Introduction
Salivary adenoid cystic carcinoma (SACC) is a common
malignant tumor of the major and minor salivary glands, accounting
for 30% of salivary gland epithelial malignant tumors. Due to its
biological characteristics, such as nerve and vascular invasion,
distant metastasis and high recurrence rate, the 15-year survival
rate of patients is only 40% (1-3). At
present, the effective treatment for SACC mainly includes surgery
and radiotherapy. Little is known about the targeted therapy for
identifying potential molecular abnormalities in SACC. Further
investigation of the specific mechanism under-lying malignant
progression of SACC is urgently needed to provide new and effective
therapeutic targets.
Malignant proliferation, invasion and metastasis of
tumor cells is a complex process that requires extensive
reorganization of the cytoskeleton. A high frequency of
intracellular cytoskeleton reorganization has been observed in
cancer, including actin cytoskeleton remodeling, microtubule
rearrangement and intermediate filament changes (4-6).
Throughout the remodeling process, these transformations are
usually controlled by microtubule-related proteins (7,8).
Therefore, microtubule regulatory proteins play a potential role in
the malignant progression of tumors, such as in cell division, cell
polarization, and cell migration. Lissencephaly 1 (LIS1), also
known as platelet-activating factor acetylhydrolase 1b1 (PAFAH1B1),
is a microtubule-organizing center-associated protein that
regulates the polymerization and stability of microtubules by
mediating the motor function of dynein (9). Through in situ hybridization,
Allanson et al (10) found
that the LIS1 gene was heterozygous in patients with Miller Dieker
syndrome (MDS). The syndrome is a typical anencephalic
malformation, which is a brain malformation caused by abnormal
neuronal migration. LIS1 is a regulated adapter between
cytoskeleton-associated proteins and cytoplasmic dynein at sites
involved in cargo microtubule loading and in the control of
microtubule dynamics, which participates in a series of cell
activities, including cell migration, organelle location and
spindle assembly (9). Previous
studies have found that LIS1 is highly expressed in tumor tissues.
The expression of LIS1 in lung cancer tissue was demonstrated to be
significantly correlated with the occurrence and prognosis of lung
cancer. LIS1 promoted lung cancer cell invasion and metastasis by
regulating the aggregation of microtubules and fibronectin
(11); in cholangiocarcinoma,
miR-144 targeted LIS1 mRNA and suppressed the proliferation and
invasion of tumor cells (12).
However, the mechanism by which LIS1 regulates tumor cells is
unclear.
Previous studies have reported that there is a
physical interaction between the WD-40 domains of LIS1 and the zinc
fingers of cytoplasmic linker protein 170 (CLIP-170) and provided
evidence that LIS1 plays a novel role in mediating CLIP-170/dynein
interactions and in coordinating dynein cargo-binding and motor
activities (13). CLIP170 is a
microtubule-associated protein with a cytoskeleton-associated
protein glycine-rich (CAP-Gly) domain. The second zinc finger of
its COOH-terminus has been implicated in the interaction with
microtubule plus-end-tracking proteins (+TIPs) to participate in
various cellular processes, including regulation of microtubule
dynamics, cell migration and intracellular transport (14). Several previous studies have
implicated CLIP-170 in the pathogenesis of cancer. CLIP-170 was
revealed to increase the ability of paclitaxel to block cell cycle
progression at mitosis and to induce apoptosis in breast cancer
cells (15). A previous study by
the authors demonstrated that arsenic trioxide (ATO) disrupted the
zinc finger of CLIP170 to disturb the LIS1/NDEL1/dynein microtubule
dynamic complex, thus inhibiting the ability of migration and
invasion in head and neck cancer (16). However, the specific downstream
mechanism of the LIS1/CLIP170 complex regulating the progression of
tumor cells remains unknown.
Cell division control protein 42 homolog (Cdc42) is
a member of the Rho family of small GTPases and a master regulator
of the actin cytoskeleton, controlling cell motility, polarity and
cell cycle progression (17).
Cdc42 is upregulated in several human cancer cell lines and its
expression has been demonstrated to be correlated with tumor stage,
lymph node metastasis, and patient survival (18,19).
A previous study revealed that Cdc42-mediated phosphorylation of
CLIP-170 was essential for the normal function of this protein
during cell cycle progression (20). CLIP-170 interacted with IQ motif
containing GTPase activating protein 1 (IQGAP1), an effector of
Cdc42, leading to a polarized microtubule array and formation of
cell tight junctions (TJ) (21).
The direct downstream effector of Cdc42 was revealed to regulate
cell migration and invasion via actin polymerization, pseudopodia
formation, and matrix metalloproteinase (MMP) secretion (22). Inhibition of Cdc42 in
Ras-transformed cells was shown to decrease oncogenic signaling via
Akt, thus contributing to the reduction of cancer malignancy
(23). Therefore, the role of
Cdc42 in cancer has been well established but its mechanism with
the LIS1/CLIP170 complex has yet to be fully elucidated.
In the present study, the expression and
distribution of LIS1 in SACC was first detected. Next, the effects
of LIS1 on the proliferation, apoptosis, invasion and metastasis of
SACC were studied, in vivo and in vitro. On this
basis, the molecular mechanism by which LIS1 interaction with the
+TIP, CLIP-170, regulates the malignant progression of SACC through
the Cdc42 signaling pathway was further explored. The results
provide mechanistic insights into microtubule-associated cellular
processes and their pathological function in SACC, and also provide
a theoretical foundation for a new therapeutic target.
Materials and methods
Human SACC tissues
A total of 30 human SACC tissues and 20 normal and
adjacent tissues were collected at the Second Affiliated Hospital
of Dalian Medical University from June 2017 to September 2019. The
patients diagnosed with SACC were included with no restrictions in
terms of age and sex, but excluded with treatment of radiotherapy
or chemotherapy. The related clinical information is listed in
Table SI. The procedures of the
present study concerning human subjects were approved (approval no.
DY2021-005) by the Medical Ethics Committee of Dalian Medical
University (Dalian, China), and written informed consent was
provided by each patient.
Immunohistochemistry (IHC) and
evaluation
Immunohistochemical staining was performed according
to procedures described in a previous study by the authors
(24). Briefly, 4-µm thick
sections were deparaffinized in dimethylbenzene and rehydrated in
graded ethanol after baking at 65°C for 1 h. Following antigen
retrieval, 3% hydrogen peroxide solution was used to inactivate
endogenous peroxidase for 15 min at room temperature. After being
blocked with goat serum for 30 min at 37°C, tissues were incubated
overnight at 4°C with a primary antibody: LIS1 (1:200; product code
ab2607; Abcam); Ki67 (1:3,000; cat. no. 27309-1-AP; ProteinTech
Group, Inc.); Pan-CK (1:3,000; cat. no. 26411-1-AP; ProteinTech
Group, Inc.) MMP2 (1:200; product code ab86607; Abcam); MMP9
(1:200; product code ab38898; Abcam); and MMP14 (1:100; product
code ab78738; Abcam). The following day, the secondary antibody and
horseradish peroxidase streptavidin (both contained in a kit; 1:1;
cat. no. SAP-9100; ZSGB-BIO, Inc.) were added to the slices for 30
min at 37°C. The sections were then incubated with
3,3′-diaminobenzidine (DAB; ZSGB-BIO, Inc.) as a chromogen
substrate and counterstained with hematoxylin for 3 min at room
temperature. Finally, the slices were dehydrated with graded
ethanol, cleared with xylene and mounted with neutral gum (Solarbio
Science & Technology, Co., Ltd.). For the evaluation, images
were obtained using a phase microscope (Olympus Corporation), and
the integral optical density (IOD) and the positive
immunohistochemical staining region were measured with Image-Pro
Plus 7.0 software (Media Cybernetics, Inc.). Protein expression was
semi-quantitatively calculated and classified based on the
intensity of staining and the percentage of stained cells as
previously described (25).
Cell culture and transfection
The SACC-LM cell line was a kind gift from Professor
Liu (Affiliated Stomatological Hospital of Fudan University,
Shanghai, China) (3) and was
cultured in DMEM/F12 supplemented with 10% fetal bovine serum (FBS;
ScienCell Research Laboratories, Inc.), 100 U/ml penicillin and 100
U/ml streptomycin (Gibco; Thermo Fisher Scientific, Inc.) at 37°C
in a humidified atmosphere with 5% CO2. The plasmids,
including the pc-DNA3.1 vector and plasmids encoding LIS1 and
CLIP170 were constructed by Changsha YouBio Co., Ltd. The small
interfering RNAs (siRNAs) were purchased from Shanghai GenePharma
Co., Ltd. and the target sequences are provided in Table SII. The cells were seeded into
6-well plates at 4×105 cells per well and cultured to
70-80% confluence. Lipofectamine 3000 Transfection Reagent (3.75
µl; cat. no. L3000015; Invitrogen; Thermo Fisher Scientific,
Inc.) was used for cell transfection with 2,500 ng plasmids or 37.5
pmol siRNAs. The transfection system was incubated for 8 h at 37°C.
The subsequent experiment was performed at 24 h after transfection.
In addition, siRNA scramble (si-control, Shanghai GenePharma Co.,
Ltd.) was used as the negative control.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from SACC-LM cells with
RNAiso Plus (TRIzol; Takara Bio, Inc.), and then, reverse
transcription into cDNA was performed using a PrimeScript RT
reagent Kit (Takara Bio, Inc.) according to the manufacturer's
instructions. The primer sequences for RT-qPCR are provided in
Table SIII. GAPDH was used as the
internal control for each experiment. RT-qPCR was performed with
SYBR Premix Ex Taq (Tli RNaseH Plus; Takara Bio, Inc.). The PCR
conditions were as follows: Initial denaturation at 95°C for 30
sec, followed by 40 cycles of denaturation at 95°C for 5 sec,
annealing at 55°C for 30 sec and elongation at 72°C for 30 sec, and
then a final extension at 72°C for 5 min. The data were collected
with a TP800 system (Takara Bio, Inc.) and analyzed using the
2−ΔΔCq comparative method (26).
Cell viability assay
Cell proliferation was assessed using Cell Counting
Kit-8 (CCK-8; APeXBio Technology, LLC). According to the
manufacturer's instructions, cells were seeded in a 96-well plate
at a density of 1×103 cells/well in 100 µl of
culture medium for 0-48 h. CCK-8 solution (10 µl) was added
to each well of the plate, and then, the plate was incubated for 1
h in an incubator at 37°C. Finally, the absorbance was measured at
490 nm using a microplate reader (Shanghai Flash Spectrum
Biotechnology Co., Ltd.).
5′-Ethynl-2′-deoxyuridine (EdU) staining
assay
An EdU assay kit (product code ab219801; Abcam) was
used to assess cell proliferation. Following cell dissociation, the
cells were diluted to 1×106 cells/ml with culture medium
and labeled with EdU solution for 4 h. The cells were then fixed
with 4% paraformaldehyde for 15 min at room temperature,
permeabilized in permeabilization buffer and incubated with EdU
reaction mix at room temperature for 30 min. Finally, the number
and proportion of EdU-incorporated cells were analyzed on a flow
cytometer (FACSVerse; BD Biosciences) at Ex/Em=491/520 nm. The data
were analyzed using Cell Quest software v5.1 (BD Biosciences).
Terminal deoxynucleotidyl transferase
dUTP nick-end labeling (TUNEL) staining
For cell samples, following transfection, the cells
were seeded onto coverslips. After adherence, the cells were fixed
with 4% paraformaldehyde for 20 min at 37°C and then permeabilized
with 0.25% Triton X-100 in PBS for 5 min. For 5 µm paraffin
sections, after deparaffinization and rehydration, the tissues were
permeabilized with 20 µg/ml Proteinase K in PBS for 5 min
according to the instructions of a TUNEL Bright Apoptosis Detection
Kit (cat. no. A112-01; Vazyme Biotech, Co., Ltd.) and a previous
study by the authors (27).
Briefly, the sections were incubated with the Equilibration Buffer
for 30 min at room temperature and then marked with the labeling
mix buffer for 1 h at 37°C. Finally, the sections were mounted with
4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI; 1:1,000; cat.
no. 10236276001; Roche Diagnostics) at 37°C for 10 min, and five
visual fields were randomly selected for observation using
fluorescence microscopy (Olympus Corporation).
Wound healing assay
Following transfection with LIS1 or si-CLIP170,
SACC-LM cells were seeded in 6-well plates at a density of
5×105 cells per well with or without treatment of ML141
(Cdc42 inhibitor; MedChemExpress). A 200-µl pipette tip was
used to create scratches in the cell monolayer when cells were
merged to 75-80% confluence. The cells were then washed with PBS
and cultured in serum-free medium. Images were obtained using a
light microscope (Olympus Corporation) at 0, 12 and 24 h to record
the wound area. Finally, the area of cell migration was quantified
using Image-Pro Plus 7.0 software.
Cell migration and invasion assays
For the cell migration and invasion assays, cells
(5×103/well) in 200 µl serum-free medium were
directly seeded into the upper chambers (pore size, 8 µm;
Corning, Inc.) coated with (at 37°C for 1 h; for the invasion
assay) or without (for the migration assay) Matrigel (Corning,
Inc.). Subsequently, 800 µl medium with 20% FBS was added to
the lower chambers. Following culture for 12 or 24 h in an
incubator, the cells were fixed with 4% paraformaldehyde (Solarbio
Science & Technology, Co., Ltd.) for 20 min at 37°C and then
stained with 1% crystal violet (Beijing Coolibo Technology Co.,
Ltd.) for 10 min at room temperature. The upper surface of the
membranes was wiped with a cotton swab to remove the
non-penetrating cells. Under a phase microscope at a high
magnification (×200), five fields in each sample were randomly
selected, and the number of migrated or invasive cells was
counted.
Immunofluorescence (IF) staining
Invadopodia of tumor cells were detected by
colocalization of cortactin and F-actin according to a previous
study by the authors (28). Cells
were fixed with 4% paraformaldehyde for 20 min at 37°C, washed
three times with PBS, and then permeabilized with 0.25% Triton
X-100 in PBS for 15 min at room temperature. Following washing, the
cells were blocked with 2.5% bovine serum albumin (BSA; Solarbio
Science & Technology, Co., Ltd.) for 1 h at 37°C and stained
with the primary antibody, cortactin (1:1,000; product code
ab81208; Abcam) overnight at 4°C. Following three washes, the cells
were incubated with Alexa Fluor 488 secondary antibody (1:400; cat.
no. A-11034; Thermo Fisher Scientific, Inc.) and
rhodamine-conjugated phalloidin (1:400; product no. 40734ES75;
Yeasen Biotechnology Co., Ltd.) at 37°C for 1 h. Finally, the cell
nuclei were counterstained with DAPI (Roche) for 5 min at room
temperature away from light, and the coverslips were mounted with
fluorescence decay resistance medium (Solarbio Science &
Technology, Co., Ltd.). The sections were observed and images were
captured using a confocal microscope (Leica Microsystems, Inc.).
For evaluation, five random high-power fields were selected, and
the threshold was set based on the staining of the negative
control. Colocalization of cortactin and F-actin was considered to
indicate invadopodia formation. The area of invadopodia per cell
was quantitatively calculated using Image-Pro Plus 7.0
software.
Western blotting
Cells (2×106) were lysed using
radioimmunoprecipitation assay lysis buffer (RIPA; Solarbio Science
& Technology, Co., Ltd.) and assessed with a bicinchoninic acid
protein assay kit (BCA; Beyotime Institute of Biotechnology).
Subsequently, 20 µg protein was loaded into a 10%
polyacrylamide gel, separated by electrophoresis and transferred to
a polyvinylidene difluoride membrane (PVDF; Millipore; Merck KGaA).
The membrane was blocked with 5% non-fat milk for 1 h at room
temperature and incubated with a primary antibody at 4°C overnight:
GAPDH (1:6,000; cat. no. 60004-1-lg; ProteinTech Group, Inc.); LIS1
(1:1,000; product code ab2607; Abcam); CLIP170 (1:1,000; product
code ab61830; Abcam) MMP2 (1:1,000; product code ab86607; Abcam);
MMP9 (1:1,000; product code ab38898; Abcam); MMP14 (1:500; product
code ab78738; Abcam); BCL2 (1:1,500; cat. no. 12789-1-AP;
ProteinTech Group, Inc.); cyclin D1 (1:250; cat. no. sc-8396; Santa
Cruz Biotechnology, Inc.); Cdc42 (1:1,000; product code ab187643;
Abcam) and then the secondary HRP-conjugated antibody (1:2,000;
cat. no. ZB-2305; ZSGB-BIO, Inc.) at room temperature for 1 h.
Finally, the proteins on the membranes were visualized using a
Supersignal West Femto Kit (cat. no. 34094; Thermo Fisher
Scientific, Inc.) and scanned with an imager (ChemiDoc™ Touch
Imaging System; Bio-Rad Laboratories, Inc.). The ImageJ software
(version 1.51j8; National Institutes of Health) was used for
densitometric analysis.
Recombinant lentiviruses and stable cell
lines
Full-length cDNA encoding human LIS1 was cloned into
pLenti-CMV-EGFR-Puro with an HA tag. The LIS1 short hairpin RNA
(sh-LIS1) and shRNA scramble as the negative control (sh-control)
were constructed using GV248-EGFP-Puro. The sh-LIS1 target sequence
was 5′-GCA TGT GGT AGA ATG CAT T-3′, and the sh-control target
sequence was 5′-TTC TCC GAA CGT GTC ACG T-3′. The 3rd generation
system was used to recombine lentiviruses, and 2.5 µg
transfer plasmid, 1.25 µg pMDLg/RRE, 0.75 µg VSVG and
0.5 µg pRSV-Rev were transfected into 293FT cells (cat. no.
R70007; Thermo Fisher Scientific, Inc.) in a 6-cm dish for 8 h at
37°C. The culture medium with recombinant lentiviruses was
collected after 24 and 48 h of transfection. Lentiviruses with
plasmids and sh-RNA were provided by Shanghai GeneChem Co., Ltd.
and used to infect SACC-LM cells at a multiplicity of infection
(MOI) of 10 for 48 h at 37°C. Stable cell lines were selected with
10 µg/ml puromycin (Beijing Coolibo Technology Co., Ltd.)
for 4 weeks. The expression of LIS1 in SACC-LM cells was detected
by qPCR and western blotting.
In vivo animal experiments
A total of 40 female nude mice (BALB/c; 4 weeks old;
weight, 18-20 g) were obtained from Dalian Medical University
Laboratory Animal Center. The animal experiments were approved
(approval no. AEE20017) by the Animal Experimental Ethics Committee
of Dalian Medical University. The 40 mice were housed at 23±2°C and
50±10% humidity, and under a 12-h light/dark cycle with access to
food and water ad libitum. The mice were divided into eight
groups randomly (4 groups for the xenograft models and 4 groups for
the metastasis models). For the xenograft models, 1×107
SACC cells stably transfected with vector, LIS1, sh-control and
sh-LIS1, respectively, in 100 µl PBS were subcutaneously
injected into the right flank of the mice (n=5 per group) under
anaesthetization by an intra-peritoneal injection of sodium
pentobarbital (30 mg/kg). The volume of the tumor (length x
width2/2) (29) and the
weight of the mice were monitored every two days throughout the
study period. In compliance with the Guide for the Animal Care and
Use Committee (ACUC) from the US National Institutes of Health, all
tumor burdens were not >10% body weight of the mice, and the
maximum tumor volume was 751.04 mm3. After 1 month, the
20 mice were euthanized through the intravascular administration of
an overdose of sodium pentobarbital (>100 mg/kg) followed by
cervical dislocation. Euthanasia was confirmed by the loss of vital
signs (respiration and heartbeat cessation), and the harvested
tumors were separated and fixed with 4% paraformaldehyde at 4°C for
48 h for subsequent histochemical staining. For the metastasis
models, 5×106 SACC cells stably transfected with vector,
LIS1, sh-control and sh-LIS1, respectively, in 100 µl PBS
were injected into the mice via the tail vein (n=5 per group) under
anaesthetization by an intraperitoneal injection of sodium
pentobarbital (30 mg/kg). The mice were weighed and observed every
other day. After one month, an IVIS Spectrum system (PerkinElmer,
Inc.) was used to detect tumor metastasis by tracking cells with
stable green fluorescent protein. Living Image Software (version
4.4; PerkinElmer, Inc.) was used to quantify tumor metastasis.
According to the humane endpoints in ACUC, among the 20 metastasis
model mice, 10 mice were euthanized through the intravascular
administration of an overdose of sodium pentobarbital (>100
mg/kg) followed by cervical dislocation in advance due to adverse
complications (loss of body weight >20% and uncoordinated
movement) after tail vein injection of tumor cells, and the other
10 mice were euthanized at two months after injection. The lungs of
the mice were then isolated and fixed for subsequent
immunohistochemical staining.
Co-immunoprecipitation (IP)
Following transfection, the cells in 6-well plates
were lysed with 300 µl IP lysis buffer (Thermo Fisher
Scientific, Inc.) per well at 4°C for 5 min. The lysis buffer was
then centrifuged for 13,000 × g at 4°C for 10 min to obtain the
supernatant. All samples were processed using a Pierce
Co-Immunoprecipitation (Co-IP) Kit (cat. no. 88804; Thermo Fisher
Scientific, Inc.) according to the manufacturer's recommendations.
Cell lysates were incubated with a primary antibody: LIS1 (1:50;
product code ab2607, Abcam); or CLIP170 (1:50; product code
ab61830; Abcam), overnight at 4°C, and then, the antigen and
antibody complexes were combined with 250 µg protein A/G
magnetic beads at room temperature for 1 h. After the beads were
collected by a magnetic stand, the complexes were eluted for 10
min, and the expression of immunoprecipitated proteins was detected
by western blotting.
Statistical analysis
All statistical analyses were performed using
GraphPad Prism 7 (GraphPad Software, Inc.). Unpaired two-tailed
t-test was used for two groups, and one-way analysis of variance
followed by Tukey's post hoc test was used for multiple
comparisons. Kaplan-Meier with log-rank testing was performed for
the survival analysis of the mice. All experiments were performed
at least in triplicate. All data are presented as the mean ± SEM.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Abundant expression of LIS1 in SACC
To evaluate whether LIS1 is a therapeutic target for
a promising SACC treatment strategy, the expression and
distribution of LIS1 in the tissues and adjacent normal tissues
(ANTs) of patients with SACC were examined using IHC staining. As
shown in Fig. 1A, the expression
of LIS1 was markedly increased in most SACC tissues compared with
ANTs. The results also revealed that the positive staining area of
LIS1 was mainly localized in the cytoplasm of cancer cells rather
than in the nucleus. By analyzing the mean density of LIS1, it was
determined that there was significantly higher LIS1 expression in
SACC tissues than in ANTs. SACC consists of two main cell types:
Ductal and modified myoepithelial cells. These two types of cells
exhibited three basic growth patterns: Cribriform, tubular, and
solid. Among the three patterns, it was determined that the
expression level of LIS1 was higher in solid SACC tissues (low
differentiation) than in the other two pattern types (high
differentiation) (Fig. 1B).
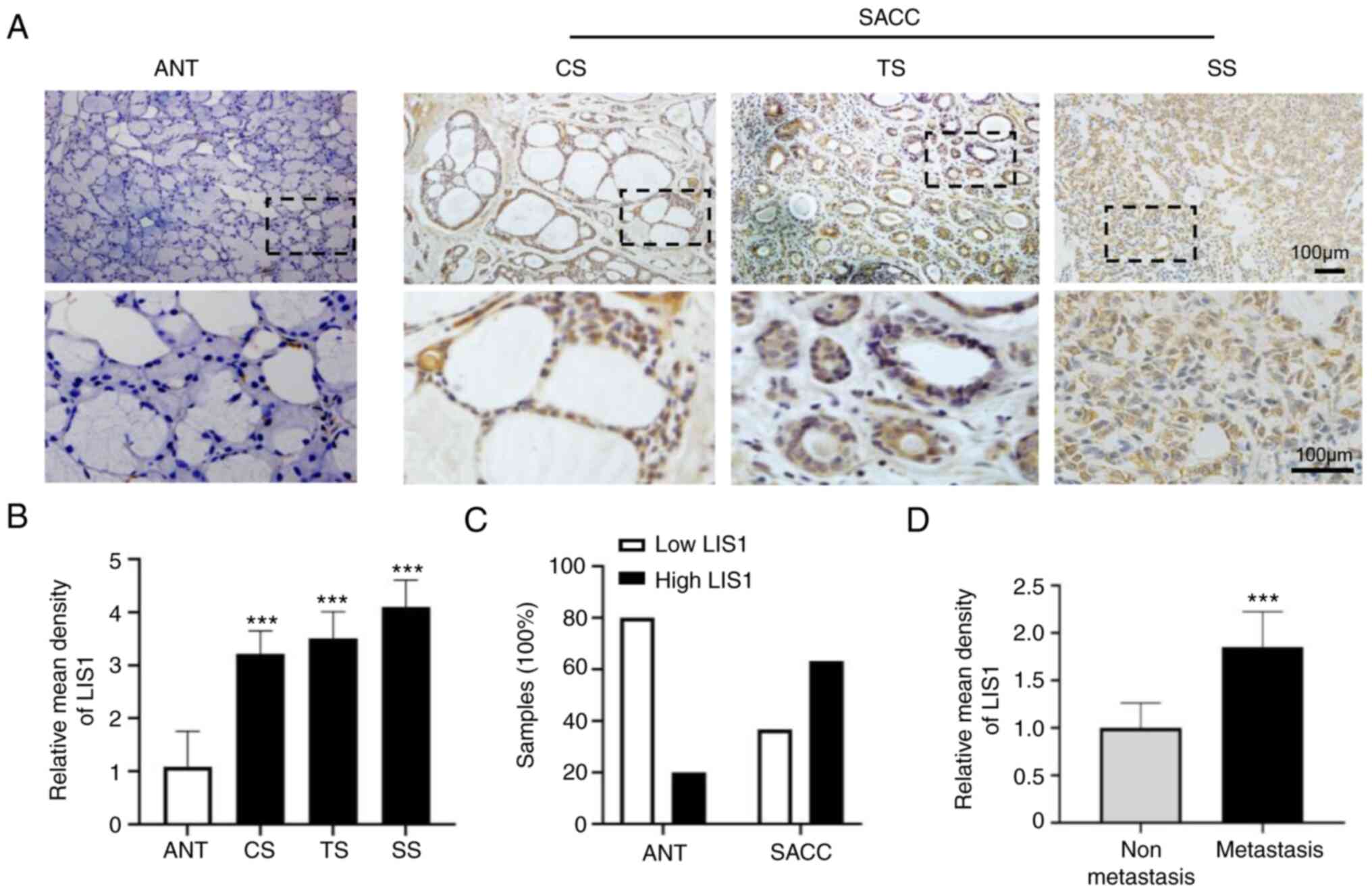 | Figure 1Abundant expression of LIS1 in human
SACC tissues. (A) Immunohistochemical staining of LIS1 in ANT, CS,
TS, and SS tissue. Shown in the bottom row are enlarged images of
the dashed region above. Scale bar, 100 µm. (B) Quantitative
analysis of LIS1 expression in ANT and SACC samples. (C) The
proportion of ANT and SACC samples with low or high LIS1
expression. (D) Quantitative analysis of LIS1 expression in
metastatic and non-metastatic SACC samples. Mean ± SEM;
***P<0.001. LIS1, lissencephaly 1; SACC, salivary
gland adenoid cystic carcinoma; ANT, adjacent normal tissue; CS,
cribriform SACC; TS, tubular SACC; SS, solid SACC. |
In addition, the proportion of high and low LIS1
expression levels were analyzed in ANTs and SACC (Table SI). The results revealed that in
ANTs, the proportion with low and high LIS1 expression was 80 and
20%, respectively. By contrast, low expression of LIS1 accounted
for only 36.67%, and high expression of LIS1 was as high as 63.33%
in SACC samples (Fig. 1C).
Moreover, the expression level of LIS1 was examined in metastatic
and non-metastatic samples and it was determined that the
expression of LIS1 was significantly higher in metastatic samples
than in non-metastatic samples (Fig.
1D). In summary, the aforementioned results indicated that LIS1
was highly expressed in SACC and that its level was positively
associated with the malignancy of SACC.
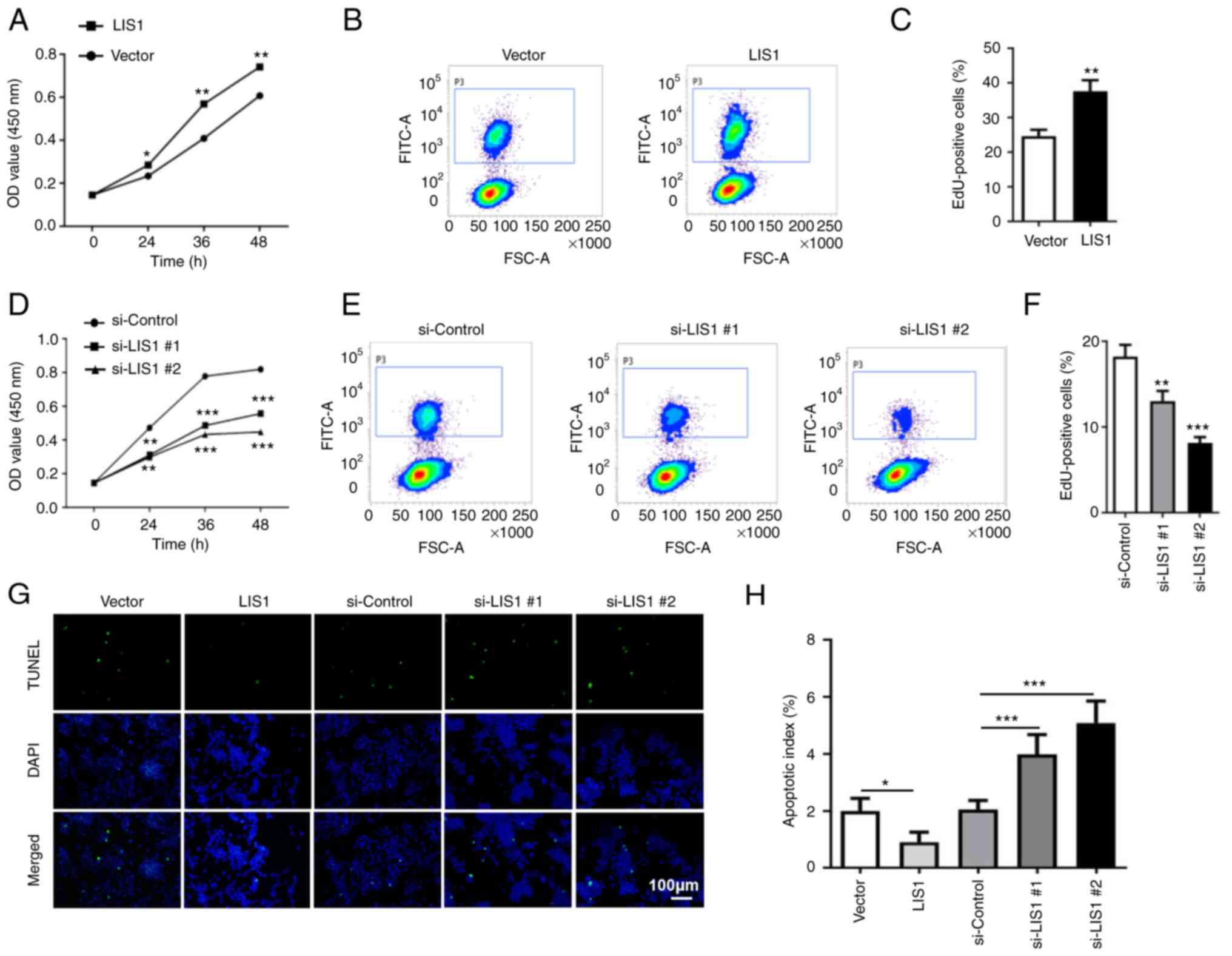
LIS1 promotes proliferation and
anti-apoptosis of SACC-LM cells
To investigate the effect of LIS1 on SACC-LM cells,
LIS1 overexpression plasmids were first constructed for transient
transfection and siRNAs targeting the human LIS1 sequence were
employed to overexpress and knock down LIS1 in SACC-LM cells,
respectively (Fig. S1A-D). As
shown in Fig. 2A and D, CCK-8
assays revealed that overexpression of LIS1 significantly enhanced
the proliferation ability of tumor cells compared with the control
group, while knockdown of LIS1 (si-LIS1) significantly suppressed
cell proliferation. EdU incorporation assays revealed that the
percentage of EdU-positive cells was higher at 48 h after
transfection with the LIS1 plasmid than after transfection with the
vector plasmid (Fig. 2B and C). In
the si-LIS1 group, the percentage of EdU-positive cells was
significantly decreased compared with that in the si-control group
(Fig. 2E and F). The effect of
LIS1 was further explored on the apoptosis of SACC-LM cells using
TUNEL assays at 48 h after transfection. The results showed that
the ratio of apoptotic cells was significantly reduced in tumor
cells with overexpressing LIS1 in contrast to the control group.
Conversely, the ratio of apoptotic cells was increased in tumor
cells with knockdown of LIS1 expression (Fig. 2G and H). Therefore, the expression
level of LIS1 was positively associated with the proliferation of
tumor cells and negatively associated with apoptosis, indicating
that LIS1 can promote tumor cell proliferation and anti-apoptosis
in SACC.
LIS1 enhances migration and invasion of
SACC-LM cells
As the key cellular microtubule dynein, LIS1 is
highly relevant to cell movement (30,31).
To identify the effect of LIS1 on the invasion and metastasis of
SACC cells, wound healing assays were first used to detect
horizontal cell migration. The results revealed that the migration
rate of LIS1 cells was faster than that of vector cells, while that
of si-LIS1 cells was markedly slower than that of si-control cells
(Fig. 3A-C), and the differences
were statistically significant. Moreover, the chemotactic migration
and invasion abilities of SACC-LM cells were assessed using a
Transwell system. As revealed in Fig.
3D-F, the number of cells that penetrated the Matrigel-coated
or uncoated membrane increased following overexpression of LIS1 and
decreased after knockdown of LIS1 expression in SACC-LM cells.
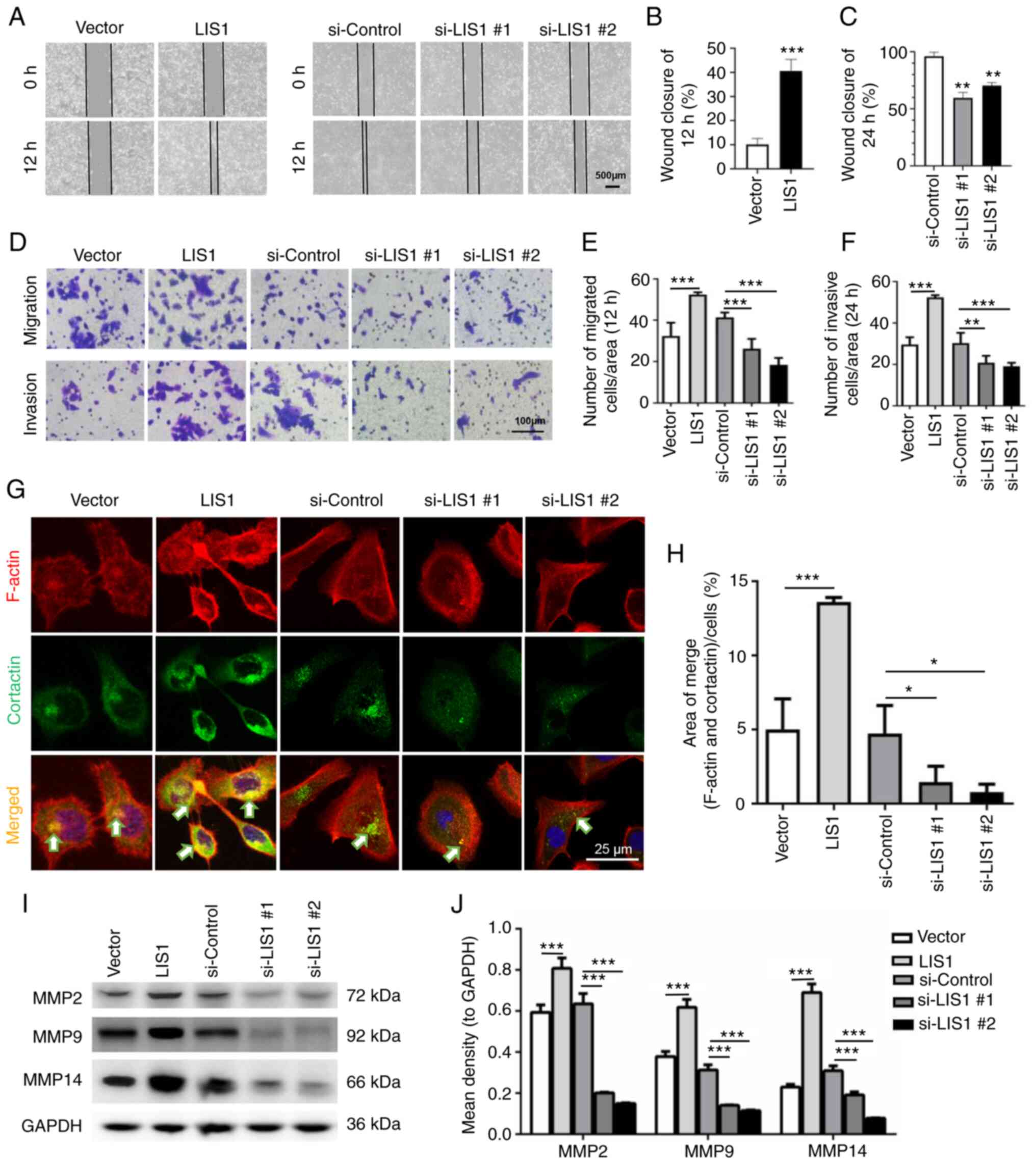 | Figure 3LIS1 promotes the migration and
invasion of SACC-LM cells. (A) Wound healing assays were used to
test the horizontal migration ability of SACC-LM cells with
overexpressed (12 h after scratching) or knocked down (24 h after
scratching) LIS1 expression. Scale bar, 500 µm. (B and C)
The wound closure area of cells following (B) LIS1 overexpression
and (C) LIS1 knockdown was quantitatively analyzed. (D) The effect
of LIS1 overexpression and knockdown on chemotactic migration and
invasion of SACC-LM cells was assessed using Transwell chambers.
For migration, cells were seeded in the upper chambers for 12 h.
For invasion, cells were seeded in the upper chambers coated with
Matrigel for 24 h. Scale bar, 100 µm. (E and F) Quantitative
statistical analysis of the number of penetrated cells after (E) 12
h of migration and (F) 24 h of invasion. (G) IF double staining of
cortactin and F-actin was performed to detect invadopodia in
SACC-LM cells after transfection with the LIS1 plasmid or si-LIS1.
F-actin was conjugated with Alexa Fluor 568, cortactin was
conjugated with Alexa Fluor 488, and the merged images (yellow)
show colocalization of F-actin (red) and cortactin (green)
indicating invadopodia formation. Scale bar, 25 µm. (H)
Quantitative data of the area of invadopodia per cell determined by
colocalization of cortactin and F-actin. (I) MMP2, MMP9, and MMP14
protein expression in SACC-LM cells with overexpressed or knocked
down LIS1 expression was assessed via western blotting. (J)
Quantitative analysis of MMP protein expression. Mean ± SEM;
*P<0.05, **P<0.01 and
***P<0.001. LIS1, lissencephaly 1; SACC, salivary
gland adenoid cystic carcinoma; si-, small interfering RNA; MMP,
matrix metalloproteinase. |
MMPs are enzymes that can degrade the components of
the extracellular matrix (ECM). Studies have demonstrated that MMP2
and MMP9 are secreted into the extracellular space via invadopodia
(32). MMP14 is usually located on
the surface of the invadopodia of tumor cells, degrades the ECM and
is required for the activation of MMP2 and MMP9 (33). Therefore, the formation of
invadopodia is an important factor that promotes tumor cell
migration and invasion. Next, through double IF staining, it was
observed whether LIS1 could regulate invadopodia in SACC-LM cells.
As revealed in Fig. 3G,
colocalization of cortactin and F-actin was considered to indicate
the presence of invadopodia in SACC-LM. The results revealed that
the number of invadopodia in LIS1-overexpressed cells was
significantly increased and that in LIS1-knockdown cells it was
decreased compared with the relative control groups. Quantitative
analysis of the invadopodia-positive area/cells further confirmed
that LIS1 promoted the formation of invadopodia in SACC-LM cells
(Fig. 3H). The expression levels
of MMPs was then detected via western blotting. As expected, LIS1
markedly enhanced the expression of MMP2, MMP9 and MMP14. The
expression of MMP14 in the LIS1 overexpression group was nearly
double that in the vector group. By contrast, the levels of MMPs
were suppressed when the expression of LIS1 was knocked down in
SACC-LM cells (Fig. 3I and J).
These results indicated that LIS1 may regulate the migration and
invasion of tumor cells by mediating the formation of invadopodia
in SACC.
LIS1 promotes tumor growth in a xenograft
mouse model of SACC
To corroborate the role of LIS1 in the promotion of
tumor cells in vivo, SACC xenograft models were established
via subcutaneous injection of SACC-LM cells in nude mice (Fig. 4A). LIS1 overexpression and
knockdown stably transfected cell lines were primarily constructed
with green fluorescent protein via recombinant lentiviruses. The
overexpression and knockdown efficiencies of the stable cell lines
are presented in Fig. S2.
Following injection in the xenograft models, the tumor volumes and
mouse weights were measured every other day (Fig. 4C). The tumor growth curve began to
exhibit different trends at approximately 15 days after injection.
The xenografts with LIS1-overexpressing cells grew faster than
those with vector cells. Subsequently, one month later, the mice
were euthanized, and the tumors were completely isolated. As
revealed in Fig. 4B, the tumor
volumes with high LIS1 expression were larger than those with low
LIS1 expression. Next, the xenografts were weighed and
statistically analyzed, and the results further indicated that the
weight of tumors in the LIS1-overexpressed group was approximately
1.5 times that in the vector group, while the weight of tumors in
the sh-control group was almost twice that in the sh-LIS1 group
(Fig. 4D). Moreover, TUNEL assays
and Ki67 immunohistochemical staining were performed to detect the
tumor tissue sections. The results of the TUNEL assay demonstrated
that knockdown of LIS1 significantly induced tumor cell apoptosis
in vivo, and the number of apoptotic tumor cells was
approximately 5 times that in the sh-control group. Compared with
the vector group, the number of Ki67-positive cells was
significantly increased in the LIS1 overexpression group (Fig. 4E-G). Collectively, the in
vivo results demonstrated that LIS1 promoted the proliferation
and inhibited the apoptosis of SACC cells, which was consistent
with the in vitro results.
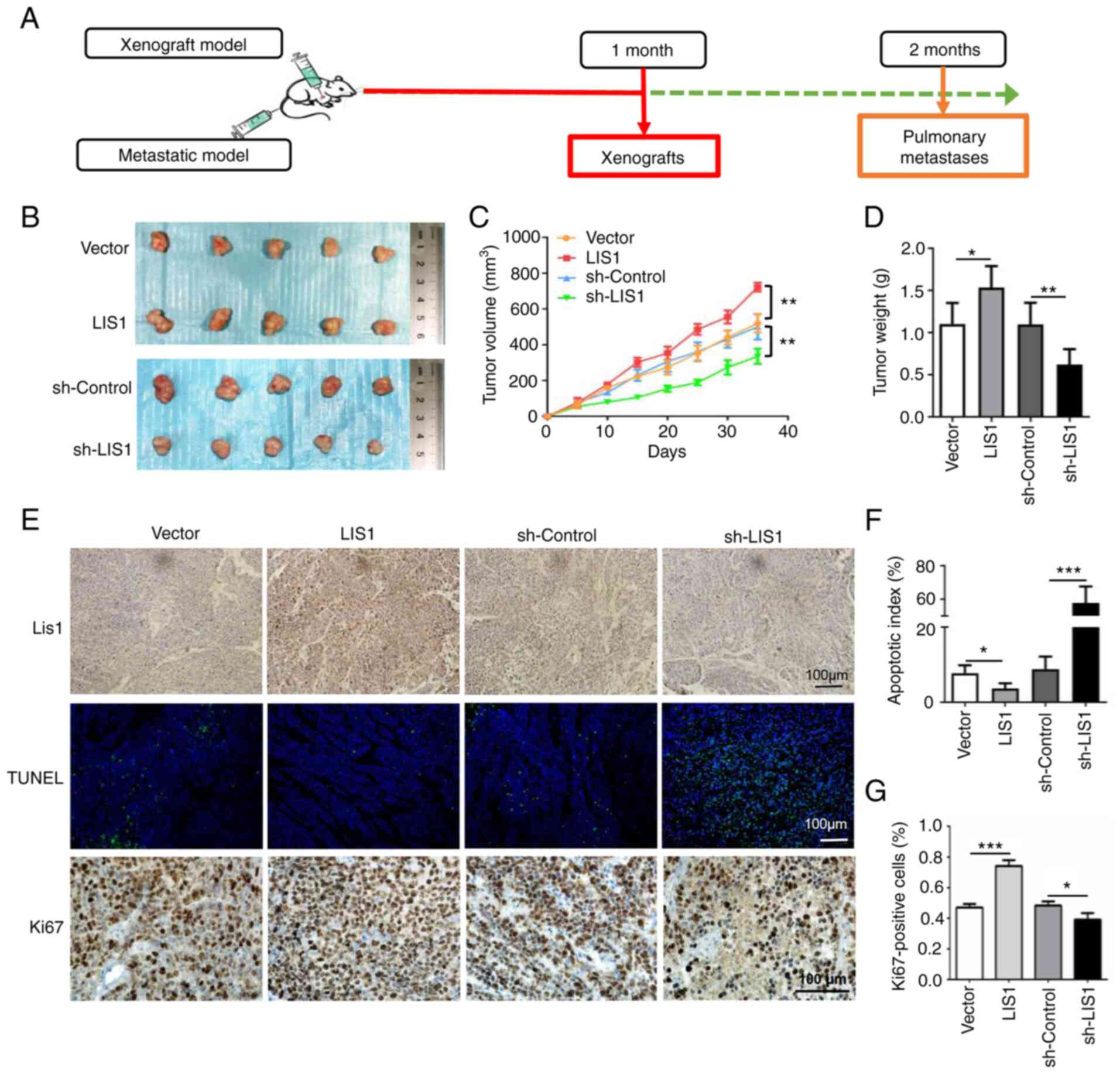 | Figure 4LIS1 enhances tumor growth in a SACC
xenograft mouse model. (A) Schematic representation of the animal
model establishment. A total of 40 nude mice were randomly divided
into two sections, one for nude mouse xenograft tumors and the
other for metastasis models. For xenograft models, 1×107
cells in 100 µl PBS were subcutaneously injected into the
right flank of mice (n=5 per group). Subsequently, one month later,
the xenograft tumors were removed. (B) Images of xenograft tumors
harvested from nude mice following injection of tumor cells
(vector, LIS1 overexpression, sh-Control and sh-LIS1 cells). (C)
The volume of xenograft tumors was measured every five days, and
the changes in the tumor weight of mice wered recorded. (D) The
weight of xenograft tumors was measured when the xenografts were
removed 35 days after injection of the tumor cells. (E) A TUNEL
assay was performed to assess cell apoptosis in the tumor tissues,
and the expression of Ki67 in xenograft tumors was detected by
immunohistochemistry. Scale bar, 100 µm. (F) Quantitative
statistical analysis of TUNEL assay results. (G) Quantitative
statistics for the percentage of Ki67-positive cells. Mean ± SEM;
*P<0.05, **P<0.01 and
***P<0.001. LIS1, lissencephaly 1; SACC, salivary
gland adenoid cystic carcinoma; PBS, phosphate-buffered saline;
sh-, short hairpin RNA; TUNEL, terminal deoxynucleotidyl
transferase dUTP nick-end labeling. |
LIS1 enhances the metastatic potential of
SACC cells in vivo
To further clarify whether LIS1 can promote the
metastasis of SACC cells in vivo, a SACC metastasis model
was constructed using mice. Following injection of SACC-LM cells
into nude mice via the tail vein, the survival rate of the mice was
continuously monitored for two months. The results showed that the
mortality of mice in the LIS1 overexpression group was the highest,
and this was the first experimental group with the death of a
mouse. In the LIS1 overexpression group, the first mouse succumbed
at approximately one month after injection, and the mouse mortality
was as high as 80% at two months. Among the four groups, the
survival rate was the highest in the sh-LIS1 group, and the first
mouse death occurred approximately one and a half months after
injection (Fig. 5A). Therefore,
the survival curve revealed that reducing the level of LIS1 in SACC
cells significantly prolonged the life of mice with metastatic
SACC. Because the constructed stable cell line exhibited green
fluorescence, live animal imaging was performed one month after the
injection to observe the metastasis of tumor cells in each group.
As shown in Fig. 5B, after tail
vein injection of SACC-LM cells, the tumor cells mainly
metastasized to the lungs of mice. The fluorescence intensity in
the LIS1 overexpression group was significantly stronger than that
in the vector group, while the fluorescence intensity in the
sh-LIS1 group was significantly weaker than that in the sh-control
group. A total of two months after establishment of the model, the
lung tissue of mice was completely isolated for observation. As
revealed in Fig. 5C, in the LIS1
overexpression group, numerous metastatic and necrotic areas were
found in the lungs, and the number of metastatic nodules in the
lungs was the highest. The morphology of the lung lobes was
destroyed, and the color of the lungs was gray. In the sh-LIS1
group, the number of lung metastases was decreased compared with
the sh-control group, and the morphology and color of the lungs
were normal.
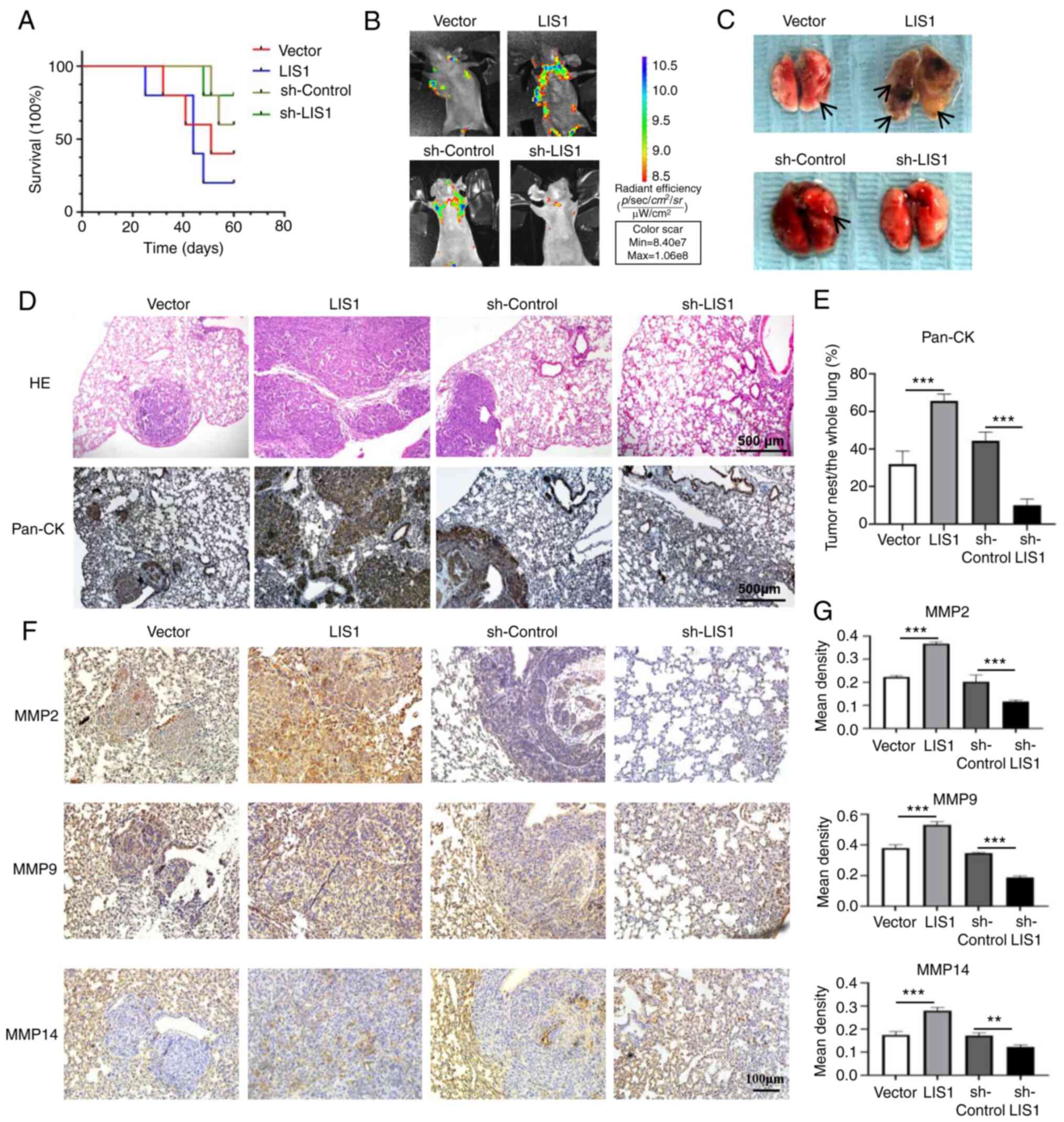 | Figure 5LIS1 enhances tumor metastasis in a
SACC metastasis nude mouse model. For the metastatic models,
5×106 cells in 100 µl PBS were injected into the
nude mice via the tail vein (n=5 per group). (A) Kaplan-Meier
survival curve of the mice after injection of LIS1 overexpression
or knockdown SACC cells via the tail vein. (B) The Living Image
system was used to observe metastasis 30 days after intravenous
injection of tumor cells. (C) Representative images of the lungs
isolated from metastatic nude mice. (D) The lungs from SACC-LM
metastatic model mice were stained with H&E, and
immunohistochemical staining to detect Pan-CK was performed. Scale
bar, 500 µm. (E) Quantitative analysis of the percentage of
tumor area in the whole lung tissue according to
immunohistochemical staining of Pan-CK. (F) The expression levels
of MMP2, MMP9, and MMP14 in the lungs of the SACC-LM metastatic
model mice were detected by immunohistochemical staining. (G)
Quantitative analysis of immunohistochemical staining of MMPs.
Scale bar, 100 µm. Mean ± SEM; **P<0.01 and
***P<0.001. LIS1, lissencephaly 1; SACC, salivary
gland adenoid cystic carcinoma; PBS, phosphate-buffered saline;
H&E, hematoxylin and eosin; MMP, matrix metalloproteinase; sh-,
short hairpin RNA. |
After embedding and sectioning, hematoxylin and
eosin (H&E) and Pan-CK IHC staining of mouse lung tissue was
performed. Compared with the vector group, the tumor cells in the
LIS1 overexpression group exhibited severe lung metastasis, forming
multiple large cancer nests. Compared with the sh-control group,
the cancer nests in lung tissue in the sh-LIS1 group were rare, and
the pulmonary alveolar structure was normal (Fig. 5D and E). Next, the expression of
MMPs in mouse lung tissues was detected via IHC staining. As
revealed in Fig. 5F and G, the
levels of MMP2, MMP9 and MMP14 in the LIS1 overexpression group
were higher than those in the vector group, while the levels of
MMP2, MMP9 and MMP14 in the sh-LIS1 group were lower than those in
the sh-control group, and the differences were significant. These
results indicated that LIS1 significantly regulated the expression
of MMPs and promoted lung metastasis of SACC tumor cells in
vivo.
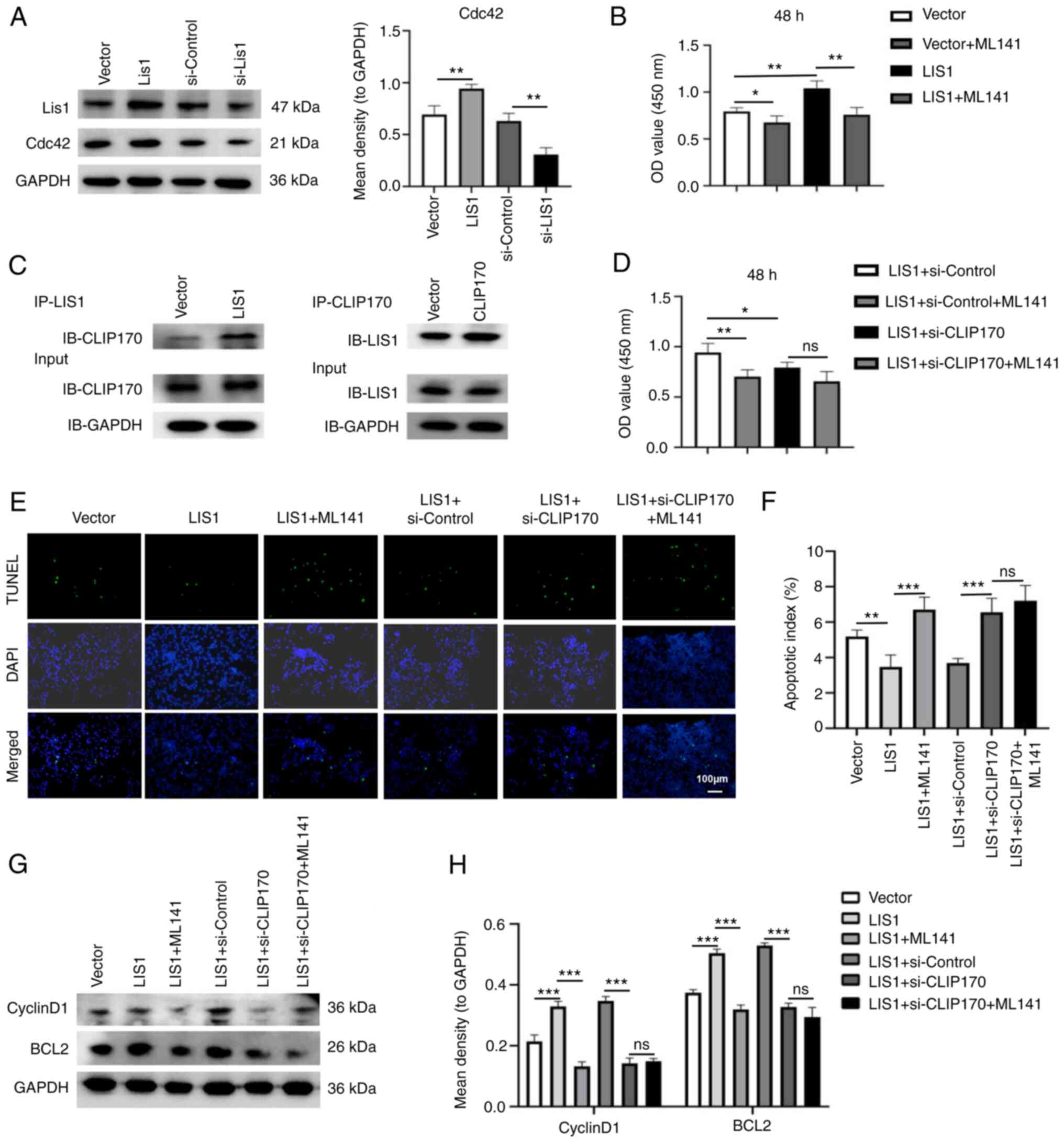
LIS1 interacts with CLIP-170 to regulate
the proliferation and apoptosis of SACC cells through the Cdc42
signaling pathway
Cdc42 is a well-known member of the Ras homolog
(Rho) family. It regulates crucial cellular processes, including
the cell cycle, and cell cytoskeleton organization (34). To investigate whether LIS1 can
regulate the Cdc42 pathway, the expression of Cdc42 in SACC cells
was assessed using western blotting. The results revealed that the
expression of LIS1 was positively associated with Cdc42 in SACC-LM
cells (Fig. 6A). Following
overexpression of LIS1, the inhibitor of Cdc42 was added in SACC-LM
cells. The results of the CCK-8 assay revealed that the
proliferation ability of tumor cells was significantly decreased
compared with the LIS1 overexpression group, indicating that Cdc42
may be a pivotal downstream factor of LIS1 in SACC-LM cells
(Fig. 6B). Studies have shown that
the WD-40 repeat domain of the LIS1 protein can interact with the
second zinc finger motif at the COOH-terminus of CLIP170 to
regulate the dynamic balance of microtubules and microfilaments,
thus playing an essential role in cell division and cell migration
(13,16). To verify whether LIS1 and CLIP170
can interact with each other in SACC-LM cells, Co-IP was performed.
When LIS1 was overexpressed in SACC-LM cells, the level of CLIP170
expressed with IP-LIS1 was significantly higher than that in the
vector group, and vice versa, indicating that LIS1 and CLIP170
could bind together in SACC-LM cells (Fig. 6C). Next, the siRNA-CLIP170 sequence
was used to knock down CLIP170 in SACC-LM cells (Fig. S1E and F). Using CCK-8 and TUNEL
assays, when the expression of CLIP170 was knocked down after
overexpression of LIS1, the proliferation of tumor cells was
significantly decreased, and the apoptosis of tumor cells was
increased compared with the LIS1 overexpression group.
Interestingly, when Cdc42 inhibitor was added in SACC-LM cells with
double-transfection of LIS1 and si-CLIP170, the proliferation of
cells was not significantly inhibited, and the apoptosis level was
also not increased compared with the LIS1 and si-CLIP170
double-transfected group, suggesting that LIS1 needs to interact
with CLIP170 to activate Cdc42, so as to enhance the proliferation
and anti-apoptosis ability of tumor cells (Fig. 6D-F). Moreover, the expression
levels of the proliferation-related gene cyclin D1 and the
apoptosis-related gene BCL2 were further detected via western
blotting (Fig. 6G and H).
Overexpression of LIS1 in SACC cells upregulated cyclin D1 and
BCL2, while knockdown of CLIP170 after overexpression of LIS1 in
tumor cells significantly reduced the levels of cyclin D1 and BCL2.
However, compared with the LIS1 and si-CLIP170 double-transfection
group, the expression of cyclin D1 and BCL2 in tumor cells was not
significantly decreased in the LIS1 + si-CLIP170 + ML141 group.
These results demonstrated that LIS1 interacted with CLIP170 to
form the LIS1/CLIP170 protein complex, which could activate the
Cdc42 signaling pathway to promote the proliferation and
anti-apoptosis of SACC cells.
LIS1 interacts with CLIP170 to modulate
metastasis of SACC cells through the Cdc42 signaling pathway
Numerous studies have demonstrated that Cdc42 is
closely related to cell migration. Cao et al (35) revealed that Cdc42 regulates the
invasive ability of breast cancer cells by regulating the formation
of lamellipodia and expression of MMPs. First, wound healing and
Transwell assays were used to verify whether the LIS1/CLIP170
complex could regulate the migration and invasion of SACC cells via
the Cdc42 pathway. The results revealed that compared with the LIS1
overexpression group, knockdown of CLIP170 after overexpression of
LIS1 in SACC cells significantly reduced the migration and invasion
of tumor cells, while the Cdc42 inhibitor could not significantly
decrease the migration and invasion of tumor cells in the
double-transfection of LIS1 and si-CLIP170 group (Fig. 7A-D). In addition, through double IF
staining, changes in the formation of invadopodia were detected in
SACC cells after treatment. The results showed that when CLIP170
was knocked down after overexpression of LIS1, the number of
invadopodia formed by tumor cells was significantly reduced.
Concurrently, it was determined that the number of invadopodia in
LIS1 + si-CLIP170 + ML141 group was not less than that in the LIS1
+ si-CLIP170 group (Fig. 7E and
F). Moreover, the expression of MMP proteins was assessed via
western blotting, and the results were consistent with the
aforementioned results (Fig. 7G and
H). Therefore, these results demonstrated that LIS1 may
interact with CLIP170 to form the LIS1/CLIP170 protein complex and
then activate Cdc42 to regulate the expression of MMPs by
regulating the formation of invadopodia in SACC cells, thus
promoting degradation of the ECM to enhance metastasis.
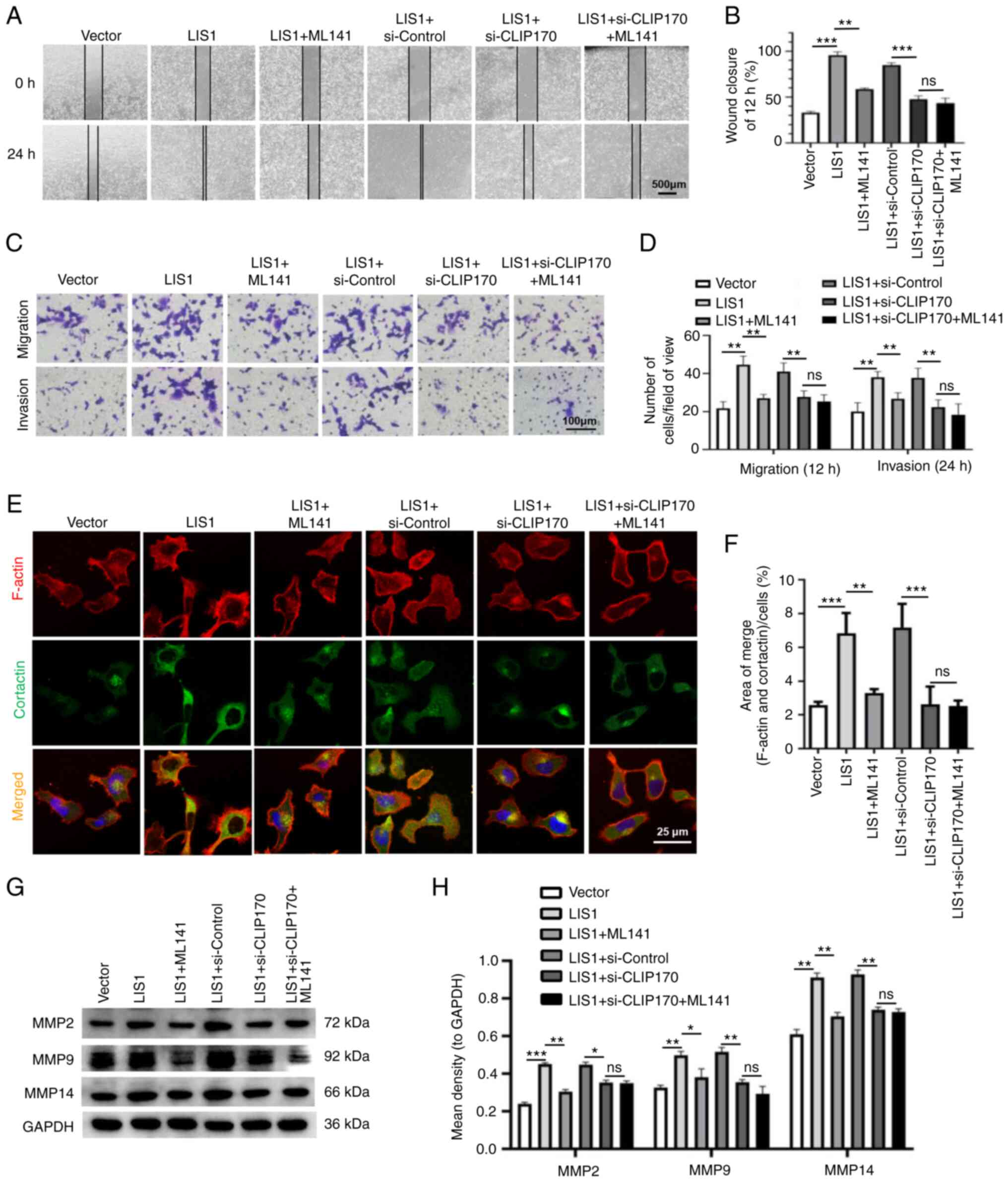 | Figure 7Protein complex LIS1/CLIP170
regulates SACC-LM cell migration and invasion through the Cdc42
signaling pathway. Tumor cells were transfected with vector or LIS1
and then transfected with si-control or si-CLIP170, and ML141, as
an inhibitor of Cdc42, was added into SACC-LM cells. (A) Wound
healing assays were performed to assess the horizontal migration
ability of SACC-LM cells. Scale bar, 500 µm. (B)
Quantitative statistical analysis of the wound healing assay
results. (C) The chemotactic migration and Matrigel invasion of
SACC-LM cells were measured using Transwell assays. Scale bar, 100
µm. (D) Quantitative analysis of the migrated cells at 12 h
and the cells that invaded through the Matrigel membrane at 24 h.
(E) Invadopodia formation in SACC-LM cells was detected by IF
double staining. The merged images (yellow) show the colocalization
of cortactin (green) and F-actin (red). Scale bar, 25 µm.
(F) Quantitative statistics of the area of invadopodia per cell
determined by the colocalization of cortactin and F-actin. (G)
MMP2, MMP9, and MMP14 protein levels in SACC-LM cells were measured
via western blotting. (H) Quantitative analysis of MMP protein
levels. Mean ± SEM; *P<0.05, **P<0.01
and ***P<0.001. LIS1, lissencephaly 1; CLIP170,
cytoplasmic linker protein 170; SACC, salivary gland adenoid cystic
carcinoma; Cdc42, cell division control protein 42 homolog; MMP,
matrix metalloproteinase; si-, small interfering RNA; ns, not
significant. |
Discussion
Several studies have revealed that LIS1 is a key
node protein that participates in several pathways, including
association with the molecular motor cytoplasmic dynein, the reelin
signaling pathway, and the platelet-activating factor pathway
(11,12,36).
Recently, the role of LIS1 in tumors has begun to attract
attention. A previous study revealed that LIS1 can positively
regulate the migration and invasion of neuroblastoma and glioma
(37). In non-neurological tumors,
the expression level of LIS1 was also revealed to be upregulated
and promote the proliferation and metastasis of tumor cells
(11). In SACC, there are three
histological patterns (cribriform, tubular, and solid). One of the
important prognostic factors of SACC is the percentage of solid
tumor components. The larger the percentage of solid tumors, the
lower the 15-year survival rate of patients with SACC (38). In the present study, it was
demonstrated that LIS1 was highly expressed in SACC tissues. Among
the three subtypes of SACC, the level of LIS1 was the highest in
the solid type with low differentiation. In addition, LIS1
expression was significantly upregulated in metastatic SACC
compared with non-metastatic SACC. Therefore, these clinical
results indicated that LIS1 plays a critical role in the malignant
progression of SACC, suggesting that LIS1 may be a potential
therapeutic target.
LIS1 is part of a complex that interacts with
diverse cortical factors and centrosomal proteins at kinetochores
on chromosomes, mitotic spindles and the cell cortex, and has been
implicated in regulation of mitotic spindles and chromosome
segregation during mitosis (39,40).
There is a close correlation between LIS1 and cancer stem cells
(CSCs). The main characteristics of CSCs are relatively unlimited
division and proliferation, multidirectional differentiation
potential and self-renewal ability (41). Brehar et al (42) revealed that the expression of the
LIS1 gene in CD133-positive glioblastoma cells was 60 times
higher than that in CD133-negative glioblastoma cells. In addition,
Wang et al (43) reported
that LIS1 promoted endogenous stem cell regeneration in
submandibular salivary glands, and this process was mainly actioned
through the interaction of LIS1 and stem cell markers (SOX2 and
Sca-1). The results of the present study indicated that
overexpression of LIS1 significantly enhanced the proliferation and
inhibited the apoptosis of SACC-LM cells in vitro and in
vivo, while knockdown of LIS1 expression decreased the
proliferation of tumor cells and increased the level of apoptosis.
Therefore, LIS1 may control cell division and cell cycle
progression by modulating mitotic spindle assembly or stem cell
gene expression, thereby regulating the proliferation and apoptosis
of tumor cells; the specific mechanism in SACC needs to be further
studied.
Another biological feature of malignant tumors is
high recurrence and metastasis rates. In a previous study of 130
cases of SACC, the recurrence rate was 43.8% and the distant
metastasis rate was 28.5%, which is the main cause for the poor
prognosis of patients with SACC (44). Acting as a microtubule-associated
protein, LIS1 was the first gene related to neuronal
migration disorders that was cloned in humans (45). LIS1 regulates leading edge dynein,
potentially serving as a holdfast for microtubules that invade the
actin-network at the front of the cell and thus regulating the
formation of lamellipodial protrusions (46). In the process of tumor metastasis,
cancer cells must migrate into the ECM and infiltrate the blood and
lymphatic vessels (32). MMPs are
critical proteases that degrade the ECM and thus the most important
enzyme system in regulating the dynamic balance of the ECM. The
main MMP family is located in the invadopodia of cancer cells
(33). Therefore, the formation of
invadopodia plays an essential role in degradation of the ECM. In
the present study, in vitro experiments were first performed
to verify that LIS1 could promote the migration and invasion
abilities of SACC-LM cells. Through double fluorescence staining,
it was determined that LIS1 could regulate the formation of
invadopodia and was positively correlated with the expression of
MMPs, which suggests that LIS1 may promote SACC invasion and
metastasis through ECM degradation mediated by the formation of
invadopodia in tumor cells. It was further verified in vivo
that overexpression of LIS1 significantly promoted lung metastasis
of SACC, while knockdown of LIS1 significantly inhibited tumor
metastasis and prolonged the life of mice. The in vivo
results provide strong evidence of the effectiveness and
feasibility of targeting LIS1 in the treatment of SACC. Prior
literature has focused on the involvement of the MYB pathway in the
ACC mutational landscape, and there is significant evidence for its
role in ACC tumorigenesis (47,48).
A previous study reported that a protein complex composed of the
basic helix-loop-helix (bHLH) associated with MYB transcription
factors and the WD40 repeat protein initiates multiple cellular
differentiation pathways (49).
LIS1 is a WD40 repeat scaffold protein, which interacts with
components of the cytoplasmic dynein motor complex to regulate
dynein-dependent cell motility (41,50).
Therefore, whether LIS1 interacts with MYB to participate in the
malignant progression of SACC needs further study. It has been
demonstrated that LIS1 and CLIP170 colocalize at kinetochores and
tips of astral microtubules in interphase cells, nuclei of prophase
cells, and cortical sites contacting astral microtubules in mitotic
cells (13). In addition, a
previous study indicated that Cdc2-mediated phosphorylation of
CLIP170 was important for its localization at microtubule-plus ends
in the G2 phase and during G2/M transition, which is related to
CLIP170 function during cell cycle progression (20). CLIP170 depletion led to centrosome
reduplication after the S phase, suggesting that CLIP170 may be a
factor that controls cell mitosis by regulating the number of
centrosomes (20). The results of
the present study provided evidence that LIS1 and CLIP170 could
form a protein complex in SACC-LM cells. Furthermore, it was
determined that the expression of LIS1 was positively associated
with the level of Cdc42, and LIS1 could regulate the proliferation
of SACC-LM cells through activation of the Cdc42 pathway.
Interestingly, the results demonstrated that inhibition of Cdc42 in
SACC-LM cells double-transfected with LIS1 and si-CLIP170 hardly
reduced the proliferation and anti-apoptosis of tumor cells,
suggesting that the interaction between LIS1 and CLIP170 may
coordinate the tumor cell cycle, and then promote the proliferation
and anti-apoptosis of SACC cells via the Cdc42 signaling pathway.
Moreover, when the expression of CLIP170 was knocked down after
overexpression of LIS1, it was determined that the invasion and
migration ability of SACC-LM cells treated with Cdc42 inhibitor was
not significantly suppressed, indicating that the activation of
Cdc42 by LIS1 in promoting tumor cell invasion and metastasis was
also regulated through its interaction with CLIP170. A previous
study revealed that the formation of the lamellipodia necessary for
cell invasion is regulated by Cdc42, while CLIP170 can form a
complex with Rac1/Cdc42, IQGAP1 and kinesin, which regulates the
invasion ability of breast cancer cells by mediating the formation
of invadopodia (51). The
activation of Cdc42 participates in invadopodia structures and is
responsible for the production or activation of MMPs (17,52,53).
In the present study, it was revealed that the interaction between
LIS1 and CLIP170 regulated the formation of invadopodia and
degradation of the ECM through activation of the Cdc42 signaling
pathway, leading to promotion of SACC cell invasion and metastasis.
In a following study, the signaling pathway of LIS1/CLIP170/Cdc42
will be further verified in clinical samples and the specific
mechanism by which Cdc42 regulates the malignant progression of
SACC will be explored.
In conclusion, the in vivo and in
vitro experimental results demonstrated that LIS1 promoted
tumor cell proliferation, inhibited tumor cell apoptosis and
increased SACC invasion and metastatic abilities. Concurrently, it
was also revealed that LIS1 interacts with CLIP170 to form a
protein complex, which regulates tumor cell division and the
formation of invadopodia via the Cdc42 signaling pathway. It is
suggested that LIS1/CLIP170 may coordinate the polymerization and
dynamic balance of microtubules by activation of the small Rho
GTPase Cdc42, thus promoting proliferation and metastasis. The data
of the present study provide a novel mechanism underlying the
malignant development of tumors and suggest LIS1/CLIP170/Cdc42 as a
new therapeutic target for treatment of SACC.
Supplementary Data
Availability of data and materials
Publicly available datasets analyzed in the present
study can be found at NCBI GenBank (Accession number: NM_000430;
NM_198240). All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LL, ZW and NK performed all the experiments and
contributed to the collection of data. JL, DJ and LW contributed to
data analysis and interpretation. NK, DJ and LW were involved in
useful discussions and revised the manuscript. FW contributed to
partial conception and design, data analysis and interpretation,
and drafting of the manuscript. LG contributed to conception and
design, data analysis and interpretation, as well as drafting and
editing of the manuscript. LG and FW confirm the authenticity of
all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The procedures of the present study concerning human
subjects were approved (approval no. DY2021-005) by the Medical
Ethics Committee of Dalian Medical University (Dalian, China).
Written informed consent was obtained from all patients after
detailed explanation of the procedure and the intended use of the
tissues. The animal experiments were approved (approval no.
AEE20017) by the Animal Experimental Ethics Committee of Dalian
Medical University. All experiments were performed according to
standard protocols, in compliance with the Guide of the Ethics
Committee of Dalian Medical University and were conducted following
the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by grants from the Natural
Science Foundation of China (grant no. 81802706 to LG; and grant
no. 81771032 to FW), the Scientific Foundation of the Department of
Education of Liaoning Province (grant no. LZ2020035 to LG), and the
Natural Science Foundation of Liaoning Province (grant no.
2021-MS-293 to LG).
References
|
1
|
Laurie SA, Ho AL, Fury MG, Sherman E and
Pfister DG: Systemic therapy in the management of metastatic or
locally recurrent adenoid cystic carcinoma of the salivary glands:
A systematic review. Lancet Oncol. 12:815–824. 2011. View Article : Google Scholar
|
|
2
|
Su BH, Qu J, Song M, Huang XY, Hu XM, Xie
J, Zhao Y, Ding LC, She L, Chen J, et al: NOTCH1 signaling
contributes to cell growth, anti-apoptosis and metastasis in
salivary adenoid cystic carcinoma. Oncotarget. 5:6885–6895. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kong J, Tian H, Zhang F, Zhang Z, Li J,
Liu X, Li X, Liu J, Li X, Jin D, et al: Extracellular vesicles of
carcinoma-associated fibroblasts creates a pre-metastatic niche in
the lung through activating fibroblasts. Mol Cancer. 18:1752019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Al Absi A, Wurzer H, Guerin C, Hoffmann C,
Moreau F, Mao X, Brown-Clay J, Petrolli R, Casellas CP, Dieterle M,
et al: Actin cytoskeleton remodeling drives breast cancer cell
escape from natural killer-mediated cytotoxicity. Cancer Res.
78:5631–5643. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Prahl LS, Bangasser PF, Stopfer LE, Hemmat
M, White FM, Rosenfeld SS and Odde DJ: Microtubule-based control of
motor-clutch system mechanics in glioma cell migration. Cell Rep.
25:2591–2604.e8. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sharma P, Alsharif S, Fallatah A and Chung
BM: Intermediate filaments as effectors of cancer development and
metastasis: A focus on keratins, vimentin, and nestin. Cells.
8:4972019. View Article : Google Scholar :
|
|
7
|
Jung YS, Wang W, Jun S, Zhang J,
Srivastava M, Kim MJ, Lien EM, Shang J, Chen J, McCrea PD, et al:
Deregulation of CRAD-controlled cytoskeleton initiates mucinous
colorectal cancer via β-catenin. Nat Cell Biol. 20:1303–1314. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peng JM, Bera R, Chiou CY, Yu MC, Chen TC,
Chen CW, Wang TR, Chiang WL, Chai SP, Wei Y, et al: Actin
cytoskeleton remodeling drives epithelial-mesenchymal transition
for hepatoma invasion and metastasis in mice. Hepatology.
67:2226–2243. 2018. View Article : Google Scholar
|
|
9
|
DeSantis ME, Cianfrocco MA, Htet ZM, Tran
PT, Reck-Peterson SL and Leschziner AE: Lis1 has two opposing modes
of regulating cytoplasmic dynein. Cell. 170:1197–1208.e12. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Allanson JE, Ledbetter DH and Dobyns WB:
Classical lissencephaly syndromes: Does the face reflect the brain?
J Med Genet. 35:920–923. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lo FY, Chen HT, Cheng HC, Hsu HS and Wang
YC: Overexpression of PAFAH1B1 is associated with tumor metastasis
and poor survival in non-small cell lung cancer. Lung Cancer.
77:585–592. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang R, Chen Y, Tang C, Li H, Wang B, Yan
Q, Hu J and Zou S: MicroRNA-144 suppresses cholangiocarcinoma cell
proliferation and invasion through targeting platelet activating
factor acetylhydrolase isoform 1b. BMC Cancer. 14:9172014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Coquelle FM, Caspi M, Cordelières FP,
Dompierre JP, Dujardin DL, Koifman C, Martin P, Hoogenraad CC,
Akhmanova A, Galjart N, et al: LIS1, CLIP-170's key to the
dynein/dynactin pathway. Mol Cell Biol. 22:3089–3102. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jakka P, Bhargavi B, Namani S, Murugan S,
Splitter G and Radhakrishnan G: Cytoplasmic linker protein CLIP170
negatively regulates TLR4 signaling by targeting the TLR adaptor
protein TIRAP. J Immunol. 200:704–714. 2018. View Article : Google Scholar
|
|
15
|
Sun X, Li D, Yang Y, Ren Y, Li J, Wang Z,
Dong B, Liu M and Zhou J: Microtubule-binding protein CLIP-170 is a
mediator of paclitaxel sensitivity. J Pathol. 226:666–673. 2012.
View Article : Google Scholar
|
|
16
|
Gao L, Xue B, Xiang B and Liu KJ: Arsenic
trioxide disturbs the LIS1/NDEL1/dynein microtubule dynamic complex
by disrupting the CLIP170 zinc finger in head and neck cancer.
Toxicol Appl Pharmacol. 403:1151582020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Murphy NP, Binti Ahmad Mokhtar AM, Mott HR
and Owen D: Molecular subversion of Cdc42 signalling in cancer.
Biochem Soc Trans. 49:1425–1442. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Murphy NP, Mott HR and Owen D: Progress in
the therapeutic inhibition of Cdc42 signalling. Biochem Soc Trans.
49:1443–1456. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chernichenko N, Omelchenko T, Deborde S,
Bakst RL, He S, Chen CH, Gusain L, Vakiani E, Katabi N, Hall A and
Wong RJ: Cdc42 mediates cancer cell chemotaxis in perineural
invasion. Mol Cancer Res. 18:913–925. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang X, Li H, Liu XS, Deng A and Liu X:
Cdc2-mediated phosphorylation of CLIP-170 is essential for its
inhibition of centrosome reduplication. J Biol Chem.
284:28775–28782. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fukata M, Watanabe T, Noritake J, Nakagawa
M, Yamaga M, Kuroda S, Matsuura Y, Iwamatsu A, Perez F and Kaibuchi
K: Rac1 and Cdc42 capture microtubules through IQGAP1 and CLIP-170.
Cell. 109:873–885. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maldonado MDM and Dharmawardhane S:
Targeting Rac and Cdc42 GTPases in cancer. Cancer Res.
78:3101–3111. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stengel KR and Zheng Y: Essential role of
Cdc42 in Ras-induced transformation revealed by gene targeting.
PLoS One. 7:e373172012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bai J, Li L, Kou N, Bai Y, Zhang Y, Lu Y,
Gao L and Wang F: Low level laser therapy promotes bone
regeneration by coupling angiogenesis and osteogenesis. Stem Cell
Res Ther. 12:4322021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gao L, Zhang W, Zhong WQ, Liu ZJ, Li HM,
Yu ZL and Zhao YF: Tumor associated macrophages induce epithelial
to mesenchymal transition via the EGFR/ERK1/2 pathway in head and
neck squamous cell carcinoma. Oncol Rep. 40:2558–2572.
2018.PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
27
|
Zhang Y, Bai Y, Bai J, Li L, Gao L and
Wang F: Targeting soluble epoxide hydrolase with TPPU alleviates
irradiation-induced hyposalivation in mice via preventing apoptosis
and microcirculation disturbance. Adv Ther. 3:20001152020.
View Article : Google Scholar
|
|
28
|
Gao L, Wang FQ, Li HM, Yang JG, Ren JG, He
KF, Liu B, Zhang W and Zhao YF: CCL2/EGF positive feedback loop
between cancer cells and macrophages promotes cell migration and
invasion in head and neck squamous cell carcinoma. Oncotarget.
7:87037–87051. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li HM, Yang JG, Liu ZJ, Wang WM, Yu ZL,
Ren JG, Chen G, Zhang W and Jia J: Blockage of glycolysis by
targeting PFKFB3 suppresses tumor growth and metastasis in head and
neck squamous cell carcinoma. J Exp Clin Cancer Res. 36:72017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Markus SM, Marzo MG and McKenney RJ: New
insights into the mechanism of dynein motor regulation by
lissencephaly-1. Elife. 9:e597372020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen W, Wang W, Sun X, Xie S, Xu X, Liu M,
Yang C, Li M, Zhang W, Liu W, et al: NudCL2 regulates cell
migration by stabilizing both myosin-9 and LIS1 with Hsp90. Cell
Death Dis. 11:5342020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gorshtein G, Grafinger O and Coppolino MG:
Targeting SNARE-mediated vesicle transport to block
invadopodium-based cancer cell invasion. Front Oncol.
11:6799552021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Seano G and Primo L: Podosomes and
invadopodia: Tools to breach vascular basement membrane. Cell
Cycle. 14:1370–1374. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Qadir MI, Parveen A and Ali M: Cdc42: Role
in cancer management. Chem Biol Drug Des. 86:432–439. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cao H, Eppinga RD, Razidlo GL, Krueger EW,
Chen J, Qiang L and McNiven MA: Stromal fibroblasts facilitate
cancer cell invasion by a novel invadopodia-independent matrix
degradation process. Oncogene. 35:1099–1110. 2016. View Article : Google Scholar
|
|
36
|
Reiner O, Sapoznik S and Sapir T:
Lissencephaly 1 linking to multiple diseases: Mental retardation,
neurodegeneration, schizophrenia, male sterility, and more.
Neuromolecular Med. 8:547–565. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Suzuki SO, McKenney RJ, Mawatari SY,
Mizuguchi M, Mikami A, Iwaki T, Goldman JE, Canoll P and Vallee RB:
Expression patterns of LIS1, dynein and their interaction partners
dynactin, NudE, NudEL and NudC in human gliomas suggest roles in
invasion and proliferation. Acta Neuropathol. 113:591–599. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Perzin KH, Gullane P and Clairmont AC:
Adenoid cystic carcinomas arising in salivary glands: A correlation
of histologic features and clinical course. Cancer. 42:265–282.
1978. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yingling J, Youn YH, Darling D, Toyo-Oka
K, Pramparo T, Hirotsune S and Wynshaw-Boris A: Neuroepithelial
stem cell proliferation requires LIS1 for precise spindle
orientation and symmetric division. Cell. 132:474–486. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Moon HM, Youn YH, Pemble H, Yingling J,
Wittmann T and Wynshaw-Boris A: LIS1 controls mitosis and mitotic
spindle organization via the LIS1-NDEL1-dynein complex. Hum Mol
Genet. 23:449–466. 2014. View Article : Google Scholar
|
|
41
|
Brehar FM, Dragomir MP, Petrescu GED and
Gorgan RM: Fighting cancer stem cell fate by targeting LIS1 a WD40
repeat protein. Front Oncol. 9:11422019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Brehar FM, Gafencu AV, Trusca VG, Fuior
EV, Arsene D, Amaireh M, Giovani A and Gorgan MR: Preferential
association of lissencephaly-1 gene expression with
CD133+ glioblastoma cells. J Cancer. 8:1284–1291. 2017.
View Article : Google Scholar :
|
|
43
|
Wang XY, Yu J, Zhang Y, Zhang FY, Liu KJ
and Xiang B: Phenylephrine alleviates 131I damage in submandibular
gland through promoting endogenous stem cell regeneration via
lissencephaly-1 upregulation. Toxicol Appl Pharmacol.
396:1149992020. View Article : Google Scholar
|
|
44
|
He S, Li P, Zhong Q, Hou L, Yu Z, Huang Z
and Chen X, Fang J and Chen X: Clinicopathologic and prognostic
factors in adenoid cystic carcinoma of head and neck minor salivary
glands: A clinical analysis of 130 cases. Am J Otolaryngol.
38:157–162. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Reiner O, Carrozzo R, Shen Y, Wehnert M,
Faustinella F, Dobyns WB, Caskey CT and Ledbetter DH: Isolation of
a Miller-Dieker lissencephaly gene containing G protein
beta-subunit-like repeats. Nature. 364:717–721. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kholmanskikh SS, Dobrin JS, Wynshaw-Boris
A, Letourneau PC and Ross ME: Disregulated RhoGTPases and actin
cytoskeleton contribute to the migration defect in Lis1-deficient
neurons. J Neurosci. 23:8673–8681. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Miller LE, Au V, Mokhtari TE, Goss D,
Faden DL and Varvares MA: A contemporary review of molecular
therapeutic targets for adenoid cystic carcinoma. Cancers (Basel).
14:9922022. View Article : Google Scholar
|
|
48
|
Biyanee A, Yusenko MV and Klempnauer KH:
Src-family protein kinase inhibitors suppress MYB activity in a
p300-dependent manner. Cells. 11:11622022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ramsay NA and Glover BJ: MYB-bHLH-WD40
protein complex and the evolution of cellular diversity. Trends
Plant Sci. 10:63–70. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cao S, Lu X, Wang L, Qian X, Jin G and Ma
H: The functional polymorphisms of LIS1 are associated with acute
myeloid leukemia risk in a Han Chinese population. Leuk Res.
54:7–11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Suzuki K and Takahashi K: Regulation of
lamellipodia formation and cell invasion by CLIP-170 in invasive
human breast cancer cells. Biochem Biophys Res Commun. 368:199–204.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sipes NS, Feng Y, Guo F, Lee HO, Chou FS,
Cheng J, Mulloy J and Zheng Y: Cdc42 regulates extracellular matrix
remodeling in three dimensions. J Biol Chem. 286:36469–36477. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yamaguchi H, Lorenz M, Kempiak S,
Sarmiento C, Coniglio S, Symons M, Segall J, Eddy R, Miki H,
Takenawa T and Condeelis J: Molecular mechanisms of invadopodium
formation: The role of the N-WASP-Arp2/3 complex pathway and
cofilin. J Cell Biol. 168:441–452. 2005. View Article : Google Scholar : PubMed/NCBI
|