Introduction
Glioblastoma (GBM), known as grade IV astrocytoma,
is a fatal brain cancer affecting adults with a median survival
between 14–17 months (1). In a
recent review, the existing criteria used for its classification
were summarized based on histologic features (astrocytoma of grades
I, II, III and IV, named as GBM), molecular subtypes
(Proneural/Neural, Classical, and Mesenchymal), or gene mutations
such as TP53, PTEN, Neu-rofibromin-1 and epidermal growth factor
receptor (EGFR) or isocitrate dehydrogenase (IDH) enzyme mutations
(2). This solid tumor is
characterized by a stroma containing cells with a different
phenotype, blood vessels and infiltrating cells, which confer
epigenetic and genetic heterogeneity to the mass. This is directly
responsible for the cancer progression and results in a difficult
therapeutic approach (3).
Hypoxic niches are a common feature of solid tumors.
In these areas, hypoxia triggers aberrant angiogenesis and promotes
stem cell population growth in the cancer mass. These
hypoxia-driven events are related to a poor prognosis as well as
therapy resistance in patients with GBM (4,5).
More specifically, the hypoxic microenvironment induces a
transcription of hypoxia-inducible factors (HIFs), such as HIF-1α,
that translocases into the nucleus and binds to the constitutive
HIF-1β subunit, forming a heterodimeric complex. This complex
activates the hypoxia response elements, triggering the activation
of numerous downstream target genes such as vascular endothelial
growth factor (VEGF), which is involved in the neoangiogenesis
process (6,7). Thus, the identification of molecules
targeting the tumor hypoxic pathway could improve GBM regression.
At present, the standard therapy consists of a multimodal approach
including surgery, radiotherapy and chemotherapy with Temozolomide
(TMZ) (8,9). This latter is a DNA alkylating
agent, which induces the destruction of cancer cells by blocking
DNA replication (10). However,
TMZ has shown different side effects including hepatic impairment
and myelosuppression (11).
Previously, it has been demonstrated that the
pituitary adenylate cyclase-activating polypeptide (PACAP) is
involved in GBM malignancy. It regulates cell invasion and
interferes with epithelial-mesenchymal transition (EMT), a process
implicated in invasiveness of cancer cells towards surrounding
tissue (12–14). This peptide is expressed both in
the central nervous system (CNS) and in peripheral organs where
PACAP exerts numerous biological effects depending on the
physio-pathological conditions (15–21). The multitude of PACAP effects
depends on its binding to the G protein-coupled receptors known as
PAC1 (PAC1-R), VPAC-1, and VPAC-2 receptors (VPAC1-R and VPAC2-R),
through which it activates the adenylate cyclase (AC) and/or
phospholipase C pathways (22).
PACAP effects in the CNS are also mediated by the induction of the
activity-dependent neuroprotective protein (ADNP), an intracellular
astrocyte-derived neurotrophic factor (23–25). ADNP intracellular stimulation
occurs in a bimodal manner based on PACAP concentration (26–28). In fact, sub-picomolar
concentrations of PACAP induce ADNP release via PAC1-R activation,
whereas nanomolar concentrations induce ADNP expression mediated
through VPAC1-R stimulation (29–31). ADNP is a protein essential for
brain development and cognitive activity (32,33). Furthermore, its involvement in
different tumors such as malignant peripheral nerve sheath tumors
(34), colon (35), breast (36), ovarian (37) and bladder cancer (38) and high-grade serous carcinoma
(39) has been previously
reported. However, the functional role of ADNP in other human
cancer development remains poorly characterized, and no evidence
exists regarding its involvement in GBM. In the present study, the
ADNP involvement in this malignancy was investigated for the first
time, in particular its role in tumor hypoxic areas. The results
revealed that ADNP is expressed in most glial cells of human GBM
sections, particularly in hypoxic areas. To investigate whether
ADNP interferes with hypoxia-mediated aberrant angiogenesis, human
GBM cells were exposed to a hypoxic mimetic agent, deferoxamine
(DFX), and were treated with the smallest active element of ADNP,
known as NAP. It is an 8-amino acid peptide synthesized from ADNP
and contains its neuroprotective active site. NAP is largely used
in different in vitro and in vivo studies showing
protective activity already at femtomolar concentrations (40). Moreover, its chemical structure
allows it to cross the cell membrane where it binds to microtubules
and protects astrocytes and neurons (41,42). The present results suggested that
ADNP exerts modulatory activity in GBM progression.
Materials and methods
Human GBM sample and cell lines
The frozen sections of one human GBM sample were
provided from Anatomic Pathology, Department of Medical and
Surgical Sciences and Advanced Technologies ‘G.F. Ingrassia’,
University of Catania. The present study was approved (approval no.
CRAM-015-2020; 16 March 2020) by the ethics committee of the
Research Center on Motor Activities (CRAM) of the University of
Catania (Catania, Italy). The single GBM sample was processed after
the patient (female; 61 years-old; date of sample collection,
25/04/14) had signed the informed consent. The surgical sample was
submitted for frozen sections at the Anatomic Pathology Laboratory
from our Institution and the following inclusion criteria were
adopted: i) representative and viable tumor tissue had to be
present; ii) it must not have contained neither necrosis nor
extensive hemorrhagic changes; and iii) the frozen tissue had to be
sufficient to obtain additional sections for immunohistochemical
and immunofluorescence analyses.
Further analyses were also performed by using human
GBM cell lines of unknown origins, U87MG (cat. no. HTB-14) and A172
(cat. no. CRL-1620), obtained from the American Type Culture
Collection (ATCC). These cell lines were cultured in Dulbecco's
modified Eagle's medium (DMEM) with 10% of heat-inactivated fetal
bovine serum (FBS), 100 U/ml penicillin and 100 µg/ml streptomycin
(all from Sigma-Aldrich; Merck KGaA) and incubated at 37°C in a
humidified atmosphere with 5% CO2 as previously
described (43). DFX (100 µM;
Sigma-Aldrich; Merck KGaA) was used to mimic hypoxic conditions. In
particular, U87MG cells were cultured for 24 h in normoxia or
DFX-induced hypoxia with or without NAP (New England Peptide, Inc.)
at different concentrations of 10 nM, 100 nM and 1 µM. The use of
exogenous administration of DFX, representing a hypoxia-mimetic
iron chelator, offers the advantage of allowing the experimenter to
open the culture plate or dish numerous times without altering the
hypoxic conditions respect to the cell incubation method in the
hypoxic chamber.
Immunohistochemical assay
One surgically resected tumor sample was included in
OCT and fresh-frozen sections were cut at 5 µM and fixed in 4%
paraformaldehyde for 30 min at room temperature. Subsequently, the
inhibition of the endogenous peroxidase activity was performed as
previously described (44) by
using 3% H2O2 in methanol for 10 min. The
sections were then incubated with 1% bovine serum albumin (BSA)
(cat. no. A-3294; Sigma-Aldrich; Merck KGaA) in PBS for 1 h at room
temperature in order to reduce non-specific staining. Subsequently,
they were incubated overnight at 4°C with rabbit anti-ADNP primary
antibody (1:50; cat. no. NBP1-89236; Novus Biologicals, LLC). Then,
the sections were incubated at 4°C for 30 min with secondary
antibodies conjugated to polymer-HRP (LSAB+ System-HRP; cat. no.
K0609; Dako; Agilent Technologies, Inc.). The immunoreaction was
revealed by incubating at room temperature the sections for 5 min
with the 3,3′-diaminobenzidine (DAB) solution (DAB substrate kit;
cat. no. SK-4100; Vector Laboratories, Inc.). The sections were
incubated for 3 min at room temperature with hematoxylin that was
used as a nuclear counterstain. The stained sections were
dehydrated through graded alcohol, cleared in xylene and covered
with neutral balsam. The sections were examined with a Zeiss
Axioplan light microscope (Carl Zeiss AG) and images were captured
with a digital camera (AxioCam MRc5; Carl Zeiss AG).
Immunofluorescence assay
To determine the distribution of ADNP, GFAP, and
HIF-1α, immunofluorescence analysis was performed as previously
described (45). One surgically
resected tumor sample was included in OCT and fresh-frozen sections
were cut at 5 µM and fixed in 4% paraformaldehyde for 30 min.
Subsequently, the inhibition of the endogenous peroxidase activity
was performed as previously described (44), by using 3%
H2O2 in methanol for 10 min. The sections
were then incubated with 1% BSA dissolved in PBS for 1 h in order
to reduce non-specific staining. They subsequently were incubated
overnight with at 4°C with rabbit anti-ADNP (1:50), mouse
anti-HIF-1α (1:50; cat. no. NB100-105; Novus Biologicals, LLC) and
mouse anti-GFAP (1:100; cat. no. IF03L, Calbiochem; Merck KGaA)
antibody.
Cells were cultured on glass cover slips, fixed in
4% paraformaldehyde in PBS for 15 min at room temperature,
permeabilized with 0.2% Triton X-100 (cat. no. sc-29112; Santa Cruz
Biotechnology, Inc.), blocked with 0.1% BSA in PBS and then probed
with rabbit anti-ADNP (1:50) and mouse anti-HIF-1α (1:50) antibody.
Signals were revealed with Alexa Fluor 488-conjugated goat
anti-rabbit (1:20,000; cat. no. A-11008) or Alexa Fluor
594-conjugated goat anti-mouse (1:30,000; cat. no. A-21203; both
from Thermo Fisher Scientific, Inc.) secondary antibodies for 1 h
at room temperature (shielded from light). DNA was counterstained
with 4,6-diamidino-2-phenylindole (DAPI; cat. no. 940110; Vector
Laboratories, Inc.). After a series of washes in PBS and
double-distilled water, the fixed cells were cover-slipped with
Vectashield mounting medium (Vector Laboratories, Inc.).
Immunolocalization was analyzed by confocal laser scanning
microscopy (Zeiss LSM700). Green and blue signals were respectively
detected with laser light at 488 nm/10 mW and 405 nm/5 mW by using
the objective ‘PLANAPOCHROMAT’ 63×/1,40 OIL DIC M27. Each scan was
individually digitalized by a high sensitivity photomultiplier tube
using the following acquisition setup: Gain master: 776; digital
offset: −202; digital gain: 1.0. All acquisitions were performed
with ZEN-2010 software (Zeiss GmbH).
Western blot analysis
Proteins were extracted with buffer containing 20 mM
Tris (pH 7.4), 2 mM EDTA, 0.5 mM EGTA, 50 mM mercaptoethanol, 0.32
mM sucrose and a protease inhibitor cocktail (Roche Diagnostics) as
previously described (43). The
total cell lysates were homogenized, sonicated twice for 20 sec,
and then protein concentrations were determined by the Quant-iT
Protein Assay kit (cat. no. Q33211; Invitrogen; Thermo Fisher
Scientific, Inc.). The protein homogenate (~35 µg) was diluted in
Laemmli buffer (cat. no. 1610747; Bio-Rad Laboratories, Inc.),
heated at 70°C for 10 min, separated by electrophoresis by using
4–15% precast polyacrylamide gel Mini-PROTEAN TGX™ Precast Protein
Gels (cat. no. 4561084) and subsequently transferred in
nitrocellulose membrane (cat. no. 1704158; both from Bio-Rad
Laboratories, Inc.). The Precision Plus Protein Standard (cat. no.
161-0373; Bio-Rad Laboratories, Inc.) was used to monitor
electrophoresis. The proteins transferred onto nitrocellulose
membrane were blocked for 1 h at room temperature with Odyssey
Blocking Buffer (cat. no. 927-70001; LI-COR Biosciences), and
incubated overnight at 4°C with rabbit anti-ADNP (1:200), mouse
anti-HIF-1α (1:200), goat polyclonal anti-VEGF (1:200; cat. no.
sc-1836) and rabbit polyclonal anti-β-tubulin (cat. no. sc-9104;
both from Santa Cruz Biotechnology, Inc.). The secondary antibodies
[goat anti-rabbit IRDye 800CW (1:20,000; cat. no. 926-32211),
donkey anti-goat IRDye 800CW (1:20,000; cat. no. 926-32214) and
goat anti-mouse IRDye 680CW (1:30,000; cat. no. 926-68020D; all
from LI-COR Biosciences)] were used. Blots were visualized with an
Odyssey Infrared Imaging System (Odyssey; http://www.bioagilytix.com/li-cor-for-quantitative-western-blots/)
and the densitometric analyses of blots were performed by using the
ImageJ software version 1.46 (Nstional Institutes of Health).
Values were normalized to β-tubulin that was used as a loading
control.
ELISA
VEGF-A release in conditioned media was measured by
using the ELISA sandwich enzymatic method with a specific
anti-VEGF-A antibody (human VEGF-A; cat. no. ELH-VEGF; RayBiotech,
Inc.) coated on a 96-well plate, according to the manufacturer's
guidelines. Briefly, confluent U87MG cells cultured in media
supplemented with 1% FBS were treated for 24 h with DFX with or
without 100 nM NAP. Standards or supernatants from samples were
pipetted into the wells containing the immobilized anti-VEGF-A
antibody. Then, wells were washed before adding biotinylated
anti-human VEGF antibody. Following incubation, the unbound
biotinylated antibody was washed off, and HRP-conjugated
streptavidin was pipetted in each well. After an additional wash
step, a 3,3′,5,5′-tetramethylbenzidine substrate solution was added
to each well, resulting in a blue coloration proportional to the
amount of bound VEGF. Finally, the stop solution was added, and the
colorimetric intensity of the blue substrate now turned yellow was
measured at 450 nm. The mean absorbance was calculated for each set
of duplicate standards, controls and samples, and the average zero
standard optical density was subtracted.
Conditioned medium (CM) preparation
and tube formation assay
The U87MG cells were cultured in a medium containing
1% FBS, representing CM1 (control) or containing either 100 nM NAP
(CM2) or 100 µM DFX (CM3) or DFX + 100 nM NAP (CM4). They were
incubated for 24 h at 37°C. Subsequently, the CMs were collected
and after centrifugation at 15,000 × g for 5 min at room
temperature the supernatants were frozen at −80°C until use. By
adding 95 µl of Geltrex matrix for each well in a plate of 24 wells
for 30 min at 37°C, polymerization was enabled. Murine
micro-vascular endothelial cells (H5V) were cultured overnight in a
starved medium. Therefore, the H5V were seeded into the layer of
Geltrex matrix and cultured in a medium containing 200 µl of CM1,
CM2, CM3 or CM4 at 37°C for 24 h. A total of 5 randomly selected
fields of view were captured with a digital camera (Canon) attached
to a light inverted microscope (Axio Observer A1; Carl Zeiss AG).
Tube number per field was calculated as percentage of control
(%).
Wound healing assay
U87MG cells were cultured in a six-well plate at a
density of 5×104 cells/well, then were scratched with a
200 µl pipette tip and wound closure was followed. Cells were
incubated in a 1%-FBS medium with or without 100 nM NAP either in
normoxia or in DFX-induced hypoxia (DFX 100 µM) and the distance
that the advancing cells had moved into the cell-free (wound) area
was measured after 24 h. Quantitative measurement of the wound area
was performed under a light inverted microscope and calculated
using ImageJ software version 1.46 (National Institutes of Health).
Data are represented as % wound closure measured 24 h after
scratching, observed in two random fields per well, in duplicate
wells and expressed as a percentage respect to control (%).
Statistical analysis
To analyze the results, GraphPad Prism version 6
software (GraphPad Software, Inc.) was used and data are
represented as the mean ± standard error of the mean (SEM). An
unpaired, two-tailed Student's t-test was performed for comparisons
between two groups. One-way analysis of variance was used to
compare differences among three or more groups. Statistical
significance was determined with the Tukey-Kramer post hoc test.
Co-localization of ADNP with HIF-1α was analyzed using Mander's
overlap coefficient and unpaired t-tests. The level of significance
for all statistical tests was set at P≤0.05.
Results
ADNP expression in GBM tissue and
cells
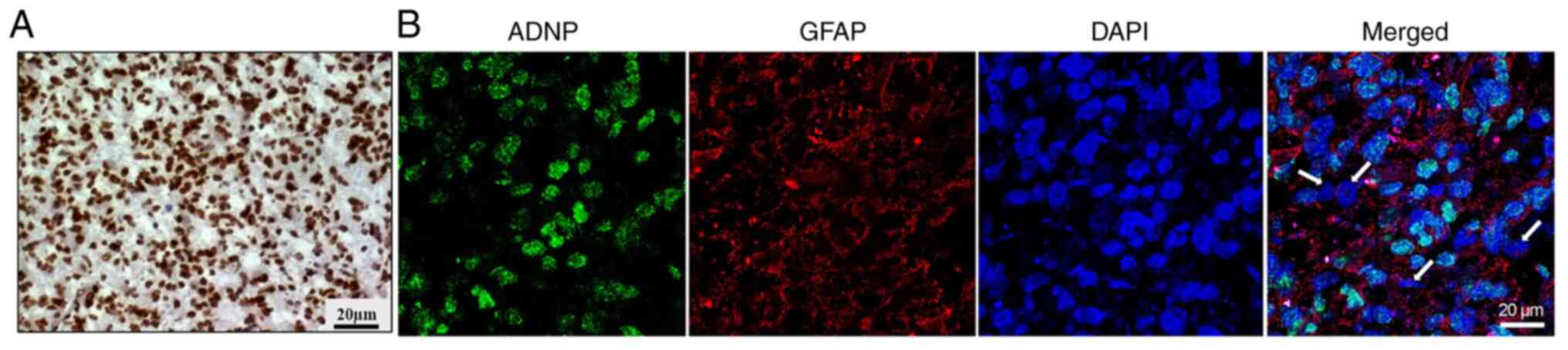
ADNP expression was detected in a GBM sample by
immunohistochemical analysis (Fig.
1A). The analyzed GBM was classified as IDH-wild-type tumors
obtained from the original tumor of one patient. Since the cancer
tissue is characterized by high cellular heterogeneity (46–48), to clarify in an improved way the
phenotype of the cells expressing ADNP, double-labeling staining
was conducted by using the selected marker for ADNP with that
against GFAP. As revealed in Fig.
1B, most of the cells were immune-positive to both GFAP (red
fluorescence) and ADNP (green fluorescence). To investigate the
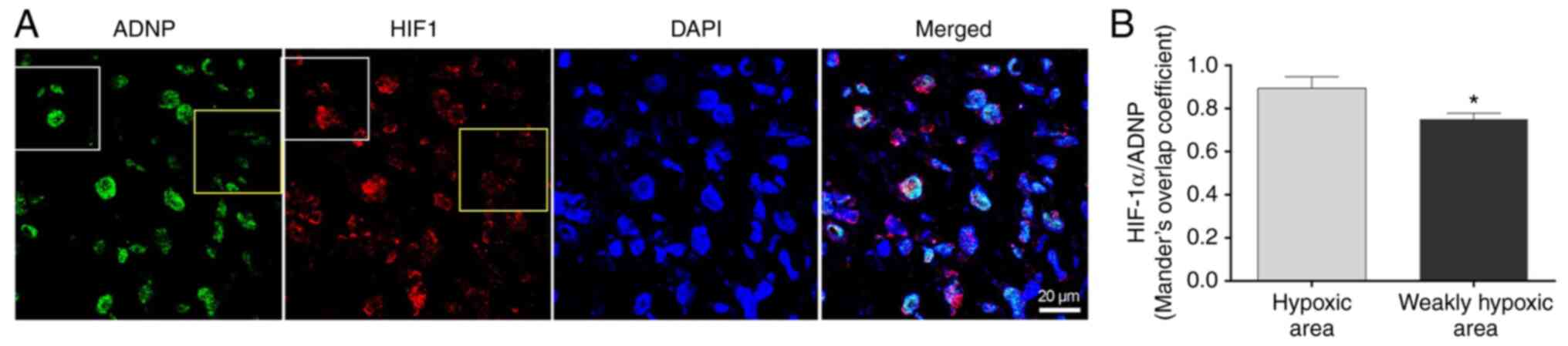
ADNP expression in the hypoxic areas of GBM, its tissue
co-expression with HIF-1α was detected by performing
double-immunofluorescence analysis on human GBM sections (Fig. 2A). The white square indicate the
hypoxic area displaying enhanced levels of HIF-1α and the yellow
square to include the non-hypoxic area (Fig. 2A). The immunofluorescence signals
detected for ADNP in hypoxic areas were quantified through the
Mander's overlap coefficient. It was revealed that ADNP expression
was significantly reduced in weakly hypoxic areas compared with
hypoxic areas (Fig. 2B;
P<0.05).
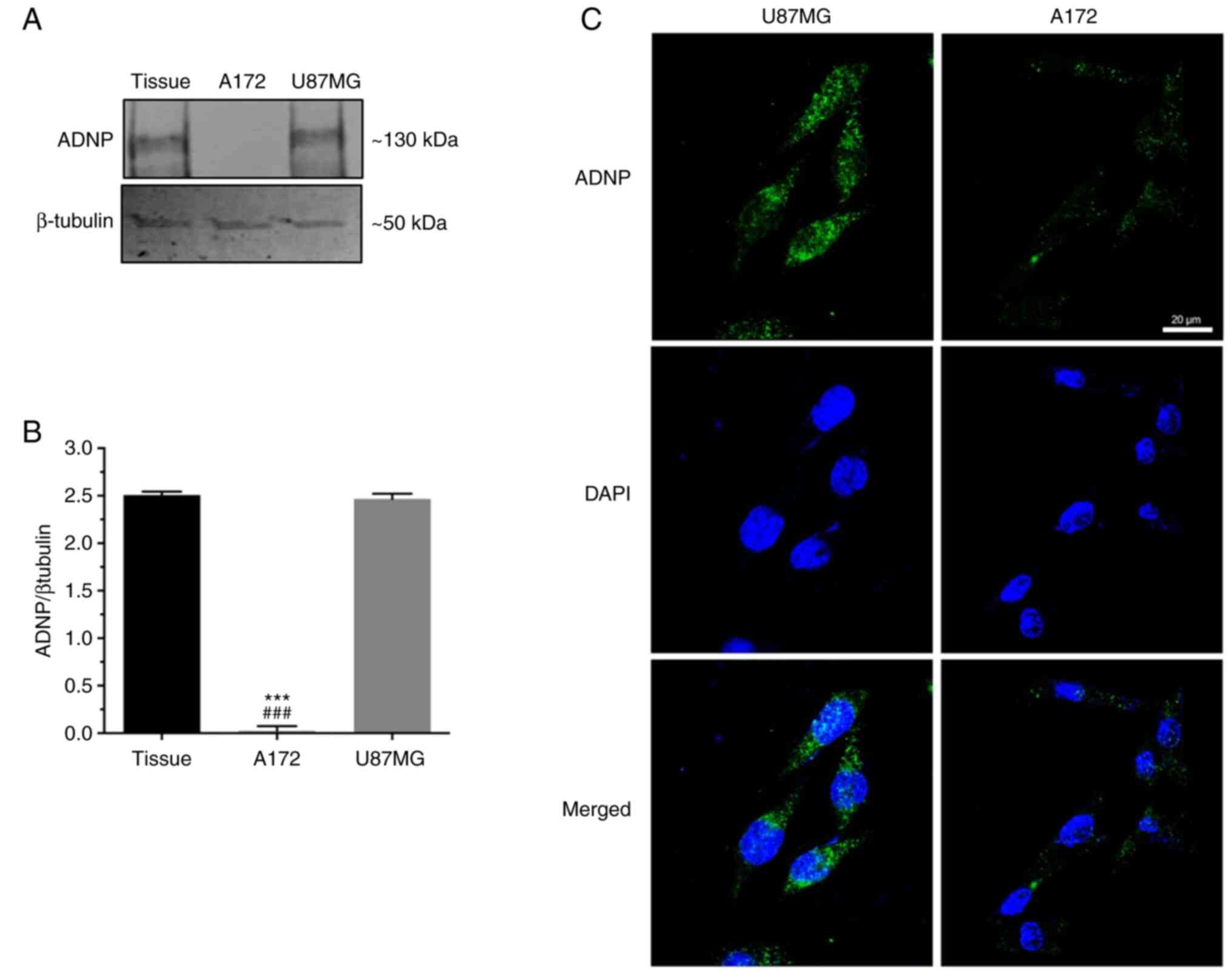
To characterize the ADNP role in this tumor, further
experiments were carried out in vitro by using A172 and
U87MG GBM cell lines. By performing western blot analysis, a
similar protein expression profile was observed between GBM tissue
and U87MG cells. By contrast, no signal was detected in A172 cells
(Fig. 3A and B). This data was
corroborated by immunofluorescence analysis revealing an ADNP
immune-positive signal in the U87MG cells both in nuclear and in
cytoplasmic compartments (Fig.
3C), in contrast to very low immunoreactivity detected in A172
cells. This evidence suggested that peptide expression depends on
cell genotype (49).
Role of ADNP on the hypoxic angiogenic
pathway
Due to the important role of the hypoxic
microenvironment on tumor progression, it was investigated whether
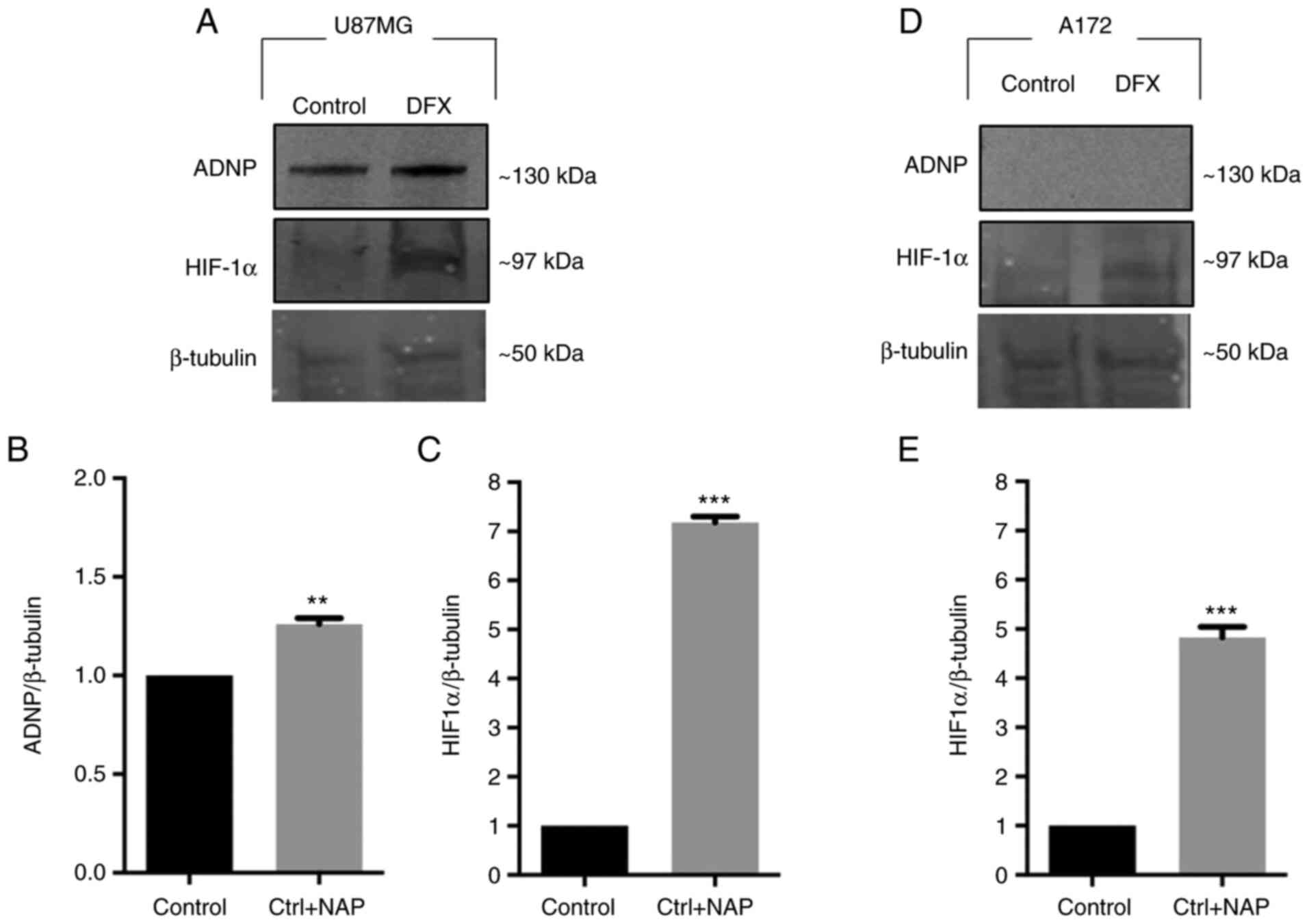
hypoxic insult affects ADNP expression in U87MG and A172 cells. In
a preliminary study, the HIF-1α expression at different time points
after DFX treatment was analyzed to verify the hypoxia status.
Based on the results, 24 h was selected as a time point, as in this
time frame the HIF-1α levels were higher than levels of the other
examined time points of 12, 24 and 48 h (data not shown). As
revealed in Fig. 4A and B, DFX
treatment significantly increased ADNP expression compared with
untreated U87MG cells (**P<0.01 vs. Control). By contrast, A172
cells did not express ADNP either in basal conditions or after DFX
treatment (Fig. 4D). Based on
this evidence, U87MG were selected cells to conduct further
experiments. The detection of HIF-1α levels was used as a positive
control to confirm that hypoxia occurs (Fig. 4C and E; ***P<0.001 vs.
Control). To understand the role of ADNP on hypoxia-induced GBM
progression in an improved way, the effect of NAP, the active
fragment of ADNP, was analyzed at different concentrations in U87MG
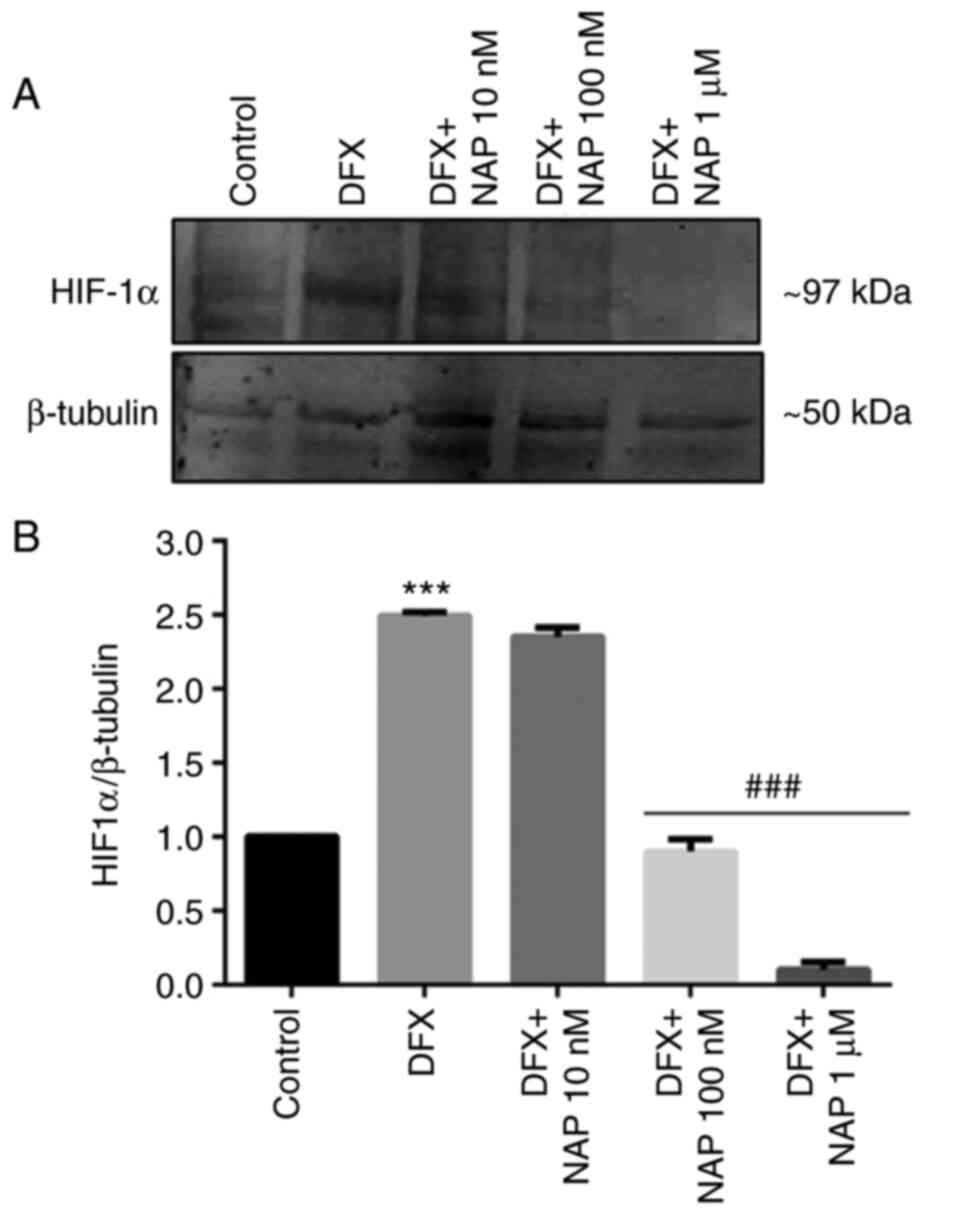
cell line cultured in DFX. As demonstrated in Fig. 5, 100 nM NAP represents the lower
concentration that significantly affected HIF-1α levels by reducing
its expression as compared with DXF-treated cells
(###P<0.01 vs. DFX). Based on this result, 100 nM of
NAP was used to study the role of ADNP on the hypoxic/angiogenic
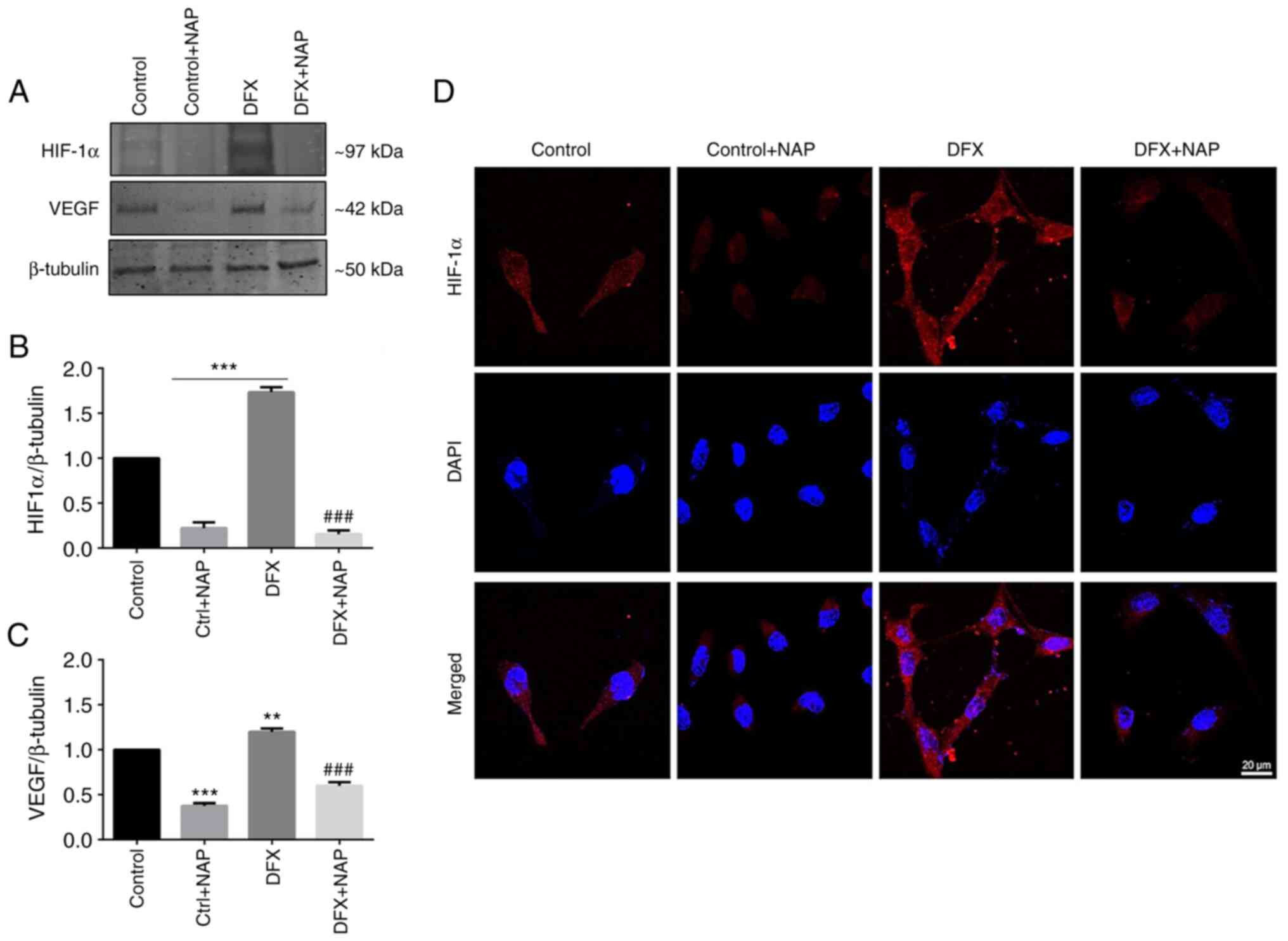
pathway in U87MG cells. DFX-induced hypoxia significantly increased
HIF-1α levels in respect to the control group (Fig. 6A and B; ***P<0.001 vs.
Control). Exogenous administration of the peptide significantly
reduced HIF-1α expression in DFX-treated cells (Fig. 6A and B; ###P<0.01
vs. DFX). This data was corroborated by immunofluorescence analysis
(Fig. 6D) since the high HIF-1α
immunoreactivity displayed in the DFX-treated group was
downregulated following NAP exogenous administration. To
characterize the effect of NAP on the hypoxia-angiogenic pathway,
VEGF expression and release were measured in human GBM cells
cultured for 24 h in a medium containing DFX. Data revealed
increased intracellular VEGF levels (Fig. 6A) as well as its secretion
(Table I) in the DFX-treated
group compared with control cells (**P<0.001 vs. Control;
Fig. 6C). NAP treatment
significantly reduced VEGF expression (Fig. 6C) and release (Table I) in the culture medium of cells
grown under normoxia or DFX-induced hypoxia (***P<0.001 vs.
Control; ###P<0.001 vs. DFX).
 | Table I.VEGF content in the CM deriving from
U87MG cells. The VEGF levels were detected in supernatants and
expressed in pg/ml. Data resulting from three independent
experiments are represented as the mean ± SEM. |
Table I.
VEGF content in the CM deriving from
U87MG cells. The VEGF levels were detected in supernatants and
expressed in pg/ml. Data resulting from three independent
experiments are represented as the mean ± SEM.
| U87MG cell
line-derived conditioned media | CM1 (Control) Mean
+ SEM | CM2 (Control + NAP)
Mean + SEM | CM3 (DFX) Mean +
SEM | CM4 (DFX + NAP)
Mean + SEM |
|---|
| VEGF (pg/ml) | 3,784±107 |
2,495±47a |
6,427±108a |
2,622±63b |
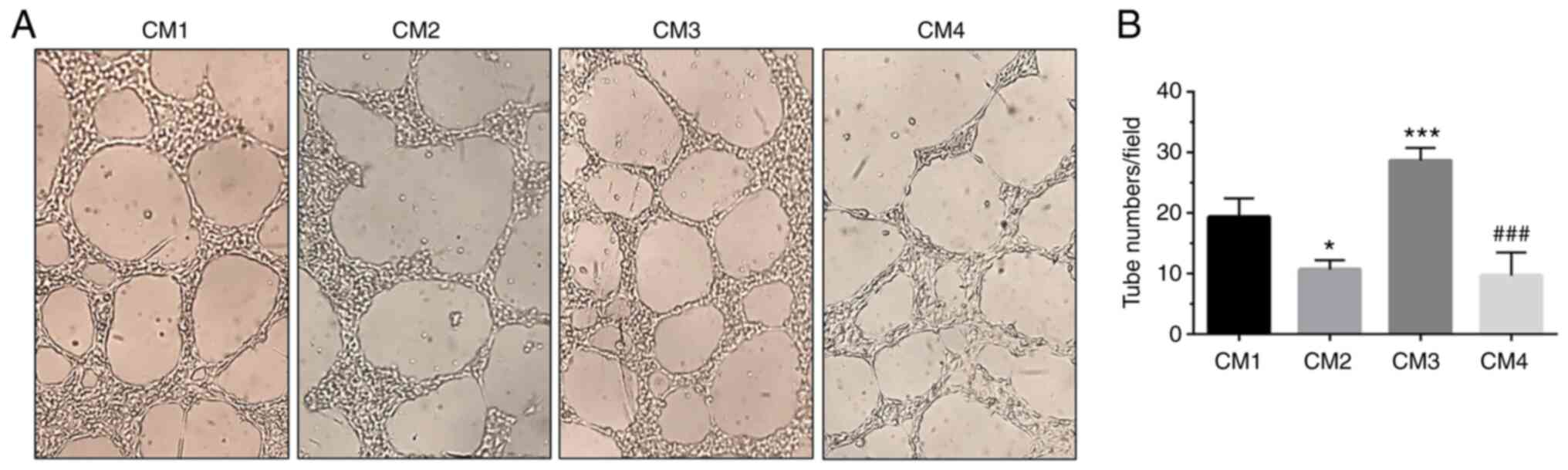
To further analyze the involvement of NAP in the
angiogenesis process, the number of tube-like structures formed by
H5V microvascular endothelial cells cultured in a CM derived from
U87MG cells cultured under normoxia or DFX-induced hypoxia and
treated with NAP was measured. The results revealed that the number
of tube-like structures was increased in cells cultured in CM3,
derived from GBM cells exposed to DFX and containing 6,427 pg/ml
VEGF, as compared with CM1 cultured cells containing 3784 pg/ml
VEGF (Fig. 7A and B;
***P<0.001 vs. CM1). The number of new vessels was significantly
reduced in H5V cells cultured in CM4, deriving from DFX +
NAP-treated U87MG cells and containing 2,622 pg/ml VEGF (Fig. 7A and B; ###P<0.001
vs. CM3). The aforementioned data confirmed that NAP interferes
with the angiogenic process by reducing new vessel formation.
Since hypoxia-driven tumor progression is strictly
linked to an increased cell migration, a preliminary study was also
conducted to characterize ADNP effect on invasion potential of GBM
cells cultured under hypoxia. As revealed in Fig. S1, NAP treatment of DFX-exposed
cells significantly decreased the percentage of wound closure as
compared with DFX-treated group.
Discussion
Uncontrolled cell proliferation in GBM forms the
hypoxic area which promotes aberrant angiogenesis and cancer stem
cell proliferation inside the tumor mass. Previous findings have
highlighted the anti-invasive role exerted by PACAP in GBM
(13,14,50,51). In addition to activation of its
related receptors, this peptide also acts indirectly through
intracellular stimulation of ADNP (23,30,31). The latter is implicated in brain
development during embryogenesis. It has also been identified that
its loss-of-function mutation is related to carcinogenesis
(52–54). Previously, its involvement in
various tumors has been demonstrated, even though its role remains
controversial depending on the type of cancer. It acts as a tumor
suppressor in triple-negative breast cancer (36), as oncogene in ovarian and bladder
cancer (37,38) as well as onco-suppressor or
oncogene in colorectal cancer (35). To date, no findings are available
regarding ADNP implication in GBM. In particular, it was
demonstrated in the present study that it is overexpressed in most
glial cells of human GBM. This evidence is in consistency with
previous studies reporting an increased ADNP expression in tumors
(38,39). Its upregulation was associated
with a poor prognosis in bladder cancer, where it prompted tumor
growth through activation of AKT signaling and induced cisplatin
resistance by promoting cancer cell migration and EMT (38,55). Furthermore, it is involved in the
death resistance of malignant peripheral nerve sheath tumor cells
(34). Notably, higher ADNP
immunoreactivity was detected in the hypoxic area of the tumor mass
by suggesting a direct relation between its upregulation and the
hypoxic microenvironment. Specifically, the hypoxic niches are
generated in the tumor when increased cell proliferation leads to
growth of tissue mass, not supported by an adequate oxygen supply.
These areas present tissue necrosis and aberrant neoangiogenesis
sustained by activation of HIF-VEGF system. To understand the
biological impact of ADNP overexpression in hypoxic niches of GBM,
an in vitro study was performed by using U87MG GBM cells
showing a similar protein expression pattern as compared with the
human GBM sample. These cells exposed to DFX, mimicking
microenvironmental hypoxia, overexpressed ADNP. This result could
be attributed to their tumorigenic potential considering that ADNP
overexpression was only revealed in this cell line whereas was
undetectable in A172 cells. These results could be attributed to
the different genotype of these cell lines. Consistently, U87MG
overexpress nestin and vimentin, two markers of immature astroglia
cells and malignancy, whereas the A172 cells are less tumorigenic
since they do not express these markers (49).
To characterize the role of ADNP in the hypoxic
niches of the tumor, its smallest active element, NAP owing a
chemical structure allowing cell membrane penetration was used
(26,52,53). Its protective effects are widely
reported in literature, demonstrating its implication in numerous
disorders (56,57). By treating DFX-exposed cells with
NAP, it was demonstrated that ADNP interferes with tumor malignancy
by downregulating hypoxia-VEGF pathway. In a previous study, it was
demonstrated that cancer cells of hypoxic niches release numerous
factors in the extracellular microenvironment such as VEGF that
actively participates to aberrant angiogenesis (58). This biological process involves
migration of endothelial cells toward the extracellular matrix,
where they cooperate to create a new lumen (58). In the present study, it was
demonstrated that cancer cells release VEGF in the extracellular
medium, which triggers neovascularization process as revealed by
increased formation of tube-like structures when H5V endothelial
cells were cultured in CM derived from GBM cells exposed to DFX
(CM3). CM derived from U87MG cells treated with NAP contains a
reduced amount of VEGF which, in turn, it is related to a reduced
formation of tube-like structures.
The preliminary study conducted is in line with
previous evidence demonstrating that induction of ADNP expression
in colon cancer leads to the inhibition of tumor growth, which was
also correlated with prolonged survival of the animals (35). In the aforementioned type of
cancer, ADNP acted as a negative regulator of WNT signaling, one of
the main factors responsible for colon tumor development. Moreover,
the silencing of ADNP increased cell proliferation and tumor
progression in xenografts in vivo. It is worth noting that
patients with high levels of ADNP survived during the follow-up
period, while moderate or negative ADNP expression was related to a
high frequency of cancer-related death. These data suggested that
ADNP could be considered an onco-suppressor in colon cancer.
In a previous study, enhanced expression of PACAP
and PAC1R was demonstrated in hypoxic areas of GBM (14). Based on the present results, it
cannot be excluded that the aforementioned effects are mediated by
PACAP binding to its receptor PAC1R which stimulates ADNP formation
in the hypoxic niches of GBM. At the light of this aspect, it is
planned to further characterize the role of PACAP-ADNP axis in
GBM.
In conclusion, the present results suggested that
ADNP may act as a tumor suppressor in GBM, in particular, in the
hypoxic niches by interfering with the aberrant angiogenesis.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the STARTING GRANT 2020,
titled ‘Regulatory effect of PACAP-ADNP axis and its involvement in
modulation of Glioblastoma multiforme’, Department of Drug Science,
University of Catania. The present study was also supported by
National Research, Development and Innovation Fund (grant nos.
K135457, TKP 2021-AGE-16 and ELKH-TKI-14016).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
AGD, GMa and VD conceptualized the study. BM, SG,
GMu, SS and CF provided methodology. EP provided technical support.
AGD and CF performed software analysis. AGD and GM conducted
investigation. RC and GB provided resources. AGD and GMa performed
data curation. AGD, GMa, DR and VD wrote the original draft. VD and
DR wrote, reviewed and edited the manuscript. GMu, VD and DR
supervised the study. AGD and DR acquired funding. AGD, GMa, BM,
SG, SS and CF, EP, RC, GB, GMu, DR and VD made substantial
contributions to conception and design of the present study,
acquisition, analysis and interpretation of data. AGD, GMa, RC, GB,
DR and VD were involved in drafting the manuscript or revising it
critically for important intellectual content. AGD, GMa and VD
confirm the authenticity of all the raw data. All authors have read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved (approval no.
CRAM-015-2020; 16 March 2020) by the ethics committee of the
Research Center on Motor Activities (CRAM) of the University of
Catania (Catania, Italy). Written informed consent was obtained
from the subject involved in the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wesseling P and Capper D: WHO 2016
classification of gliomas. Neuropathol Appl Neurobiol. 44:139–150.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
D'Amico AG, Maugeri G, Vanella L, Pittalà
V, Reglodi D and D'Agata V: Multimodal role of PACAP in
glioblastoma. Brain Sci. 11:9942021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wenger A, Vega SF, Kling T, Bontell TO,
Jakola AS and Carén H: Intratumor DNA methylation heterogeneity in
glioblastoma: Implications for DNA methylation-based
classification. Neuro Oncol. 21:616–627. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Semenza GL: Intratumoral hypoxia,
radiation resistance, and HIF-1. Cancer Cell. 5:405–406. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vaupel P and Mayer A: Hypoxia in cancer:
Significance and impact on clinical outcome. Cancer Metastasis Rev.
26:225–239. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maugeri G, D'Amico AG, Reitano R, Magro G,
Cavallaro S, Salomone S and D'Agata V: PACAP and vip inhibit the
invasiveness of glioblastoma cells exposed to hypoxia through the
regulation of HIFs and EGFR expression. Front Pharmacol. 7:1392016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Maugeri G, D'Amico AG, Rasà DM, Saccone S,
Federico C, Cavallaro S and D'Agata V: PACAP and VIP regulate
hypoxia-inducible factors in neuroblastoma cells exposed to
hypoxia. Neuropeptides. 69:84–91. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Anjum K, Shagufta BI, Abbas SQ, Patel S,
Khan I, Shah SAA, Akhter N and Hassan SSU: Current status and
future therapeutic perspectives of glioblastoma multiforme (GBM)
therapy: A review. Biomed Pharmacother. 92:681–689. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Touat M, Idbaih A, Sanson M and Ligon KL:
Glioblastoma targeted therapy: Updated approaches from recent
biological insights. Ann Oncol. 28:1457–1472. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Weller M, van den Bent M, Tonn JC, Stupp
R, Preusser M, Cohen-Jonathan-Moyal E, Henriksson R, Rhun EL,
Balana C, Chinot O, et al: European association for neuro-oncology
(EANO) guideline on the diagnosis and treatment of adult astrocytic
and oligodendroglial gliomas. Lancet Oncol. 8:e315–e329. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cochaud S, Chevrier L, Meunier AC, Brillet
T, Chadéneau C and Muller JM: The vasoactive intestinal
peptide-receptor system is involved in human glioblastoma cell
migration. Neuropeptides. 44:373–383. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cochaud S, Meunier AC, Monvoisin A,
Bensalma S, Muller JM and Chadéneau C: Neuropeptides of the VIP
family inhibit glioblastoma cell invasion. J Neurooncol. 122:63–73.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maugeri G, D'Amico AG, Saccone S, Federico
C, Rasà DM, Caltabiano R, Broggi G, Giunta S, Musumeci G and
D'Agata V: Effect of PACAP on hypoxia-induced angiogenesis and
epithelial-mesenchymal transition in glioblastoma. Biomedicines.
9:9652021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Toth D, Szabo E, Tamas A, Juhasz T,
Horvath G, Fabian E, Opper B, Szabo D, Maugeri G, D'Amico AG, et
al: Protective effects of PACAP in peripheral organs. Front
Endocrinol (Lausanne). 11:3772020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
D'Amico AG, Maugeri G, Saccone S, Federico
C, Cavallaro S, Reglodi D and D'Agata V: PACAP modulates the
autophagy process in an in vitro model of amyotrophic lateral
sclerosis. Int J Mol Sci. 21:29432020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lauretta G, Ravalli S, Szychlinska MA,
Castorina A, Maugeri G, D'Amico AG, D'Agata V and Musumeci G:
Current knowledge of pituitary adenylate cyclase activating
polypeptide (PACAP) in articular cartilage. Histol Histopathol.
35:1251–1262. 2020.PubMed/NCBI
|
|
18
|
D'Amico AG, Maugeri G, Musumeci G, Reglodi
D and D'Agata V: PACAP and NAP: Effect of two functionally related
peptides in diabetic retinopathy. J Mol Neurosci. 71:1525–1535.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maugeri G, D'Amico AG, Musumeci G, Reglodi
D and D'Agata V: Effects of pacap on schwann cells: Focus on nerve
injury. Int J Mol Sci. 21:82332020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Castorina A, Scuderi S, D'Amico AG, Drago
F and D'Agata V: PACAP and VIP increase the expression of
myelin-related proteins in rat schwannoma cells: Involvement of
PAC1/VPAC2 receptor-mediated activation of PI3K/Akt signaling
pathways. Exp Cell Res. 322:108–121. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maugeri G, D'Amico AG, Bucolo C and
D'Agata V: Protective effect of PACAP-38 on retinal pigmented
epithelium in an in vitro and in vivo model of diabetic retinopathy
through EGFR-dependent mechanism. Peptides. 119:1701082019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vaudry D, Falluel-Morel A, Bourgault S,
Basille M, Burel D, Wurtz O, Fournier A, Chow BK, Hashimoto H,
Galas L and Vaudry H: Pituitary adenylate cyclase-activating
polypeptide and its receptors: 20 years after the discovery.
Pharmacol Rev. 61:283–357. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zusev M and Gozes I: Differential
regulation of activity-dependent neuroprotective protein in rat
astrocytes by VIP and PACAP. Regul Pept. 123:33–41. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dejda A, Sokołowska P and Nowak JZ:
Neuroprotective potential of three neuropeptides PACAP, VIP and
PHI. Pharmacol Rep. 57:307–320. 2005.PubMed/NCBI
|
|
25
|
Lelievre V, Ghiani CA, Seksenyan A,
Gressens P, de Vellis J and Waschek JA: Growth factor-dependent
actions of PACAP on oligodendrocyte progenitor proliferation. Regul
Pept. 137:58–66. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bassan M, Zamostiano R, Davidson A,
Pinhasov A, Giladi E, Perl O, Bassan H, Blat C, Gibney G, Glazner
G, et al: Complete sequence of a novel protein containing a
femtomolar-activity-dependent neuroprotective peptide. J Neurochem.
72:1283–1293. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gozes I, Bassan M, Zamostiano R, Pinhasov
A, Davidson A, Giladi E, Perl O, Glazner GW and Brenneman DE: A
novel signaling molecule for neuropeptide action:
Activity-dependent neuroprotective protein. Ann N Y Acad Sci.
897:125–135. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zamostiano R, Pinhasov A, Gelber E,
Steingart RA, Seroussi E, Giladi E, Bassan M, Wollman Y, Eyre HJ,
Mulley JC, et al: Cloning and characterization of the human
activity-dependent neuroprotective protein. J Biol Chem.
276:708–714. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li M, David C, Kikuta T, Somogyvari-Vigh A
and Arimura A: Signaling cascades involved in neuroprotection by
subpicomolar pituitary adenylate cyclase-activating polypeptide 38.
J Mol Neurosci. 27:91–105. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nakamachi T, Li M, Shioda S and Arimura A:
Signaling involved in pituitary adenylate cyclase-activating
polypeptide-stimulated ADNP expression. Peptides. 27:1859–1864.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nakamachi T, Ohtaki H, Yofu S, Dohi K,
Watanabe J, Hayashi D, Matsuno R, Nonaka N, Itabashi K and Shioda
S: Pituitary adenylate cyclase-activating polypeptide (PACAP) type
1 receptor (PAC1R) co-localizes with activity-dependent
neuroprotective protein (ADNP) in the mouse brains. Regul Pept.
145:88–95. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gozes I, Alcalay R, Giladi E, Pinhasov A,
Furman S and Brenneman DE: NAP accelerates the performance of
normal rats in the water maze. J Mol Neurosci. 19:167–170. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pinhasov A, Mandel S, Torchinsky A, Giladi
E, Pittel Z, Goldsweig AM, Servoss SJ, Brenneman DE and Gozes I:
Activity-dependent neuroprotective protein: A novel gene essential
for brain formation. Brain Res Dev Brain Res. 144:83–90. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Castorina A, Giunta S, Scuderi S and
D'Agata V: Involvement of PACAP/ADNP signaling in the resistance to
cell death in malignant peripheral nerve sheath tumor (MPNST)
cells. J Mol Neurosci. 48:674–683. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Blaj C, Bringmann A, Schmidt EM, Urbischek
M, Lamprecht S, Fröhlich T, Arnold GJ, Krebs S, Blum H, Hermeking
H, et al: ADNP is a therapeutically inducible repressor of WNT
signaling in colorectal cancer. Clin Cancer Res. 23:2769–2780.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rangel R, Guzman-Rojas L, Kodama T, Kodama
M, Newberg JY, Copeland NG and Jenkins NA: Identification of new
tumor suppressor genes in triple-negative breast cancer. Cancer
Res. 77:4089–4101. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Karagoz K, Mehta GA, Khella CA, Khanna P
and Gatza ML: Integrative proteogenomic analyses of human tumours
identifies ADNP as a novel oncogenic mediator of cell cycle
progression in high-grade serous ovarian cancer with poor
prognosis. EBioMedicine. 50:191–202. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhu S, Xu Z, Zeng Y, Long Y, Fan G, Ding
Q, Wen Y, Cao J, Dai T, Han W and Xie Y: ADNP upregulation promotes
bladder cancer cell proliferation via the AKT Pathway. Front Oncol.
10:4911292020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Turashvili G: ADNP (Activity Dependent
Neuroprotector Homeobox): A novel oncogene driving poor prognosis
in high-grade serous carcinoma. EBioMedicine. 51:1025892020.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gozes I, Morimoto BH, Tiong J, Fox A,
Sutherland K, Dangoor D, Holser-Cochav M, Vered K, Newton P, Aisen
PS, et al: NAP: Research and development of a peptide derived from
activity-dependent neuroprotective protein (ADNP). CNS Drug Rev.
11:353–368. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gozes I: Activity-dependent
neuroprotective protein: From gene to drug candidate. Pharmacol
Ther. 114:146–154. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gozes I: NAP (davunetide) provides
functional and structural neuroprotection. Curr Pharm Des.
17:1040–1044. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Maugeri G, D'Amico AG, Rasà DM, Saccone S,
Federico C, Magro G, Cavallaro S and D'Agata V: Caffeine effect on
HIFs/VEGF pathway in human glioblastoma cells exposed to hypoxia.
Anticancer Agents Med Chem. 18:1432–1439. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bonaventura G, Iemmolo R, D'Amico AG, La
Cognata V, Costanzo E, Zappia M, D'Agata V, Conforti FL, Aronica E
and Cavallaro S: PACAP and PAC1R are differentially expressed in
motor cortex of amyotrophic lateral sclerosis patients and support
survival of iPSC-derived motor neurons. J Cell Physiol.
233:3343–3351. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
D'Agata V, Grimaldi M, Pascale A and
Cavallaro S: Regional and cellular expression of the parkin gene in
the rat cerebral cortex. Eur J Neurosci. 12:3583–3588. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yao M, Li S, Wu X, Diao S, Zhang G, He H,
Bian L and Lu Y: Cellular origin of glioblastoma and its
implication in precision therapy. Cell Mol Immunol. 15:737–739.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Guichet PO, Guelfi S, Ripoll C, Teigell M,
Sabourin JC, Bauchet L, Rigau V, Rothhut B and Hugnot JP:
Asymmetric distribution of GFAP in glioma multipotent cells. PLoS
One. 11:e01512742016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kim HJ, Park JW and Lee JH: Genetic
architectures and cell-of-origin in glioblastoma. Front Oncol.
10:6154002020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Belot N, Rorive S, Doyen I, Lefranc F,
Bruyneel E, Dedecker R, Micik S, Brotchi J, Decaestecker C, Salmon
I, et al: Molecular characterization of cell substratum attachments
in human glial tumors relates to prognostic features. Glia.
36:375–390. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bensalma S, Turpault S, Balandre AC, De
Boisvilliers M, Gaillard A, Chadéneau C and Muller JM: PKA at a
cross-road of signaling pathways involved in the regulation of
glioblastoma migration and invasion by the neuropeptides VIP and
PACAP. Cancers (Basel). 11:1232019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
D'Amico AG, Scuderi S, Saccone S,
Castorina A, Drago F and D'Agata V: Antiproliferative effects of
PACAP and VIP in serum-starved glioma cells. J Mol Neurosci.
51:503–513. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gozes I and Ivashko-Pachima Y: ADNP: In
search for molecular mechanisms and innovative therapeutic
strategies for frontotemporal degeneration. Front Aging Neurosci.
7:2052015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Gozes I, Yeheskel A and Pasmanik-Chor M:
Activity-dependent neuroprotective protein (ADNP): A case study for
highly conserved chordata-specific genes shaping the brain and
mutated in cancer. J Alzheimers Dis. 45:57–73. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Jo YS, Kim MS, Yoo NJ, Lee SH and Song SY:
ADNP encoding a transcription factor interacting with BAF complexes
exhibits frameshift mutations in gastric and colorectal cancers.
Scand J Gastroenterol. 51:1269–1271. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Xie Y, Zhu S, Zang J, Wu G, Wen Y, Liang
Y, Long Y, Guo W, Zang C, Hu X, et al: ADNP prompts the
cisplatin-resistance of bladder cancer via TGF-β-mediated
epithelial-mesenchymal transition (EMT) pathway. J Cancer.
12:5114–5124. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Scuderi S, D'Amico AG, Castorina A,
Federico C, Marrazzo G, Drago F, Bucolo C and D'Agata V: Davunetide
(NAP) protects the retina against early diabetic injury by reducing
apoptotic death. J Mol Neurosci. 54:395–404. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
D'Amico AG, Scuderi S, Maugeri G,
Cavallaro S, Drago F and D'Agata V: NAP reduces murine
microvascular endothelial cells proliferation induced by
hyperglycemia. J Mol Neurosci. 54:405–413. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Bergers G and Benjamin LE: Tumorigenesis
and the angiogenic switch. Nat Rev Cancer. 3:401–410. 2003.
View Article : Google Scholar : PubMed/NCBI
|