32,33). Thus, it was hypothesized that the
increased MVD may also influence the number of monocytes recruited
by the crosstalk between cancer and stroma. In the present study,
CD34 was used to examine the MVD in the skin invasion front,
central area and bottom invasion front of OSCC, which suggested
that the crosstalk between VSCC-SCs/SCC-SCs and HSC-3 cells
promoted the MVD in skin invasion front, central area and bottom
invasion front of OSCC, and that the crosstalk between SCC-SCs and
HSC-3 cells exerted a more prominent promoting effect than the
crosstalk between VSCC-SCs and HSC-3 cells. These data indicated
that the crosstalk between cancer and the stroma promoted the
infiltration of macrophages into the TME of OSCC by promoting MVD,
and SCC-SCs exerted a more prominent promoting effect than
VSCC-SCs. CD45 was used to label the monocytes to examine the
effects of VSCC-SCs, SCC-SCs and HDFs on the infiltration of
CD45(+) monocytes. The results revealed that the crosstalk between
SCC-SCs and HSC-3 exerted a more prominent promoting effect on the
infiltration of CD45(+) monocytes into the TME of OSCC than
VSCC-SCs in the skin invasion front, whereas there was a minimal
difference in the bottom invasion front, which may be caused by the
complex structures in the bottom invasion front.
A recent study indicated that TAMs may be reliable
markers to assay the aggressiveness of OSCC (34). In addition, CAFs can promote
monocyte differentiation and the activation by secreting IL6 and
GM-CSF (35). Thus, both cancer
cells and CAFs may possibly promote the activation of TAMs in the
TME. In the present study, the crosstalk between SCC-SCs and HSC-3
cells exerted a more prominent promoting effect on the activation
and fusion of macrophages than VSCC-SCs in vitro. The size
of macrophages increased following activation by the crosstalk
between SCC-SCs and HSC-3 cells. After the fusion of macrophages,
not only will the size of cells enlarge, but the number of nuclei
will also increase. Furthermore, IF staining was used to evaluate
F4/80 and CD163 expression to identify the characteristics of
activated macrophages and multinucleated giant cells. These results
suggested that the characteristics of these two cell types were
similar to M2 type TAMs. In addition, MTS assay was used to assay
the proliferative ability in the different groups, which
demonstrated that both the VSCC-SCs and SCC-SCs inhibited the
proliferation of macrophages, and the SCC-SCs exerted a more
prominent promoting effect than the VSCC-SCs. Both the VSCC-SCs and
SCC-SCs promoted macrophage proliferation following crosstalk with
HSC-3 cells in vitro on days 2 and 3. Following crosstalk
with HSC-3, the effect of SCC-SCs on the proliferation of
macrophages significantly increased, whereas the effect of VSCC-SCs
on the proliferation of macrophages decreased significantly. Based
on Giemsa staining, IF and MTS assay, both the VSCC-SCs and SCC-SCs
primarily promoted the activation and differentiation of
macrophages into M2 type TAMs and SCC-SCs exerted a more prominent
promoting effect than VSCC-SCs. Following crosstalk with HSC-3
cells, both VSCC-SCs and SCC-SCs mainly promoted the proliferation
and differentiation of macrophages into M2 type TAMs with SCC-SCs
exerting a more prominent promoting effect than VSCC-SCs. In
addition, the effects of VSCC-SCs and SCC-SCs on the migration of
macrophages increased significantly following crosstalk with HSC-3
cells in vitro, and the SCC-SCs exerted a more prominent
effect than the VSCC-SCs in promoting changes in the shape of
macrophages from a spindle to a round shape. Based on Giemsa
staining, the round-shaped macrophages were activated macrophages
compared with spindle-shaped macrophages. It is considered that the
spindle-shaped macrophages were more closely associated with the
migration ability. After migration, the crosstalk between cancer
and different subtype cancer stroma could induce the activation of
macrophages, resulting in morphological changes from a spindle to a
round shape. The number of round-shaped macrophages in the RAW264.7
+ HSC-3 + SCC-SCs group was the highest, followed by that in the
RAW264.7 + HSC-3 + VSCC-SCs group. Therefore, both the VSCC-SCs and
SCC-SCs had a similar effect on the migration of macrophages, and
the SCC-SCs had a more prominent effect on the activation of
macrophages than the VSCC-SCs following crosstalk with HSC-3 cells
in vitro.
Given that the pre-macrophages (BMDCs) and
macrophages are CD11b(+) (36),
IHC staining was used to observe CD11b expression to determine the
effects of VSCC-SCs and SCC-SCs on the infiltration of CD11b(+) M0
type macrophages into the TME of OSCC following crosstalk with
HSC-3 cells in vivo. The crosstalk between SCC-SCs and HSC-3
exerted a more prominent promoting effect on the infiltration of
CD11b(+) TAMs than VSCC-SCs in the skin and bottom invasion fronts,
while HDFs exerted a minimal effect. M2 type TAMs have
anti-inflammatory and pro-tumor functions, and IHC staining can be
used to examine the expression of CD206 and CD163 to assay the
infiltration of M2 type TAMs into the TME of OSCC (37). Recent studies have indicated that
OSCC-derived exosomes and CAFs play crucial roles in the
polarization and infiltration of M2 type TAMs (38,39). Previous studies by the authors
indicated that both VSCC-SCs and SCC-SCs promoted the progression
of OSCC, which was more relevant with the M2 type TAMs rather than
M1 type TAMs (19,20). Thus, the present study mainly
focused on M2 type macrophages and did not perform staining for M1
markers to examine the M2/M1 ratio. In the present study, the
crosstalk between SCC-SCs and HSC-3 cells exerted a more prominent
promoting effect on the infiltration of CD163(+) M2 type TAMs in
the skin invasion front than VSCC-SCs, whereas the HDFs exerted a
minimal effect. However, there was a negative result in the bottom
invasion front, which may be caused by complex structures such as
bone, muscle and brain in bottom invasion front. In a previous
study by the authors, it was reported that the cancer stroma
promotes bone destruction by inducing osteoclasts on the bone
surface (23). In addition, it
was found that crosstalk between HSC-3 cells and VSCC-SCs/SCC-SCs
promoted the fusion of macrophages into multinucleated giant cells.
Therefore, it was hypothesized that factors released from the bone
destroyed by the cancer/stroma complex caused the assembled
macrophages to differentiate into osteoclasts or promote the fusion
of macrophages into multinucleated giant cells rather than M2
macrophages. Given that the VSCC-SCs and SCC-SCs can promote the
proliferation and migration of macrophages following crosstalk with
HSC-3 cells in vitro, the VSCC-SCs and SCC-SCs have immense
potential to promote the infiltration of TAMs, by promoting the
proliferation and migration following crosstalk with HSC-3 cells
in vivo. In addition, after being mixed with HSC-3 cells to
construct the animal model, the VSCC-SCs and SCC-SCs gradually
disappeared. Therefore, it is quite difficult to determine whether
the VSCC-SCs and SCC-SCs changed into cancerous cells through
mesenchymal transition or provide the room for the direct
infiltration of TAMs through the blood vessels inside the
tumor.
IL1B plays a crucial role in the crosstalk between
TAMs and multitype cancers; however, it has been poorly
investigated in OSCC (40-42).
It has been shown that the expression of BMP4 in TAMs is increased
following co-culture with cancer cells, which in turn enhanced the
invasion of gastric cancer cells (43). The TGF-β/BMP signaling pathway
also plays a key role in the crosstalk between pancreatic cancer
and TAMs (44). Therefore, BMP4
may be involved in the differential effects of VSCC-SCs and SCC-SCs
on the infiltration of TAMs into the TME of OSCC. IL6 was closely
associated with the polarization of M2 type TAMs and OSCC
progression, which may be involved in the differential effects of
VSCC-SCs and SCC-SCs on the infiltration of TAMs into the TME of
OSCC (45). CAFs can recruit M2
type TAMs by secreting CXCL12 in the TME of OSCC, which may be
involved in the differential effects of VSCC-SCs and SCC-SCs on the
infiltration of TAMs into the TME of OSCC (39).
In conclusion, the present study demonstrated that
both VSCC-SCs and SCC-SCs promoted the differentiation,
proliferation and migration of macrophages following crosstalk with
HSC-3 cells in vitro. In addition, both VSCC-SCs and SCC-SCs
promoted the MVD and infiltration of CD45(+) monocytes, CD11b(+) M0
type macrophages, and CD163(+) M2 type TAMs into the TME of OSCC
following crosstalk with HSC-3 cells in vivo. The SCC-SCs
exerted a more prominent promoting effect than the VSCC-SCs.
Finally, IL1B, BMP4, IL6, and CXCL12 may be involved in the
differential effects of VSCC-SCs and SCC-SCs on the infiltration of
TAMs. These findings provide a potential regulatory mechanism
underlying the effects of the cancer stroma on the infiltration of
TAMs into the TME of OSCC, which may contribute to the development
of novel treatment strategies for patients with OSCC by targeting
TAMs.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available in the Gene Expression Omnibus
repository (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?&acc=GSE164374).
Author's contributions
QS designed the outline of the study. All authors
(QS, KT, HK, MWO, SS, MF, KN and HN) conducted experiments and data
analysis. QS and KT were involved in the preparation of the
manuscript. QS wrote the manuscript. KN, KN and HN supervised the
study and contributed to data interpretation and manuscript
revision. KT, KN and HN confirmed the authenticity of all raw data.
All authors have read and agreed to the published version of the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Okayama University (project identification code:
1703-042-001). Written informed consent was obtained from all
patients. All animal experiments were conducted according to the
relevant guidelines and regulations approved by the institutional
committees at Okayama University (approval no. OKU-2017406).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by the Japan Society for
Promotion of Science (JSPS) KAKENHI Grants-in-Aid for Scientific
Research (nos. JP20K10094, JP21K10043, JP21K17089, JP19K19159,
JP20H03888 and JP22K10170).
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Watanabe T, Kamio N, Okabe T, Hayama T,
Fukai J, Watanabe A, Okada H and Matsushima K: Macrophage migration
inhibitory factor promotes inflammation in human dental pulp. J
Hard Tissue Biol. 29:9–16. 2020. View Article : Google Scholar
|
|
5
|
Suzuki T, Hayakawa T and Gomi K: GM-CSF
stimulates mouse macrophages and causes inflammatory effects in
vitro. J Hard Tissue Biol. 28:37–42. 2019. View Article : Google Scholar
|
|
6
|
Noy R and Pollard JW: Tumor-associated
macrophages: From mechanisms to therapy. Immunity. 41:49–61. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ruffell B, Affara NI and Coussens LM:
Differential macrophage programming in the tumor microenvironment.
Trends Immunol. 33:119–126. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamagata Y, Tomioka H, Sakamoto K, Sato K,
Harada H, Ikeda T and Kayamori K: CD163-positive macrophages within
the tumor stroma are associated with lymphangiogenesis and lymph
node metastasis in oral squamous cell carcinoma. J Oral Maxillofac
Surg. 75:2144–2153. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He KF, Zhang L, Huang CF, Ma SR, Wang YF,
Wang WM, Zhao ZL, Liu B, Zhao YF, Zhang WF and Sun ZJ: CD163+
tumor-associated macrophages correlated with poor prognosis and
cancer stem cells in oral squamous cell carcinoma. Biomed Res Int.
2014:8386322014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qian BZ and Pollard JW: Macrophage
diversity enhances tumor progression and metastasis. Cell.
141:39–51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mills CD: M1 and M2 macrophages: Oracles
of health and disease. Crit Rev Immunol. 32:463–488. 2012.
View Article : Google Scholar
|
|
12
|
Goswami KK, Ghosh T, Ghosh S, Sarkar M,
Bose A and Baral R: Tumor promoting role of anti-tumor macrophages
in tumor microenvironment. Cell Immunol. 316:1–10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kai K, Moriyama M, Haque ASMR, Hattori T,
Chinju A, Hu C, Kubota K, Miyahara Y, Kakizoe-Ishiguro N, Kawano S
and Nakamura S: Oral squamous cell carcinoma contributes to
differentiation of monocyte-derived tumor-associated macrophages
via PAI-1 and IL-8 production. Int J Mol Sci. 22:94752021.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Alves A, Diel L, Ramos G, Pinto A,
Bernardi L, Yates J III and Lamers M: Tumor microenvironment and
oral squamous cell carcinoma: A crosstalk between the inflammatory
state and tumor cell migration. Oral Oncol. 112:1050382021.
View Article : Google Scholar
|
|
15
|
Schmid MC, Khan SQ, Kaneda MM, Pathria P,
Shepard R, Louis TL, Anand S, Woo G, Leem C, Faridi MH, et al:
Integrin CD11b activation drives anti-tumor innate immunity. Nat
Commun. 9:53792018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Patel SG and Shah JP: TNM staging of
cancers of the head and neck: Striving for uniformity among
diversity. CA Cancer J Clin. 55:242–264. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Spiro RH, Guillamondegui O Jr, Paulino AF
and Huvos AG: Pattern of invasion and margin assessment in patients
with oral tongue cancer. Head Neck. 21:408–413. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hussein MR and Cullen K: Molecular
biomarkers in HNSCC: Prognostic and therapeutic implications.
Expert Rev Anticancer Ther. 1:116–124. 2001. View Article : Google Scholar
|
|
19
|
Takabatake K, Kawai H, Omori H, Qiusheng
S, Oo MW, Sukegawa S, Nakano K, Tsujigiwa H and Nagatsuka H: Impact
of the stroma on the biological characteristics of the parenchyma
in oral squamous cell carcinoma. Int J Mol Sci. 21:77142020.
View Article : Google Scholar :
|
|
20
|
Shan Q, Takabatake K, Omori H, Kawai H, Oo
MW, Nakano K, Ibaragi S, Sasaki A and Nagatsuka H: Stromal cells in
the tumor microenvironment promote the progression of oral squamous
cell carcinoma. Int J Oncol. 59:722021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sakakura K, Takahashi H, Kaira K, Toyoda
M, Murata T, Ohnishi H, Oyama T and Chikamatsu K: Relationship
between tumor-associated macrophage subsets and CD47 expression in
squamous cell carcinoma of the head and neck in the tumor
microenvironment. Lab Invest. 96:994–1003. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dan H, Liu S, Liu J, Liu D, Yin F, Wei Z,
Wang J, Zhou Y, Jiang L, Ji N, et al: RACK1 promotes cancer
progression by increasing the M2/M1 macrophage ratio via the NF-κB
pathway in oral squamous cell carcinoma. Mol Oncol. 14:795–807.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shan Q, Takabatake K, Kawai H, Oo MW,
Inada Y, Sukegawa S, Fushimi S, Nakano K and Nagatsuka H:
Significance of cancer stroma for bone destruction in oral squamous
cell carcinoma using different cancer stroma subtypes. Oncol Rep.
47:812022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Flecknell PA: Laboratory Animal
Anesthesia. 3rd edition. Academic Press; San Diego, CA: 2009
|
|
25
|
Adams S and Pacharinsak C: Mouse
anesthesia and analgesia. Curr Protoc Mouse Biol. 5:51–63. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
An YZ, Cho E, Ling J and Zhang X: The
Axin2-snail axis promotes bone invasion by activating
cancer-associated fibroblasts in oral squamous cell carcinoma. BMC
Cancer. 20:9872020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wei C, Yang C, Wang S, Shi D, Zhang C, Lin
X, Liu Q, Dou R and Xiong B: Crosstalk between cancer cells and
tumor associated macrophages is required for mesenchymal
circulating tumor cell-mediated colorectal cancer metastasis. Mol
Cancer. 18:642019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Duan Z and Luo Y: Targeting macrophages in
cancer immunotherapy. Signal Transduct Target Ther. 6:1272021.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xiang X, Wang J, Lu D and Xu X: Targeting
tumor-associated macrophages to synergize tumor immunotherapy.
Signal Transduct Target Ther. 6:752021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cassetta L, Fragkogianni S, Sims AH,
Swierczak A, Forrester LM, Zhang H, Soong DYH, Cotechini T, Anur P,
Lin EY, et al: Human tumor-associated macrophage and monocyte
transcriptional landscapes reveal cancer-specific reprogramming,
biomarkers, and therapeutic targets. Cancer Cell. 35:588–602.e10.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Devalaraja S, To TKJ, Folkert IW, Natesan
R, Alam MZ, Li M, Tada Y, Budagyan K, Dang MT, Zhai L, et al:
Tumor-derived retinoic acid regulates intratumoral monocyte
differentiation to promote immune suppression. Cell.
180:1098–1114.e16. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang Y, Guo Z, Chen W, Wang X, Cao M, Han
X, Zhang K, Teng B, Cao J, Wu W, et al: M2 macrophage-derived
exosomes promote angiogenesis and growth of pancreatic ductal
adenocarcinoma by targeting E2F2. Mol Ther. 29:1226–1238. 2021.
View Article : Google Scholar :
|
|
33
|
Miyata Y, Kanda S, Ohba K, Nomata K,
Hayashida Y, Eguchi J, Hayashi T and Kanetake H: Lymphangiogenesis
and angiogenesis in bladder cancer: Prognostic implications and
regulation by vascular endothelial growth factors-A, -C, and -D.
Clin Cancer Res. 12(3 Pt 1): 800–806. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mukherjee A, Spadigam A and Dhupar A:
Tumor-associated macrophages: Harbingers of aggressiveness in oral
squamous cell carcinoma. J Oral Maxillofac Pathol. 25:46–50. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cho H, Seo Y, Loke KM, Kim SW, Oh SM, Kim
JH, Soh J, Kim HS, Lee H, Kim J, et al: Cancer-stimulated CAFs
enhance monocyte differentiation and protumoral TAM activation via
IL6 and GM-CSF secretion. Clin Cancer Res. 24:5407–5421. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Okubo M, Kioi M, Nakashima H, Sugiura K,
Mitsudo K, Aoki I, Taniguchi H and Tohnai I: M2-polarized
macrophages contribute to neovasculogenesis, leading to relapse of
oral cancer following radiation. Sci Rep. 6:275482016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Weber M, Moebius P, Büttner-Herold M,
Amann K, Preidl R, Neukam FW and Wehrhan F: Macrophage polarisation
changes within the time between diagnostic biopsy and tumour
resection in oral squamous cell carcinomas-an immunohistochemical
study. Br J Cancer. 113:510–519. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pang X, Wang SS, Zhang M, Jiang J, Fan HY,
Wu JS, Wang HF, Liang XH and Tang YL: OSCC cell-secreted exosomal
CMTM6 induced M2-like macrophages polarization via ERK1/2 signaling
pathway. Cancer Immunol Immunother. 70:1015–1029. 2021. View Article : Google Scholar
|
|
39
|
Li X, Bu W, Meng L, Liu X, Wang S, Jiang
L, Ren M, Fan Y and Sun H: CXCL12/CXCR4 pathway orchestrates
CSC-like properties by CAF recruited tumor associated macrophage in
OSCC. Exp Cell Res. 378:131–138. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Eum HH, Kwon M, Ryu D, Jo A, Chung W, Kim
N, Hong Y, Son DS, Kim ST, Lee J, et al: Tumor-promoting
macrophages prevail in malignant ascites of advanced gastric
cancer. Exp Mol Med. 52:1976–1988. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chittezhath M, Dhillon MK, Lim JY, Laoui
D, Shalova IN, Teo YL, Chen J, Kamaraj R, Raman L, Lum J, et al:
Molecular profiling reveals a tumor-promoting phenotype of
monocytes and macrophages in human cancer progression. Immunity.
41:815–829. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen Q, Wang J, Zhang Q, Zhang J, Lou Y,
Yang J, Chen Y, Wei T, Zhang J, Fu Q, et al: Tumour cell-derived
debris and IgG synergistically promote metastasis of pancreatic
cancer by inducing inflammation via tumour-associated macrophages.
Br J Cancer. 121:786–795. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shen Z, Kauttu T, Cao J, Seppänen H,
Vainionpää S, Ye Y, Wang S, Mustonen H and Puolakkainen P:
Macrophage coculture enhanced invasion of gastric cancer cells via
TGF-β and BMP pathways. Scand J Gastroenterol. 48:466–472. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shen Z, Seppänen H, Kauttu T, Vainionpää
S, Ye Y, Wang S, Mustonen H and Puolakkainen P: Vasohibin-1
expression is regulated by transforming growth factor-β/bone
morphogenic protein signaling pathway between tumor-associated
macrophages and pancreatic cancer cells. J Interferon Cytokine Res.
33:428–433. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Petruzzi MN, Cherubini K, Salum FG and de
Figueiredo MA: Role of tumour-associated macrophages in oral
squamous cells carcinoma progression: An update on current
knowledge. Diagn Pathol. 12:322017. View Article : Google Scholar : PubMed/NCBI
|