Introduction
In the context of cancer, both comorbidity, the
presence of an additional long-term condition (LTC) and
multimorbidity, the presence of multiple LTCs, are a significant
consideration. Studies have indicated that LTCs are associated with
increased cancer pathogenesis (1), more advanced disease (2), lower rates of, and delays to
commencing, active treatment (1,3–6),
unfavorable survival (7–10), increased cost and use of
healthcare (11,12) and lower health-related quality of
life (13–15). Cancer treatments carry significant
side-effects and complications, rates of which are also higher in
patients with other LTCs (11–15). As such, understanding the impact
of LTCs is crucial, both at an individual level and when
undertaking large-scale health services studies.
Comorbidity has also been indicated to be associated
with demographic features, with the presence of LTC rising with age
(16,17), and in the context of an ageing
population, accurate assessment of the associated increasing levels
of comorbidity and their impact upon cancer care and outcomes, are
becoming an ever more pressing concern.
In order to support the evaluation of comorbidity,
multiple indices have been developed. These allow adjustment of
outcomes for comorbidity, both within academic studies and in
comparisons between provider organisations. These indices typically
weigh the severity of a comorbidity in a population against a
specific outcome. They have been indicated to be user-friendly,
with high test-retest reliability, and demonstrate that comorbidity
is a strong predictor of outcome (18). Among the most widely used of these
is the original Charlson Comorbidity Index (CCI). Previous
systematic reviews have indicated that the CCI is the most
frequently utilised index (19,20), a pattern which is thought to be
continuing despite the availability of multiple other comorbidity
indices.
There remain doubts about the reliability and
validity of certain indices. Of the 13 methods for measuring
comorbidity examined by de Groot et al (21), only four, including the CCI, were
indicated to have sufficient data on the clinimetric properties to
properly assess their validity and reliability. Insufficient data
were available for the others and whilst multiple types of
reliability were examined, the authors did not consider how closely
matched studies using comorbidity indices were to the studies that
validated these indices.
Mismatches have the potential to reduce the efficacy
of a comorbidity index's use. Hall (22) suggested that when selecting a
comorbidity index, researchers should look for indices that were
validated in similar patients to those of the study. Not doing so
may mean the index is not suitable for use in the study population,
leading to questions around the generalisability, reliability and
robustness of results. Even where an element of generalisation is
necessary, certain indices have performed better when investigating
specific cancer types. For instance, the Adult Comorbidity
Evaluation 27 (ACE-27) performed better than the CCI in patients
with acute myeloid leukaemia (23) and laryngeal cancer (24). Head and neck cancers were included
in the validation of ACE-27, but not CCI. This therefore arguably
supports Nitz's (25) expectation
that a disease-specific comorbidity measure would explain more
variance in the outcome than either a general or
non-cancer-specific measure.
Indices derived from different data sources have
also been explored in relation to outcomes. When comparing
diagnosis-based (comorbidities identified based on diagnoses) and
pharmacy-based (comorbidities identified based on medications from
pharmacy data) indices, diagnosis-based measures provided superior
prediction for mortality, while medication-based indices better
predicted care utilisation and costs (26). This suggests there may also be an
important need for authors to consider the alignment between study
and index in terms of data source and/or outcome when selecting an
appropriate measure of controlling for comorbidity.
At one level, the present systematic literature
review aimed to provide a snapshot of the comorbidity indices used
in retrospective observational studies of patients with cancer and
document the justifications for these choices. Beyond that, it also
aimed to evaluate the alignment between these studies and the
studies in which the indices of comorbidity were initially
developed. With cancer type, data source and outcome all
potentially influencing the effectiveness of comorbidity indices,
alignment was assessed with respect to these key parameters.
Materials and methods
Literature search
A structured search was conducted in PubMed covering
a five-year period between 23 March 2015 and 22 March 2020 in
accordance with the PRISMA guidelines (27). Studies were restricted to those
published in the English language. The search terms used were as
follows: [cancer(Title)] AND [comorb*(Title)] AND
[population(Title/Abstract) OR observational(Title/Abstract) OR
retrospective(Title/Abstract) OR regist*(Title/Abstract)].
Literature screening
Following the literature search, papers were
de-duplicated and screening based on study title and abstract was
carried out independently by two reviewers (AB and RB) to assess
against key inclusion criteria. These were as follows:
Retrospective observational studies; studies on patients with
cancer; a relationship to an outcome. While the ‘relationship to an
outcome’ criterion was defined broadly in terms of a specific
outcome, the relationship aspect was more restrictive to those
studies utilising comorbidity for predictive or effect modification
purposes. It therefore did not include studies that were restricted
to, e.g., simply describing comorbidities in cancer populations or
being used as a confounder. The decision to restrict the ‘cancer’
and ‘comorb*’ search terms to the title was made with the aim of
maximising results meeting the inclusion criteria while reducing
the proportion of those that did not. The full text was
subsequently reviewed if insufficient information was available to
include the paper based on title/abstract. Where there was
disagreement around inclusion between the two reviewers, a third
individual (KS) also assessed the paper and inclusion was based on
a majority decision.
Data extraction
Data extraction took place following this
evaluation. Two reviewers (AB and RB) obtained data pertaining to
the following five parameters: i) First, the time period covered by
the study was denoted. ii) Furthermore, the type of comorbidity
index or indices used in the paper was extracted. Both those using
established indices of comorbidity in patients with cancer and
novel study-specific measures were identified. Established indices
were denoted, whilst free text fields were used to record more
detailed information on how novel measures were calculated. This
included, for instance, the methodology for calculating the
comorbidity score and, if relevant, details of the validation
population. For established measures, paper-specific modifications
to the original methodology were also denoted. iii) In addition,
the primary cancer diagnosis/diagnoses included in the studies were
recorded. All solid-organ diagnoses were classified by primary
diagnosis, with haematological cancers and lymphomas grouped
separately. Histological subtypes and stages were not considered.
If an unselected cancer population was included in the study, the
individual diagnoses were not recorded separately. iv) The data
source for each included study was defined. This involved not only
identifying the primary data source, e.g., cancer registries or
hospital records, but also whether different sources were formally
linked. Each source, including linked data, was classified
individually. v) Outcome(s) for each paper were identified. This
included capturing both the primary outcome and any secondary
outcomes explored in the study. With a majority of established
cancer comorbidity indices validated against differing measures of
mortality, each time-specific mortality and overall survival
outcome was classified separately.
Comparisons
Comparisons were then made between the extracted
studies and the paper in which the selected comorbidity index was
initially validated. The comorbidity index validation paper was
identified from the reference lists of reviewed studies during data
extraction and confirmed via a separate literature search. For each
index validation paper, the cancer type(s), data source(s) and
outcome(s) were identified and summarised in Table I. Comparisons with respect to
these three parameters between the study paper and comorbidity
index validation paper were then made. For each parameter,
‘complete’ or ‘no’ match were primarily explored, although matches
were also classed as ‘partial’ or ‘uncertain’ where they did not
fit into either of the prime categories. Descriptive analyses were
then performed to evaluate these matches, both separately and
combined.
 | Table I.Data source, study population
[including cancer type(s)] and outcome of the validation studies
for comorbidity indices used by extracted papers included in the
systematic review. |
Table I.
Data source, study population
[including cancer type(s)] and outcome of the validation studies
for comorbidity indices used by extracted papers included in the
systematic review.
| Comorbidity
index | Paper source | Data source
(country) | Study
population | Outcome | (Refs.) |
|---|
| Adult comorbidity
evaluation 27 | Piccirillo et
al | Multi-centre
registry data (USA) | 19,268 patients at
two hospitals with lung, female breast, gastrointestinal tract,
gynecological, urinary tract, and head and neck cancers | Overall
survival | (28) |
| Aggregated
diagnosis groups | Austin et
al | Population registry
linked with claims data (Canada) | All adult residents
of Ontario (10,498,413) divided
into development and validation cohorts | 1-year
mortality | (29) |
| C3 | Sarfati et
al | Population registry
data (New Zealand) | 14,096 patients
(development), 11,014 patients (validation) diagnosed with
colorectal, uterine, ovarian, liver, stomach, female breast, kidney
or bladder cancers | 1-year non-cancer
mortality | (30) |
| CCI | Charlson et
al | Hospital
notes/records (USA) | 559 medical
patients (development), 685 breast cancer patients
(validation) | 1-year
mortality | (31) |
| Age-adjusted
CCI | Charlson et
al | Hospital
notes/records (USA) | 218 patients with
hypertension or diabetes undergoing elective surgery | 3-year and 5-year
mortality | (32) |
| Deyo-CCI | Deyo et
al | Claims data
(USA) | 27,111 patients who
underwent lumbar spine surgery | Postoperative
mortality | (33) |
| Head & Neck
CCI | Bøje et
al | Population registry
data (Denmark) | 9,388 patients with
head and neck cancer receiving radiotherapy | 5-year
mortality | (34) |
| Romano CCI | Romano et
al | Claims data
(USA) | Multiple non-cancer
diagnostic groups considered | Various | (35) |
| Elixhauser | Elixhauser et
al | Discharge data
(USA) | 1,779,167 acute
admissions across 438 hospitals | In-hospital
mortality | (36) |
| Klabunde
comorbidity index | Klabunde et
al | Population registry
linked with claims data (USA) | 28,868 males
diagnosed with prostate cancer and 14,943 females diagnosed with
breast cancer | 2-year non-cancer
mortality | (37) |
| National cancer
institute comorbidity index | Klabunde et
al | Population registry
linked with claims data (USA) | 28,868 males
diagnosed with prostate cancer and 14,943 females diagnosed with
breast cancer | 2-year non-cancer
mortality | (37) |
|
| Klabunde et
al | Population registry
linked with claims data (USA) | 26,377 female
breast, 53,503 prostate, 26,460 colorectal and 33,975 lung cancer
patients | 2-year non-cancer
mortality | (38) |
| Ovarian cancer
comorbidity index | Noer et
al | Population registry
data (Denmark) | 2,020 patients
(development), 1,975 patients (validation) diagnosed with ovarian,
peritoneal or fallopian tube cancer | Overall survival
(up to 5 years) | (39) |
| Rx-Risk-V
model | Sloan et
al | Collective registry
linked with pharmacy data (USA) | 126,075 users of
Veteran Health Administration services | Associations
between pharmacy and ICD-9 diagnostic classes | (40) |
| Simplified
comorbidity score | Colinet et
al | Case reports
(France) | 735 patients
(development), 136 patients (validation) diagnosed with non-small
cell lung cancer | 1 and 2-year
mortality | (41) |
Results
Literature search and selection
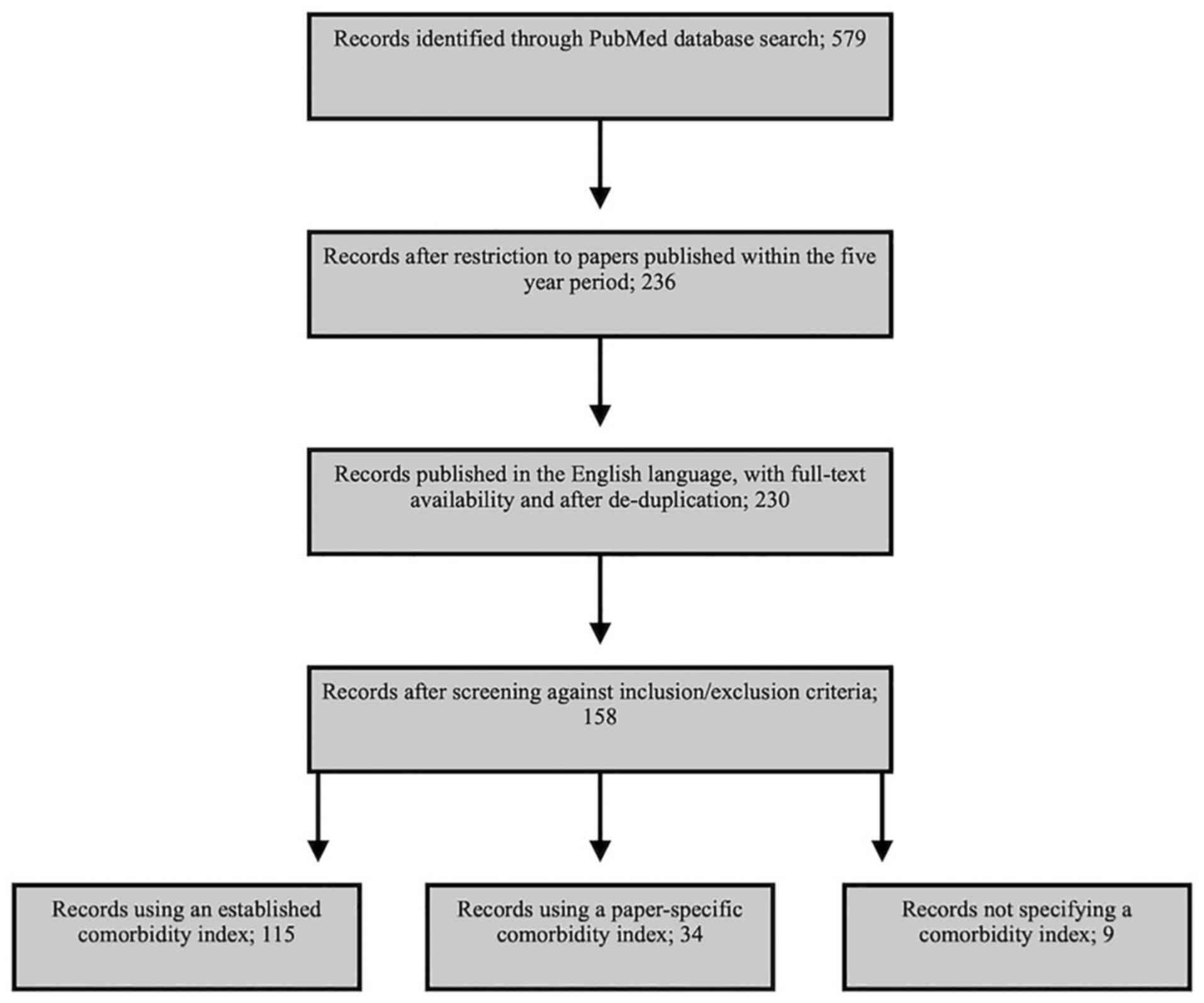
According to the search criteria, 236 studies were
identified over the five-year period selected. Of these, the full
text was available for 233 studies, while only the abstract was
available for the remaining three. Following de-duplication and
removal of non-English language papers, 230 articles remained. All
230 studies were then screened against the inclusion criteria and
this process identified a total of 158 papers to be included in the
review. A full list of these studies is provided in Data S1. Of the 158 studies included in
the review, 115 used one or more of the established comorbidity
indices or measures outlined in Table
I. Of the remaining papers, 34 used a study-specific evaluation
of comorbidity and nine papers did not specify how comorbidities
were weighted and scored (Fig.
1).
Comorbidity index
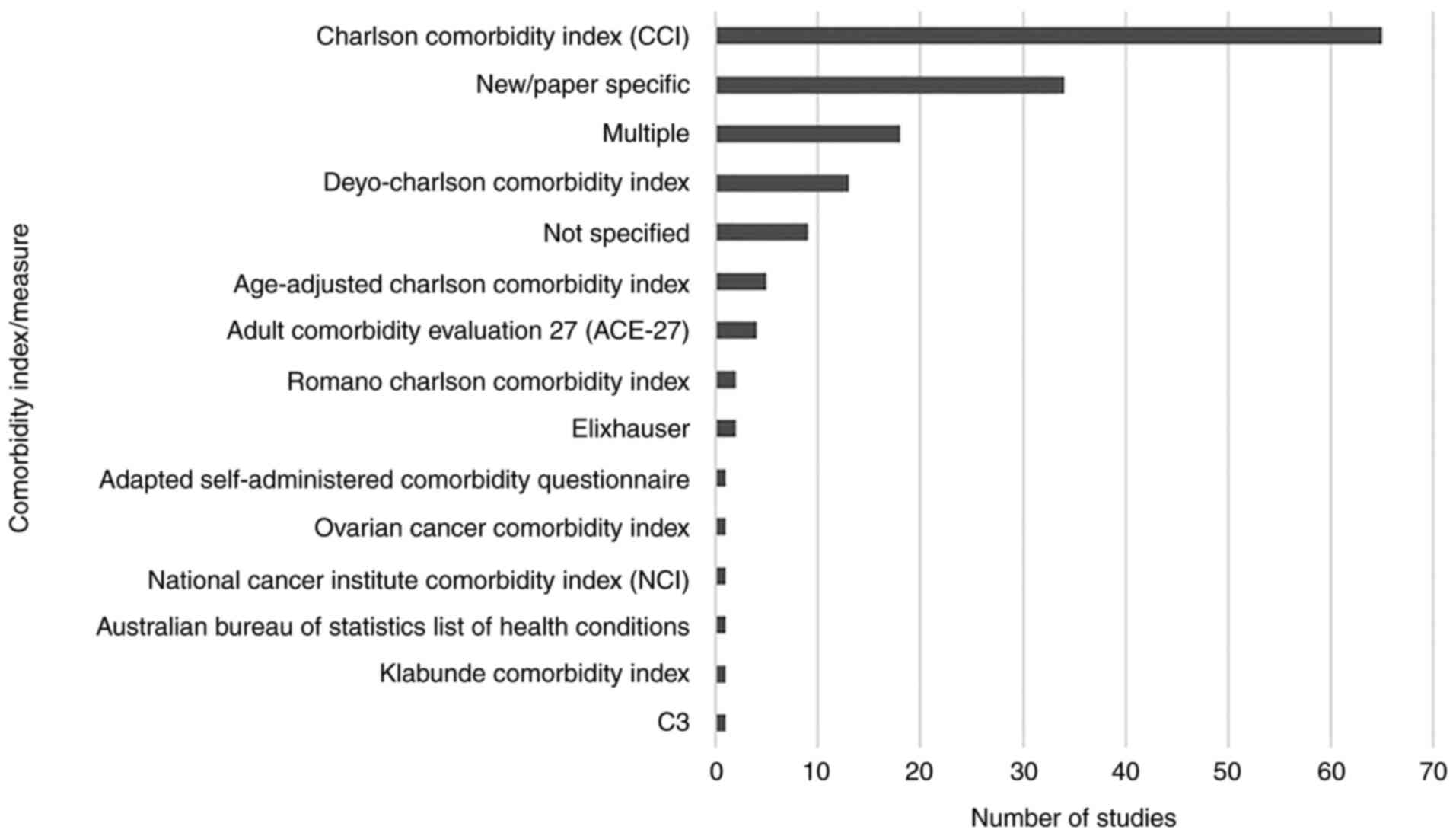
A total of 16 established indices or measures of
comorbidity were used across the papers reviewed, with 38 studies
using or including a measure of comorbidity developed specifically
for the published study. The number of papers using each index or
measure is presented in Fig. 2,
with Table I outlining basic
information about the indices identified during data extraction
where available (28–41). Of these, the CCI was the most
frequently utilised, with 65 (41.1%) papers using it alone
(Fig. 2). This increased to 79
(50.0%) papers when studies using CCI alongside others were
included. When combined with the various adaptations of the CCI,
this increased to 85 (53.8%) using one of these measures alone, or
104 (65.8%) where studies using multiple indices were included.
Other indices were used at a markedly lower frequency. While the
ACE-27 was used as the sole comorbidity index in four (2.5%)
studies, the Elixhauser index was used alone in two studies (1.3%).
When studies using multiple indices were also included, they were
each featured in eight (5.1%) of the 158 papers reviewed.
In 75.7% (n=87) of studies, no modifications to the
indices were made. Where changes were made, however, these
typically involved removing comorbidities (generally cancers) [n=7
(6.1%)] (42–48), adding and removing comorbidities
[n=6 (5.2%)] (49–54) or adding comorbidities [n=3 (2.6%)]
(55–57). Modified indices were used in five
(4.4%) studies where the nature of the modification was not
provided in the manuscript (58–62).
Of the 115 eligible papers, only 15 (13.0%) provided
a justification for the index used. Where justifications were
provided, these typically involved statements to the effect that
the index is widely used, or has been validated in populations of
cancer patients. However, whilst certain studies, such as that by
Stordeur et al (57),
provided detailed justifications with supporting references, others
provided minimal detail.
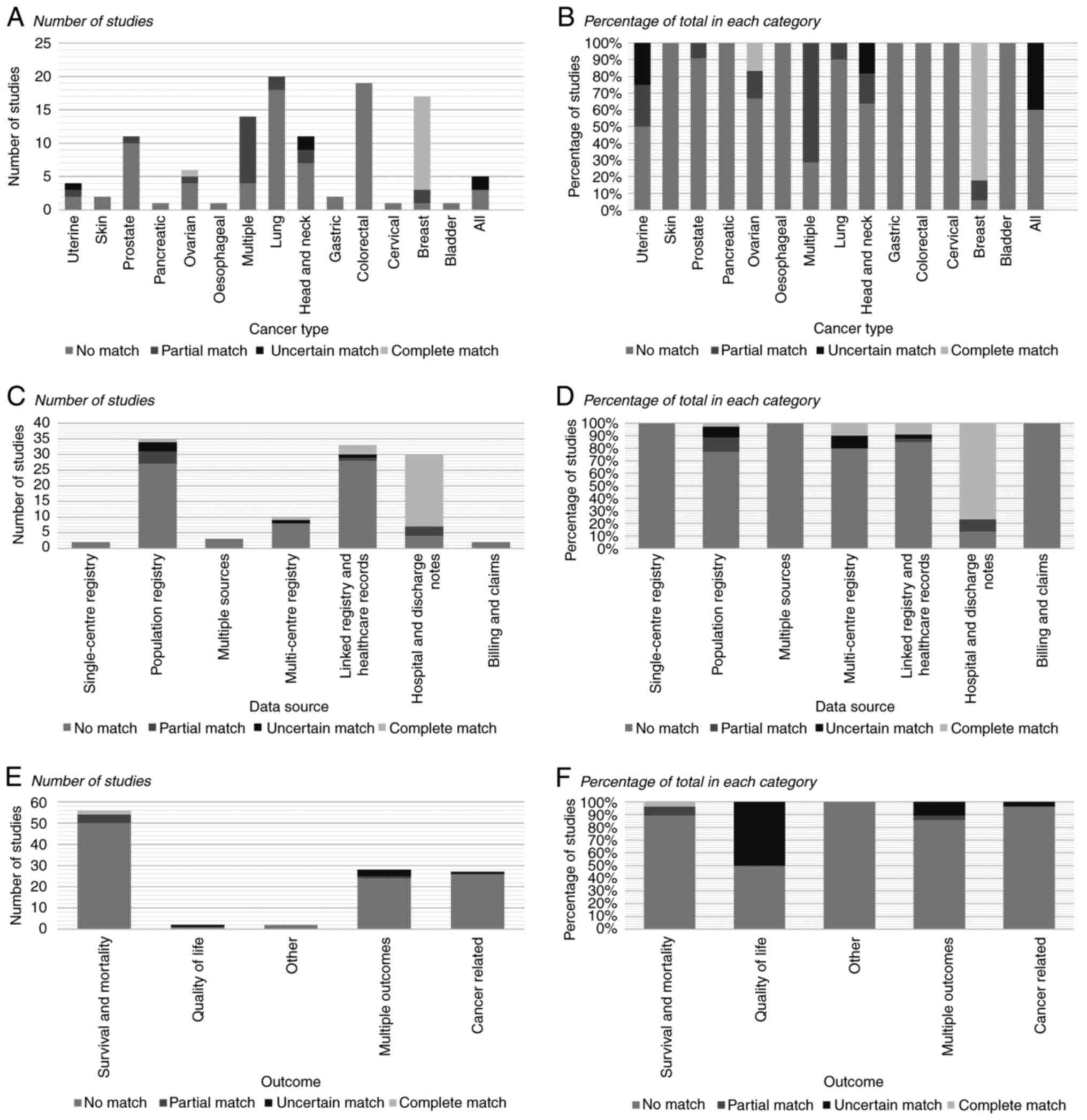
Cancer type
The cancer types used in the studies included in the
present review are presented in Fig.
3. The most common single type was colorectal cancer [n=26 of
the 158 included studies (16.5%)], followed by lung [n=25 (15.8%)],
breast [n=21 (13.3%)] and prostate cancer [n=15 (9.5%)]. A total of
16 studies (10.1%) examined multiple named cancer types.
In total, a complete match between the study paper
and comorbidity index validation paper for cancer type was observed
in only 16 (13.9%) of the 115 studies that used an established
index. In all but three studies (60,63,64), matches were with the CCI. For
other studies that had a match, these were the C3 index,
Deyo-Charlson Comorbidity Index (DCCI) and Ovarian Cancer
Comorbidity Index (OCCI). A full breakdown of matches by cancer
type is provided in Fig. 3.
Data source
Registries or other databases were the most common
data source, being used in 60 of the 158 extracted studies (38.0%).
Of these, 45 used population registries, three used single-centre
registries and 12 used multi-centre registries. Hospital and
discharge notes [n=46 (29.1%)] and linked registry and billing data
[n=44 (27.9%)] were also commonly used data sources (Fig. 3).
A complete match between the study paper and
comorbidity index validation paper for data source was observed in
26 of the 115 studies including an established measure (22.6%).
Matches by data source are detailed in Fig. 3. As with cancer type, a majority
of these matches were in studies using the CCI [n=18 (15.7%)].
There were additionally three matches in papers using the
age-adjusted CCI, and one for each of ACE-27, C3, National Cancer
Institutes and OCCI indices.
Outcome
Overall survival was the most commonly identified
outcome, being used in 45 of the 158 studies (28.5%). Other
mortality outcomes used included in-hospital/28-day, 1, 2, 3 and
5-year mortality. Multiple outcomes were examined in 35 studies
(22.2%) (Fig. 3).
There were only two studies in which there was a
complete match between the study paper and comorbidity index
validation paper for the outcome (44,65). One of these was a study using
ACE-27, while the other used the CCI.
Combined matches between study and
comorbidity index used
Overall, no studies were identified where there was
a complete match between the study paper and comorbidity index
validation paper across different cancer types, data sources and
outcomes. Of the 115 studies using an established comorbidity
index, complete matches in two domains and a partial match in the
third was observed in one study (65). Partial matches were identified for
all three domains in three studies (3.5%) (66–68), all of which used multiple
comorbidity indices. Complete or partial matches were found across
two domains in 15 studies (13.0%) and in only one domain in 37
studies (32.2%). However, 57 (49.5%) had no complete or partial
matches. For the remaining studies, the index or measure being used
did not specify the relevant information (Table I), meaning the comparison could
not be made.
Discussion
The results of the present study confirmed the
findings of Sarfati (19) and
Connolly et al (20),
highlighting the dominance of the CCI and its derivatives in
studies of cancer populations. Of the papers reviewed, none
included a complete match between the study population and
comorbidity index development population for all domains assessed.
While it is difficult for a study to achieve a match with the
comorbidity validation paper across all three domains, the most
common finding was that of no matches.
Of the papers utilising the CCI, the majority used
either an unadjusted or modified version (e.g., exclusion of a
single condition). Where a validated adaptation was selected, the
DCCI was more popular than the Romano Charlson Comorbidity index
(RCCI). This was in line with Yurkovich et al (26). It is notable, however, that both
the DCCI and RCCI were developed in non-cancer populations
(Table I). As such, despite their
relatively frequent use, it would be reasonable to question how
well suited they are for use in studies of cancer populations and
whether indices developed in cancer populations would be more
appropriate.
Studies frequently used comorbidity indices which
were developed for use in a cancer type other than that which was
the focus of the study. The CCI was validated in a population with
breast cancer, but has been used to adjust for comorbidity in
cancers with distinctly different disease trajectories and
treatment pathways. As such, the impact, and presence of,
comorbidity is likely to differ significantly. For instance,
anthracyclines are routinely used in adjuvant chemotherapy regimens
for breast cancer, whilst in other cancer types, these are used
predominantly in subsequent lines of therapy. As such, the presence
of cardiac disease is expected to have quite a different impact on
the decision making around adjuvant breast cancer treatment.
Advances in the treatment of numerous diseases have
meant that the initial weighting defined in the CCI validation
paper may not correspond to their significance now. Reflecting
these advances, a re-evaluation of the CCI weights by Quan et
al (69) saw the weights
decreased for eight diseases, reflecting improvements in their
management and outcomes. In a further four diseases, an increase in
weighting was seen. These findings suggested that the original CCI
may not always be the most appropriate index for predicting
outcomes in contemporary cancer populations. Authors therefore need
to consider both the generalisability of modified CCIs and the
requirement for updated weightings to reflect contemporaneous
outcomes.
For most studies included in the present review,
indices were largely used without detailed justification. As such,
it was not possible to fully evaluate the reasons for the
widespread use of the CCI. It is possible that its continued use is
largely down to its previous popularity. Of the nine studies which
did state a reason for selecting the CCI, five stated its
popularity without providing supporting references. The most
detailed justification came from Grønhøj et al (70), who referenced a previous Danish
National Patient Register-based study where the CCI had a high
positive predictive value.
The CCI is calculated from routinely collected
population data, meaning it is more readily accessible for
registry-based health services studies, potentially leading to its
selection for use over alternative site-specific indices. Several
considerations exist contributing to this. First, the availability
of sufficient information to derive relevant indices may influence
selection. For instance, several papers included in the present
review used the CCI in studies of patients with non-small cell lung
cancer (NSCLC), despite the Simplified Comorbidity Score (SCS)
having been validated in an NSCLC population (41). The SCS, however, includes smoking
status and alcohol consumption, which are not routinely collected
by cancer registries and discharge records among others, and would
thus prevent its use in studies using such sources, unless these
items are specifically sought. As such, whilst such an alternative
measure may seem more appropriate, this does not always relate to
practice. This should be borne in mind where new indices are
developed; the advantage of the CCI is its simplicity. Indices
aiming to replace it must recognise the limitations of the
available data.
Whilst justifications for the use of the CCI exist,
arguably its widespread use reflects a limited number of indices
designed and/or validated for specific cancer types. This was
particularly evident for two of the most common cancer types,
colorectal and prostate cancer. While Marventano et al
(71) proposed an adjusted CCI
for patients with colorectal cancer, this has remained to be
validated. An opportunity therefore exists to develop and validate
comorbidity indices that are better tailored for specific cancer
types and may better predict outcomes than existing, less
diagnosis-specific measures. In the absence of such new indices
though, a more comprehensive understanding of the relative
performance of alternative cancer-specific indices would be
valuable for identifying the most appropriate indices for certain
cancer types.
‘Data source’ proved to be the domain in which a
match was most frequently observed. Despite this, 77% of studies
had a mismatch for data source. Of the data sources identified, one
of the lesser used was billing data. While valuable and readily
available, billing data potentially capture billable comorbidities
while disregarding numerous diagnoses that are not. It is therefore
likely that the number of comorbidities from this source reflect
conditions that may be billed by the treating hospital, and thus,
have limited generalisability. This has been observed in the USA,
where the proportion of patients assigned to a Diagnosis-Related
Group (DRG), the basis on which hospitals are reimbursed, has
increased over time (72). The
authors suggest this may reflect financial incentives to improve
the management of recorded diseases that contribute to these DRGs.
As such, the use of billing data may not fully reflect patient
comorbidity.
The impact of this variation in recording is likely
to be increased between jurisdictions and where the rationale for
recording differs. Using linked registry and hospital discharge
data from Australia, Canada, Norway and the UK, Lüchtenborg et
al (73) determined that it
was possible to calculate the CCI, Elixhauser and inpatient bed day
comorbidity scores from the data captured. However, they noted that
differences in coding and hospital admission practices may make
comparability of recorded comorbidity among the countries
difficult. The potential limitations to the use of individual
comorbidity indices resulting from the data source from which they
were derived clearly warrant consideration where researchers are
seeking an appropriate measure.
Beyond the infrastructure and rationale for data
capture in a given healthcare setting, the demographics of the
population in which the comorbidity index was developed and
validated should be considered when selecting an index. The
socioeconomic and demographic differences observed among
populations may reduce generalisability, particularly when
validation has occurred based on a single hospital or limited
geographical area. For instance, compared with Caucasians, those of
South Asian ethnicity are at a higher risk of type two diabetes and
hypertension (74). Ethnicity has
also been associated with outcome, with patients of Asian ethnicity
having better survival from lung cancer compared with Caucasians
(75), whilst Asian females aged
15–64 years have reduced breast cancer survival (75). With indices including the CCI,
ACE-27 and Elixhauser validated in patients from a limited number
of locations, it may be important for authors to consider
population generalisability when selecting an index. This is also
true for the patient age range, with older patients likely to have
both greater comorbidity and a different impact of comorbidity when
compared with younger patients.
The final area in which a match was sought was
between the outcome of the reviewed study and validation of the
relevant comorbidity index. As expected, there were a significant
number of outcomes identified and it would be impractical to expect
indices to be developed which cover the full range of those that
were able to be measured. In the domain ‘cancer type’, however, the
CCI appeared to be frequently used despite other indices
potentially being more appropriate. With regard to outcomes, only
one study using the CCI had one-year mortality as its primary
outcome (58). With overall
survival being the most frequent outcome, the OCCI may have been
more appropriate than the CCI if a study examined gynaecological
cancers, or ACE-27 for other cancer types, both of which have been
validated for this end point.
Given the large number of possible measurable
outcomes, it may be more appropriate for authors to consider
comparisons that were made between indices with respect to
different outcomes, rather than seeking a perfect match. For
instance, ACE-27 has performed better than the CCI with respect to
five-year mortality (24) and was
also found to be the best among the compared indices at predicting
overall survival (23). Unlike
Nesic et al (24), Hwang
et al (76) found the CCI
to be the most effective index in estimating hospital costs for
patients treated surgically for gastric cancer among the four
compared. However, it was less useful in predicting the length of
hospital stay. Meanwhile, Elixhauser has been indicated to be a
better predictor of both short- and long-term mortality than the
CCI (26). Such comparative
studies offer authors the opportunity to evaluate the performance
of different indices against their desired outcomes, allowing the
most appropriate to be selected.
The present review has demonstrated that, while the
CCI remains the dominant comorbidity index in studies of cancer
populations, it may not always be the most closely aligned with key
elements of the study design in terms of cancer type, data source
and outcome. These factors have the potential to make the CCI a
less effective index in predicting outcomes than others that have
been designed and/or validated for particular cancer types or with
respect to specific outcomes. Further work to examine this is key,
and particularly, to determine in which domains matches should be
prioritised, which was beyond the scope of the analyses in the
present study.
Study authors may nonetheless benefit from
questioning whether other indices are more closely aligned with
their study design prior to selecting which index to use. Table I provided in the present study may
assist with this process. They should also consider justifying
their decision in more detail, as well as discussing the potential
limitations of their choice. Registry data controllers may assist
in and facilitate the use of a wider range of indices by providing
the means for them to be calculated by authors. Going further
though, registries may also be able to recommend to researchers the
optimal index to use within the available data and in relation to
the population and outcome of interest. Finally, gaps have been
identified in terms of index development, particularly for specific
diagnostic groups. Researchers may consider the development of new
indices better tailored to not only specific diagnostic groups, but
also data sources or outcomes.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RJB initially conceived the study with AMB and KS
contributing to the refinement of the study design. AMB, KS and RJB
were all involved in literature screening and data extraction. Data
analysis was primarily performed by AMB. The manuscript was
initially drafted by AMB. KS and RJB provided critical revisions of
the manuscript prior to submission and during the revision process.
All authors read and approved the final manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sarfati D, Koczwara B and Jackson C: The
impact of comorbidity on cancer and its treatment. CA Cancer J
Clin. 66:337–350. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gurney J, Sarfati D and Stanley J: The
impact of patient comorbidity on cancer stage at diagnosis. Br J
Cancer. 113:1375–1380. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee L, Cheung WY, Atkinson E and
Krzyzanowska MK: Impact of comorbidity on chemotherapy use and
outcomes in solid tumors: A systematic review. J Clin Oncol.
29:106–17. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Janssen-Heijnen ML, Houterman S, Lemmens
VE, Louwman MW, Maas HA and Coebergh JW: Prognostic impact of
increasing age and co-morbidity in cancer patients: A
population-based approach. Crit Rev Oncol Hematol. 55:231–240.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
van de Poll-Franse LV, Houterman S,
Janssen-Heijnen ML, Dercksen MW, Coebergh JW and Haak HR: Less
aggressive treatment and worse overall survival in cancer patients
with diabetes: A large population based analysis. Int J Cancer.
120:1986–1992. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yoon SJ, Kim EJ, Seo HJ and Oh IH: The
association between Charlson comorbidity index and the medical care
cost of cancer: A retrospective study. Biomed Res Int.
2015:2593412015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Søgaard M, Thomsen RW, Bossen KS, Sørensen
HT and Nørgaard M: The impact of comorbidity on cancer survival: A
review. Clin Epidemiol. 5 (Suppl 1):S3–S29. 2013. View Article : Google Scholar
|
|
8
|
Tammemagi CM, Neslund-Dudas C, Simoff M
and Kvale P: Impact of comorbidity on lung cancer survival. Int J
Cancer. 103:792–802. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Piccirillo JF: Importance of comorbidity
in head and neck cancer. Laryngoscope. 110:593–602. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Houterman S, Janssen-Heijnen ML, Verheij
CD, Louwman WJ, Vreugdenhil G, Van Der Sangen MJ and Coebergh JW:
Comorbidity has negligible impact on treatment and complications
but influences survival in breast cancer patients. Br J Cancer.
90:2332–2337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dominick K, Bosworth H and Dudley T:
Comparison of three comorbidity indices for prediction of health
service use. Gerontologist 44: 120-; 2004
|
|
12
|
Stavrou EP, Lu CY, Buckley N and Pearson
S: The role of comorbidities on the uptake of systemic treatment
and 3-year survival in older cancer patients. Ann Oncol.
23:2422–2428. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vissers PA, Thong MS, Pouwer F, Zanders
MM, Coebergh JW and van de Poll-Franse LV: The impact of
comorbidity on health-related quality of life among cancer
survivors: Analyses of data from the PROFILES registry. J Cancer
Surviv. 7:602–613. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yun YH, Kim SH, Lee KM, Park SM and Kim
YM: Age, sex, and comorbidities were considered in comparing
reference data for health-related quality of life in the general
and cancer populations. J Clin Epidemiol. 60:1164–1175. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Smith AW, Reeve BB, Bellizzi KM, Harlan
LC, Klabunde CN, Amsellem M, Bierman AS and Hays RD: Cancer,
comorbidities, and health-related quality of life of older adults.
Health Care Financ Rev. 29:41–56. 2008.PubMed/NCBI
|
|
16
|
Piccirillo JF, Vlahiotis A, Barrett LB,
Flood KL, Spitznagel EL and Steyerberg EW: The changing prevalence
of comorbidity across the age spectrum. Crit Rev Oncol Hematol.
67:124–132. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stenholm S, Westerlund H, Head J, Hyde M,
Kawachi I, Pentti J, Kivimäki M and Vahtera J: Comorbidity and
functional trajectories from midlife to old age: The health and
retirement study. J Gerontol A Biol Sci Med Sci. 70:332–338. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Extermann M: Measuring comorbidity in
older cancer patients. Eur J Cancer. 36:453–41. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sarfati D: Review of methods used to
measure comorbidity in cancer populations: No gold standard exists.
J Clin Epidemiol. 65:924–933. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Connolly KL, Jeong JM, Barker CA,
Hernandez M and Lee EH: A systematic review of comorbidity indices
used in the nonmelanoma skin cancer population. J Am Acad Dermatol.
76:344–346.e2. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
de Groot V, Beckerman H, Lankhorst GJ and
Bouter LM: How to measure comorbidity: A critical review of
available methods. J Clin Epidemiol. 56:221–229. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hall SF: A user's guide to selecting a
comorbidity index for clinical research. J Clin Epidemiol.
59:849–855. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wass M, Hitz F, Schaffrath J, Müller-Tidow
C and Müller LP: Value of different comorbidity indices for
predicting outcome in patients with acute myeloid leukemia. PLoS
One. 11:e01645872016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nesic VS, Petrovic ZM, Sipetic SB, Jesic
SD, Soldatovic IA and Kastratovic DA: Comparison of the adult
comorbidity evaluation 27 and the Charlson comorbidity indices in
patients with laryngeal squamous cell carcinoma. J Laryngol Otol.
126:516–524. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nitz NM: Comorbidity. Understanding health
care outcomes research. Kane R: Aspen Publishing Inc.;
Gaithersburg, MD: pp. 153–174. 1997
|
|
26
|
Yurkovich M, Avina-Zubieta JA, Thomas J,
Gorenchtein M and Lacaille D: A systematic review identifies valid
comorbidity indices derived from administrative health data. J Clin
Epidemiol. 68:3–14. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Page MJ, Moher D, Bossuyt PM, Boutron I,
Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: PRISMA 2020 explanation and elaboration: Updated
guidance and exemplars for reporting systematic reviews. BMJ.
372:n1602021. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Piccirillo JF, Tierney RM, Costas I, Grove
L and Spitznagel JE Jr: Prognostic importance of comorbidity in a
hospital-based cancer registry. JAMA. 291:2441–2447. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Austin PC, Shah BR, Newman A and Anderson
GM: Using the Johns Hopkins' aggregated diagnosis groups (ADGs) to
predict 1-year mortality in population-based cohorts of patients
with diabetes in Ontario, Canada. Diabet Med. 29:1134–1141. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sarfati D, Gurney J, Stanley J, Salmond C,
Crampton P, Dennett E, Koea J and Pearce N: Cancer-specific
administrative data-based comorbidity indices provided valid
alternative to Charlson and national cancer institute indices. J
Clin Epidemiol. 67:586–595. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Charlson ME, Pompei P, Ales KL and
MacKenzie CR: A new method of classifying prognostic comorbidity in
longitudinal studies: Development and validation. J Chronic Dis.
40:373–383. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Charlson M, Szatrowski TP, Peterson J and
Gold J: Validation of a combined comorbidity index. J Clin
Epidemiol. 47:1245–1251. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Deyo RA, Cherkin DC and Ciol MA: Adapting
a clinical comorbidity index for use with ICD-9-CM administrative
databases. J Clin Epidemiol. 45:613–619. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bøje CR, Dalton SO, Primdahl H, Kristensen
CA, Andersen E, Johansen J, Andersen LJ and Overgaard J: Evaluation
of comorbidity in 9388 head and neck cancer patients: A national
cohort study from the DAHANCA database. Radiother Oncol. 110:91–97.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Romano PS, Roos LL and Jollis JG: Adapting
a clinical comorbidity index for use with ICD-9-CM administrative
data: Differing perspectives. J Clin Epidemiol. 46:1075–1079.
1081–1090. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Elixhauser A, Steiner C, Harris DR and
Coffey RM: Comorbidity measures for use with administrative data.
Med Care. 36:8–27. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Klabunde CN, Potosky AL, Legler JM and
Warren JL: Development of a comorbidity index using physician
claims data. J Clin Epidemiol. 53:1258–1267. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Klabunde CN, Legler JM, Warren JL, Baldwin
LM and Schrag D: A refined comorbidity measurement algorithm for
claims-based studies of breast, prostate, colorectal, and lung
cancer patients. Ann Epidemiol. 17:584–590. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Noer MC, Sperling CD, Antonsen SL, Ottesen
B, Christensen IJ and Høgdall C: A new clinically applicable
age-specific comorbidity index for preoperative risk assessment of
ovarian cancer patients. Gynecol Oncol. 141:471–478. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sloan KL, Sales AE, Liu CF, Fishman P,
Nichol P, Suzuki NT and Sharp ND: Construction and characteristics
of the RxRisk-V: A VA-adapted pharmacy-based case-mix instrument.
Med Care. 41:761–774. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Colinet B, Jacot W, Bertrand D, Lacombe S,
Bozonnat MC, Daures JP and Pujol JL; oncoLR health network, : A new
simplified comorbidity score as a prognostic factor in
non-small-cell lung cancer patients: Description and comparison
with the Charlson's index. Br J Cancer. 93:1098–1105. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dobbins TA, Badgery-Parker T, Currow DC
and Young JM: Assessing measures of comorbidity and functional
status for risk adjustment to compare hospital performance for
colorectal cancer surgery: A retrospective data-linkage study. BMC
Med Inform Decis Mak. 15:552015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Genther DJ and Gourin CG: Effect of
comorbidity on short-term outcomes and cost of care after head and
neck cancer surgery in the elderly. Head Neck. 37:685–693. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hsieh MC, Thompson T, Wu XC, Styles T,
O'Flarity MB, Morris CR and Chen VW: The effect of comorbidity on
the use of adjuvant chemotherapy and type of regimen for curatively
resected stage III colon cancer patients. Cancer Med. 5:871–880.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Islam KM, Jiang X, Anggondowati T, Lin G
and Ganti AK: Comorbidity and survival in lung cancer patients.
Cancer Epidemiol Biomarkers Prev. 24:1079–1085. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kuwada K, Kuroda S, Kikuchi S, Yoshida R,
Nishizaki M, Kagawa S and Fujiwara T: Sarcopenia and comorbidity in
gastric cancer surgery as a useful combined factor to predict
eventual death from other causes. Ann Surg Oncol. 25:1160–1166.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lin J, McGlynn KA, Nations JA, Shriver CD
and Zhu K: Comorbidity and stage at diagnosis among lung cancer
patients in the US military health system. Cancer Causes Control.
31:255–261. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
van Eeghen EE, Bakker SD, van Bochove A
and Loffeld RJ: Impact of age and comorbidity on survival in
colorectal cancer. J Gastrointest Oncol. 6:605–612. 2015.PubMed/NCBI
|
|
49
|
Ahern TP, Horváth-Puhó E, Spindler KL,
Sørensen HT, Ording AG and Erichsen R: Colorectal cancer,
comorbidity, and risk of venous thromboembolism: Assessment of
biological interactions in a Danish nationwide cohort. Br J Cancer.
114:96–102. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Belot A, Fowler H, Njagi EN,
Luque-Fernandez MA, Maringe C, Magadi W, Exarchakou A, Quaresma M,
Turculet A, Peake MD, et al: Association between age, deprivation
and specific comorbid conditions and the receipt of major surgery
in patients with non-small cell lung cancer in England: A
population-based study. Thorax. 74:51–59. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cao KI, Salviat F, Laki F, Falcou MC,
Carton M, Poortmans P, Fourquet A and Kirova YM: Outcomes of
postoperative radiation therapy for breast cancer in older women
according to age and comorbidity status: An observational
retrospective study in 752 patients. J Geriatr Oncol. 9:600–605.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cao KI, Waechter L, Carton M and Kirova
YM: Outcomes of exclusive radiation therapy for older women with
breast cancer according to age and comorbidity status: An
observational retrospective study. Breast J. 26:976–980. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Murawski M, Walter J and Schwarzkopf L:
Assessing the lung cancer comorbidome: An analysis of German claims
data. Lung Cancer. 127:122–129. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Niazi SK, Naessens JM, White L, Borah B,
Vargas ER, Richards J, Cabral S, Clark MM and Rummans T: Impact of
psychiatric comorbidities on health care costs among patients with
cancer. Psychosomatics. 61:145–153. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Mohammadi M, Cao Y, Glimelius I, Bottai M,
Eloranta S and Smedby KE: The impact of comorbid disease history on
all-cause and cancer-specific mortality in myeloid leukemia and
myeloma-a Swedish population-based study. BMC Cancer. 15:8502015.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sarfati D, Gurney J, Lim BT, Bagheri N,
Simpson A, Koea J and Dennett E: Identifying important comorbidity
among cancer populations using administrative data: Prevalence and
impact on survival. Asia Pac J Clin Oncol. 12:e47–e56. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Stordeur S, Schillemans V, Savoye I,
Vanschoenbeek K, Leroy R, Macq G, Verleye L, De Gendt C, Nuyts S,
Vermorken J, et al: Comorbidity in head and neck cancer: Is it
associated with therapeutic delay, post-treatment mortality and
survival in a population-based study? Oral Oncol. 102:1045612020.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Aarts MJ, Aerts JG, van den Borne BE,
Biesma B, Lemmens VE and Kloover JS: Comorbidity in patients with
small-cell lung cancer: Trends and prognostic impact. Clin Lung
Cancer. 16:282–291. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Manderbacka K, Arffman M, Suvisaari J,
Ahlgren-Rimpiläinen A, Lumme S, Keskimäki I and Pukkala E: Effect
of stage, comorbidities and treatment on survival among cancer
patients with or without mental illness. Br J Psychiatry.
211:304–309. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Noer MC, Leandersson P, Paulsen T, Rosthøj
S, Antonsen SL, Borgfeldt C and Høgdall C: Confounders other than
comorbidity explain survival differences in Danish and Swedish
ovarian cancer patients-a comparative cohort study. Acta Oncol.
57:1100–1108. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Noer MC, Sperling CD, Ottesen B, Antonsen
SL, Christensen IJ and Høgdall C: Ovarian cancer and comorbidity:
Is poor survival explained by choice of primary treatment or system
delay? Int J Gynecol Cancer. 27:1123–1133. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Skyrud KD, Bray F, Eriksen MT, Nilssen Y
and Møller B: Regional variations in cancer survival: Impact of
tumour stage, socioeconomic status, comorbidity and type of
treatment in Norway. Int J Cancer. 138:2190–2200. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Drzayich Antol D, Waldman Casebeer A,
Khoury R, Michael T, Renda A, Hopson S, Parikh A, Stein A,
Costantino M, Stemkowski S and Bunce M: The relationship between
comorbidity medication adherence and health related quality of life
among patients with cancer. J Patient Rep Outcomes. 2:292018.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Efird JT, Hunter S, Chan S, Jeong S,
Thomas SL, Jindal C and Biswas T: The association between age,
comorbidities and use of radiotherapy in women with breast cancer:
Implications for survival. Medicines (Basel). 5:622018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Binder PS, Peipert JF, Kallogjeri D,
Brooks RA, Massad LS, Mutch DG, Powell MA, Thaker PH and McCourt
CK: Adult comorbidity evaluation 27 score as a predictor of
survival in endometrial cancer patients. Am J Obstet Gynecol.
215:766.e1–766.e9. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
DeWees TA, Nikitas J, Rehman S, Bradley
JD, Robinson CG and Roach MC: Defining optimal comorbidity measures
for patients with early-stage non-small cell lung cancer treated
with stereotactic body radiation therapy. Pract Radiat Oncol.
9:e83–e89. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Göllnitz I, Inhestern J, Wendt TG,
Buentzel J, Esser D, Böger D, Mueller AH, Piesold JU,
Schultze-Mosgau S, Eigendorff E, et al: Role of comorbidity on
outcome of head and neck cancer: A population-based study in
Thuringia, Germany. Cancer Med. 5:3260–3271. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zist A, Amir E, Ocana AF and Seruga B:
Impact of comorbidity on the outcome in men with advanced prostate
cancer treated with docetaxel. Radiol Oncol. 49:402–408. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Quan H, Li B, Couris CM, Fushimi K, Graham
P, Hider P, Januel JM and Sundararajan V: Updating and validating
the Charlson comorbidity index and score for risk adjustment in
hospital discharge abstracts using data from 6 countries. Am J
Epidemiol. 173:676–682. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Grønhøj C, Kronberg Jakobsen K, Kjær E,
Friborg J and von Buchwald C: Comorbidity in HPV+ and HPV-
oropharyngeal cancer patients: A population-based, case-control
study. Oral Oncol. 96:1–6. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Marventano S, Grosso G, Mistretta A,
Bogusz-Czerniewicz M, Ferranti R, Nolfo F, Giorgianni G, Rametta S,
Drago F, Basile F and Biondi A: Evaluation of four comorbidity
indices and Charlson comorbidity index adjustment for colorectal
cancer patients. Int J Colorectal Dis. 29:1159–1169. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Gluckman TJ, Spinelli KJ, Wang M, Yazdani
A, Grunkemeier G, Bradley SM, Wasfy JH, Goyal A, Oseran A and Joynt
Maddox KE: Trends in diagnosis related groups for inpatient
admissions and associated changes in payment from 2012 to 2016.
JAMA Netw Open. 3:e20284702020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Lüchtenborg M, Morris EJA, Tataru D,
Coupland VH, Smith A, Milne RL, Te Marvelde L, Baker D, Young J,
Turner D, et al: Investigation of the international comparability
of population-based routine hospital data set derived comorbidity
scores for patients with lung cancer. Thorax. 73:339–349. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wong RJ, Chou C, Sinha SR, Kamal A and
Ahmed A: Ethnic disparities in the association of body mass index
with the risk of hypertension and diabetes. J Community Health.
39:437–445. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
National Cancer Intelligence Network
(NCIN), . Cancer Incidence and Survival By Major Ethnic Group,
England, 2002–2006. NCIN; London: 2009, https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwin7Zbruu7vAhWOURUIHTVrAgwQFjABegQIAxAD&url=http%3A%2F%2Fwww.ncin.org.uk%2Fview%3Frid%3D75&usg=AOvVaw3z7JFntDtb9_kGh3CDWurO
|
|
76
|
Hwang SW, Chambers C, Chiu S, Katic M,
Kiss A, Redelmeier DA and Levinson W: A comprehensive assessment of
health care utilization among homeless adults under a system of
universal health insurance. Am J Public Health. 103 (Suppl
2):S294–S301. 2013. View Article : Google Scholar : PubMed/NCBI
|