Introduction
Currently, tumour cell resistance to chemotherapy is
one of the leading causes of colorectal cancer (CRC)-related death
(1). Therefore, a more
comprehensive understanding of drug resistance mechanisms is
required to better combat this disease.
It is well known that hypoxia is closely related to
the occurrence and development of tumours, and is a common
phenomenon in most malignant tumours (2). Hypoxia occurs during tumorigenesis
because of insufficient oxygen supply due to irregularities or
spacing of tumour blood vessels. Cancer cells survive mainly by
adapting to the environment in response to hypoxia through a
variety of cellular mechanisms (3). Hypoxia serves important roles in
tumour initiation and progression, especially in promoting tumour
chemotherapy resistance (4). The
hypoxic microenvironment can promote chemoresistance in tumour
cells through a variety of related mechanisms, including inhibition
of apoptotic signalling pathways, which is one of the mechanisms
that leads to tumour chemoresistance (5-7). In
the hypoxic tumour microenvironment, the expression of Bcl2
increases, whereas that of proapoptotic proteins decreases, and
tumour cells overexpressing Bcl2 can lead to drug resistance due to
their decreased apoptotic ability (8). Thus, strategies that can regulate
hypoxia-mediated drug resistance in CRC have broad clinical
potential.
α-hederin is a typical pentacyclic triterpene
saponin, and a large number of studies have shown that pentacyclic
triterpene saponins have a wide range of biological activities,
such as antitumour, antiviral, anti-inflammatory and
immunoregulatory activities (9).
To date, extensive research has been carried out on the antitumour
effects of α-hederin, such as inhibition of tumour cell metastasis
and tumour immune evasion, and enhancement of chemotherapeutic drug
sensitivity (10-14). Therefore, it was hypothesized that
α-hederin may inhibit the expression of Bcl2 by reducing the
phosphorylation of AKT, thereby overcoming hypoxia-mediated drug
resistance in CRC. The present study explored the mechanism by
which α-hederin overcomes hypoxia-mediated chemoresistance in CRC
in vivo and in vitro.
Materials and methods
Cell lines and reagents
The human CRC cell lines HCT116 (TCHu 99), RKO
(TCHu116), HCT15 (TCHu133) and DLD-1 (TCHu134) were purchased from
and authenticated by The Cell Bank of Type Culture Collection of
The Chinese Academy of Sciences. All cell lines were cultured in
RPMI-1640 medium (HCT116, HCT15 and DLD-1) or DMEM (RKO) containing
10% FBS in a hypoxic cell incubator containing 1% O2
(21%O2 was used for normoxia)Culture medium and FBS were
purchased from Gibco; Thermo Fisher Scientific, Inc. The AKT
overexpression plasmid was purchased from Addgene, Inc.
Cell viability and apoptosis assays
HCT116, RKO, HCT15 and DDR-1 cells
(5×103) were inoculated into 96-well plates and, after
48 h, α-hederin (Chengdu Must Bio-Technology Co., Ltd), OXA (Chia
Tai Tianqing Pharmaceutical Group Co., Ltd.) or 5-FU (Chia Tai
Tianqing Pharmaceutical Group Co., Ltd.) were added. After 48 h of
treatment at 37°C, cell viability was evaluated using the Cell
Counting Kit 8 (CCK-8) assay (Dojindo Molecular Technologies,
Inc.), and OD was detected after incubation with CCK-8 reagent for
2 h (37°C). The IC50 values of different drugs were
mainly determined by setting a drug concentration gradient. OD
values were determined 48 h after treatment according to the
previously described method and IC50 values were
obtained according to cell survival rate. Apoptosis analysis was
performed using an apoptosis detection kit (cat. no. 556547; BD
Biosciences) according to the manufacturer's instructions.
Apoptosis was detected using a flow cytometer (FACSCalibur; BD
Biosciences), and the results were analysed by FlowJo software
(V10.8.1; FlowJo, LLC).
BrdU assay
The proliferation of CRC cells (HCT116, RKO, DLD1
and HCT15) was evaluated using the cell proliferation ELISA BrdU
kit (cat. no. 11647229001; Roche Diagnostics GmbH) following
treatment with different concentrations of α-hederin (0, 2.5, 5, 10
and 20 µM), according to the manufacturer's instructions.
The data were analysed using an ELISA reader at a wavelength of 450
nm.
JC-1 immunofluorescence assay
CRC cells (1×106) were inoculated in a
six-well cell culture plate, cultured for 24 h, followed by
treatment with different concentrations of α-hederin (0, 5, 10 and
20 µM) for 48 h at 37°C. Different groups of cells were
harvested and washed in PBS, and JC-1 (MedChemExpress) was added.
JC-1 reagent (1 µl) was added to 500 µl 1X Incubation
Buffer to provide the JC-1 working fluid, of which 500 µl
was used to treat the cells. Cells were incubated with the working
solution for 20 min. After washing the cells, one drop of cell
suspension was added to glass slides, which were slowly covered
with a cover slip, and observed under a laser confocal microscope.
In the experiment, the JC-1 dye was used as a fluorescent probe,
which is considered an indicator of mitochondrial membrane
potential. When the mitochondrial membrane potential is high, JC-1
accumulates in the matrix of mitochondria and forms polymers, which
can produce red fluorescence, whereas when the mitochondrial
membrane potential is low, JC-1 cannot gather in the mitochondrial
matrix, and at this time, JC-1 exists as a monomer that can produce
green fluorescence. Red fluorescence indicates the presence of
living cells that have maintained their mitochondrial membrane
potential, whereas green fluorescence indicates the presence of
cells that have undergone apoptosis or necrosis.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from CRC cells and mouse
tumour tissues using TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.), followed by RT and qPCR [95°C for 30 sec
(pre-denaturation), followed by 40 cycles at 95°C for 5 sec
(denaturation), 60°C for 30 sec (annealing), 72°C for 30 sec
(extension)] using the PrimeScript RT-PCR kit (Takara Bio, Inc.)
according to the manufacturer's instructions. The 2−ΔΔCq
method (15) was used to determine
the relative expression levels in each cell line in each group. The
PCR primer sequences (Accurate Biology) were as follows: Bcl2,
forward 5′-TAC CTG AAC CGG CAC CTG-3′ and reverse 5′-GCC GTA CAG
TTC CAC AAA GG-3′; Bcl-XL, forward 5′-TTG GAC AAT GGA CTG GTT GA-3′
and reverse 5′-TGG GAT GTC AGG TCA CTG AA-3′; β-actin, forward
5′-ATT GCC GAC AGG ATG CAG AA-3′ and reverse 5′-GCT GAT CCA CAT CTG
CTG GAA-3′; HIF-1α, forward 5′-ATC CAT GTG ACC ATG AGG AAT G-3′ and
reverse 5′-TCG GCT AGT TAG GGT ACA CTT C-3′.
Western blot (WB) analysis
Cells were lysed in RIPA Lysis Buffer
(MedChemExpress), after which proteins were quantified using the
BCA Protein Reagent Assay Kit (Beyotime Institute of
Biotechnology), according to the manufacturer's instructions.
Proteins (20 µg) were separated by SDS-PAGE on 10% gels and
were transferred to polyvinylidene fluoride membranes. The
membranes were blocked with 5% BSA (Beyotime Institute of
Biotechnology) with agitation at room temperature for 1 h. Finally,
the membranes were incubated with primary antibodies at 4°C
overnight and then with secondary antibodies at room temperature
for 1 h. Immunoblotting was assessed using an enhanced
chemiluminescent substrate (MilliporeSigma) and bands were
visualized using a chemiluminescence detection system (Bio-Rad
Laboratories, Inc.). The primary antibodies used were: AKT (cat.
no. ab8805; 1:500), phosphorylated (p)-AKT (cat. no. ab38449;
1:1,000), STAT3 (cat. no. ab68153; 1:1,000), p-STAT3 (cat. no.
ab76315; 1:5,000), cleaved Caspase-3 (cat. no. ab32042; 1:500),
cleaved PARP (cat. no. ab32064; 1:2,000) (all from Abcam), HIF-1α
(cat. no. 36169S; 1:1,000), Bcl2 (cat. no. 15071S; 1:1,000), Bcl-xL
(cat. no. 2764S; 1:1,000) and β-actin (cat. no. 3700S; 1:1,000)
(all from Cell Signaling Technology, Inc.). Secondary antibodies
(1:5,000) were also purchased from Cell Signaling Technology, Inc.
(anti-mouse secondary antibody, cat. no. 7076S; anti-rabbit
secondary antibody, cat. no. 7074S). Semi-quantification of protein
bands was performed using ImageJ software (version 1.8.0; National
Institutes of Health).
Transcriptome sequencing and
analysis
Transcriptome sequencing and analysis were conducted
by Shanghai OE Biotech Co., Ltd. Total cellular RNA was extracted
using the TruSeq Stranded mRNA LTSample Prep Kit (cat. no.
RS-122-2101; Illumina, Inc.), DNA was digested with DNase, cellular
mRNA was enriched with magnetic beads containing only thymine
nucleotide chains [oligo (dT)] and a breaking reagent was added to
break mRNA. Using the interrupted short fragment as a template, a
six-base random primer was used to synthesize one-strand cDNA, then
a two-strand synthesis reaction system was prepared to synthesize
two-strand cDNA, and the double-strand cDNA was purified. The
purified double-strand cDNA was then subjected to end repair, an A
tail was added and sequencing adapters were connected.
Subsequently, fragment size selection and PCR amplification were
performed. After the quality of the constructed library was
assessed using an Agilent 2100 bioanalyzer (Agilent Technologies,
Inc.), it was sequenced using the Illumina HiSeq™ 2500 sequencer
(Illumina, Inc.) to generate 125 or 150 bp paired-end data. HiSeq X
HD Reagent kit (300 cycles; cat. no. FC-501-1001; Illumina, Inc.)
was used for sequencing. Library quality was assessed using the
Agilent 2100 Bioanalyzer (Agilent Technologies, Inc.), and the
loading concentration of the final library was >0.5 ng/G for DNA
sequencing.
Subsequently, related data analysis, such as
transcript level quantification, differential gene screening,
functional enrichment and cluster analysis, was performed. Clean
reads were sequenced with the designated reference genomes using
hisat2 [(2.2.1.0) (16)] to obtain
location information on reference genomes or genes, as well as
specific sequence characteristics of sequenced samples. The known
reference gene sequences and annotation files were used to identify
the expression abundance of each protein-coding gene in each sample
by sequence similarity comparison. htseq-count software [(0.9.1)
(17)] was used to obtain the
number of reads aligned to protein-coding genes in each sample, and
cufflinks software [(2.2.1) (18)]
was used to calculate the FPKM value of protein-coding gene
expression. Differential expression analysis aims to identify
differentially expressed genes among different samples. After
obtaining differentially expressed genes, Gene Ontology (GO)
functional significance and Kyoto Encyclopedia of Genes and Genomes
(KEGG) pathway significance analyses were conducted.
ELISA
Cytochrome c and Caspase-3 levels were
measured using a Cytochrome c ELISA kit (cat. no. ab221832;
Abcam) and a Caspase-3 ELISA kit (cat. no. EK1425; Wuhan Boster
Biological Technology, Ltd.), respectively. The cell supernatant
was collected from each group according to the manufacturer's
protocol, and a microplate reader was used to measure the optical
density value at 450 nm and to calculate the experimental
results.
Patient-derived tumor xenograft (PDX)
models
A total of 10 patients with colorectal cancer (age,
55-65 years; six male patients, four female patients) were
recruited between April 2020 and April 2021. Tissue (primary tumour
tissues from patients with CRC) was collected in RPMI-1640
supplemented with penicillin and streptomycin. The mice were
anesthetized with pentobarbital sodium (50 mg/kg, i.p.) before the
tumour tissue was transplanted. Next, 2-3 mm3 blocks
were immediately subcutaneously transplanted on the backs of 15
male athymic nude mice (age, 5-6 weeks; weight, 20 g,; Shanghai
SLAC Laboratory Animal Co., Ltd.). The mice were housed at a
constant temperature of 22°C, with a relative humidity of 30% under
a 12-h light/dark cycle, and were given free access to food and
water. Tumours from the first generation of mice were harvested
after reaching ~1.5 cm in diameter and were directly reimplanted
into a new generation of nude mice for up to five generations. The
mice (n=10) were then randomly divided into the following groups:
Vehicle group and α-hederin (5 mg/kg) group (n=5 mice/group); the
drugs were administered by intraperitoneal injection 5 days/week
for 3 weeks. Remnant tumour samples (suspended in 10% DMSO plus 10%
FBS-containing RPMI-1640) were cryopreserved in liquid nitrogen for
further research on other related subjects. Euthanasia was
performed by intraperitoneal injection of 200 mg/kg pentobarbital.
The institutional animal care and use committee of Putuo Hospital,
Shanghai University of Traditional Chinese Medicine (Shanghai,
China) approved all animal experiments according to the guidelines
and protocols (approval no. AP202204011).
In vivo mouse model
The institutional animal care and use committee of
Putuo Hospital, Shanghai University of Traditional Chinese Medicine
approved all animal experiments according to the guidelines and
protocols (approval no. AP202204011). A xenograft model of CRC was
established via subcutaneous inoculation of 20 male athymic nude
mice (age, 5-6 weeks; weight, 18-22 g; Shanghai SLAC Laboratory
Animal Co., Ltd.) with 1×106 normoxic and hypoxic HCT116
CRC cells. The mice were housed at a constant temperature of 22°C,
with a relative humidity of 30% under a 12-h light/dark cycle, and
were given free access to food and water. After 2 weeks, the mice
were divided into the following groups: Hypoxia group, normoxia
group, hypoxia [α-hederin (5 mg/kg)] group and normoxia [α-hederin
(5 mg/kg)] group. The drugs were administered by intraperitoneal
injection 5 days/week for 4 weeks. Tumour volumes and mouse weight
were measured every 4 days until the mice were euthanized by
intraperitoneal injection of 200 mg/kg pentobarbital. Death was
verified by the absence of heartbeat and respiration. Tumour volume
(V) was calculated as follows: V=W2 × L × 0.5, where W
is the largest tumour diameter in centimetres and L is the second
largest tumour diameter. Tumours were weighed after excision and
fixed in 10% formalin at 4°C for 1 week for immunohistochemistry
(IHC) and haematoxylin and eosin (H&E) staining. The present
study was carried out according to the National Institutes of
Health Guide for the Care and Use of Laboratory Animals (19).
H&E staining
The paraffin-embedded tissue sections (5 µm)
were first incubated in an automatic chip baking machine at 63°V
for 30 min. Subsequently, the sections were dehydrated, incubated
with haematoxylin (5 g/l) dyeing solution for 5 min at room
temperature, washed with water, soaked in 1% hydrochloric ethanol
for 3 sec, and then washed with water for a further 30 sec. After
rinsing, the sections were incubated with 0.5% eosin dyeing
solution for 3 min at room temperature. The sections were sealed
with neutral gum and were observed under an optical microscope.
IHC
Mouse tumour tissues and tumour tissues from
patients with CRC were fixed in 10% formalin at 4°C for 1 week,
embedded in paraffin and sectioned (5 µm). The
paraffin-embedded tissue sections were incubated in an automatic
sheet baking machine at 63°C for 30 min, and then placed in an
automatic dewaxing machine for dewaxing. The sections were then
incubated with sodium citrate at high heat for 10 min and medium
heat for 5 min for antigen retrieval. Subsequently, 3%
H2O2 solution (50 µl) was added and
sections were incubated in the dark at room temperature for 10 min.
Then, 5% BSA sealing solution was added to the sections at 37°C for
30 min and excess water traces were dried. Diluted primary
antibodies were added (50 µl) and incubated at 4°C
overnight, followed by incubation with the diluted secondary
antibody (50 µl) at 37°C for 30 min. Subsequently, 50
µl SABC amplifiers (Wuhan Boster Biological Technology,
Ltd.) were added and incubated at 37°C for 30 min, and DAB was
added for colour development. After 15 sec staining with
haematoxylin, the staining was terminated and the sections were
washed with water. Finally, they were dehydrated in a gradient
alcohol series in a dehydrator and sealed with neutral gum.
Finally, staining was observed under a microscope and images were
captured. Notably, between steps, sections were washed three times
with PBS (5 min/wash). IHC was performed to assess the protein
expression levels of p-AKT (cat. no. 4060S; 1:200; Cell Signaling
Technology, Inc.), Ki67 (cat. no. ab15580; 1:300; Abcam), Bcl2
(cat. no. ab182858; 1:500; Abcam), Bcl-xL (cat. no. ab178844;
1:1,000; Abcam) and HIF-1α (cat. no. ab114977; 1:300; Abcam).
Positive staining was assessed from 10 random images of the
experimental group under an optical microscope (Leica Microsystems,
Inc.). The immunohistochemical staining results were assigned a
mean score, considering both the intensity of staining and the
proportion of tumour cells with an unequivocal positive reaction;
because each section was independently evaluated by two
pathologists, the score was the average of the scores of the two
specialists. Each section was independently assessed by two
pathologists without prior knowledge of the data. Positive
reactions were defined as those showing brown signals in the cell
cytoplasm. For HIF-1α, Bcl2 and Bcl-a staining index (values, 0-12)
was determined by multiplying the score for staining intensity with
the score for positive area. The intensity was scored as follows:
0, negative; 1, weak; 2, moderate; and 3, strong. The frequency of
positive cells was defined as follows: 0, <5%; 1, 5-25%; 2,
26-50%; 3, 51-75%; and 4, >75%. When the staining was
heterogeneous, it was scored as follows: Each component was scored
independently and summed for the results. For example, a specimen
containing 75% tumour cells with moderate intensity (3×2=6) and
another 25% tumour cells with weak intensity (1×1=1) received a
final score of 6+1=7. For statistical analysis, scores of 0-7 were
considered low expression and scores of 8-12 were considered high
expression.
Toxicity analysis
The tissues obtained from the xenograft model
(heart, liver, lung, spleen, kidney and intestine) were analysed by
H&E staining. Venous blood samples were collected from the
eyeball after euthanasia and were placed in EDTA-coated tubes for
later use in haematological studies. The samples were analysed for
white blood cells, red blood cells, alanine aminotransferase,
aspartate transaminase and other related indicators in the hospital
clinical laboratory. Whole blood samples were centrifuged at 1,000
× g for 10 min at room temperature to obtain serum. The serum was
sent to the clinical laboratory of the hospital for analysis at
room temperature within 2 h of collection. The sample size for
blood biochemical examination was >100 µl; the sample
size for blood routine examination was >150 µl.
Colony formation assay
A total of 1×103 cells were seeded in a
6-well plate, thoroughly resuspended, and cultured for 7 days in
medium containing 10% FBS. Clusters containing ≥30 cells were
counted as single colonies.
Cell transfection
HCT116 and RKO cells were digested and cell
suspensions were spread evenly in a 6-well plate in a cell
incubator. After reaching 70-90% confluence, plasmid transfection
was performed. Briefly, 125 µl Opti-MEM (Invitrogen; Thermo
Fisher Scientific, Inc.) and 7.5 µl
Lipofectamine® 3000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) were added to an Eppendorf tube and mixed well in
a biological safety cabinet. At first, 2.5 µg plasmid
(plasmid backbone, pENTER) was gently mixed with 5 µl
Lipofectamine 3000 and incubated for 5 min at room temperature.
Subsequently, it was mixed with 125 µl Opti-MEM and
incubated for 5 min at room temperature. Finally, it was mixed with
1 ml pure medium to prepare the transfection reagent. Subsequently,
the prepared transfection reagent was added to the wells and shaken
gently. Empty plasmids were used as negative controls. The cells
were returned to the cell culture incubator, and 48 h after
transfection, AKT expression levels were observed using RT-qPCR and
WB analysis.
TUNEL assay
The frozen mouse tumour tissue sections stored at
-80°C were fixed with 4% paraformaldehyde for 30 min at room
temperature. After fixation, sections were washed with PBS twice
(10 min/wash), and 0.1% Triton X-100 was prepared with PBS and used
to permeabilize tissues at room temperature for 20 min.
Subsequently, sections were again washed twice with (10 min/wash)
and TUNEL test solution was added to the tissue for 60 min at room
temperature in the dark. After staining, sections were washed once
with PBS for 5 min, sealed with anti-fluorescence quenching sealing
liquid and observed under a laser confocal microscope. The
excitation wavelength was 488 nm and the emission wavelength range
was 515-565 nm.
Statistical analysis
All data are reported as the mean ± SD from
triplicate experiments and were analysed using SPSS 22.0 (IBM
Corporation). Unpaired Student's t-test or Mann-Whitney U test was
performed for comparisons between two groups, and Tukey's post-hoc
test was performed for pairwise comparisons among multiple groups
following one-way ANOVA or two-way ANOVA. The correlation
coefficients were analysed using the Spearman's rank test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
α-hederin overcomes hypoxia-mediated drug
resistance in CRC cells
It has previously been reported that hypoxia
promotes chemoresistance. In the present study, it was revealed
that hypoxia caused CRC cell resistance to OXA and 5-FU, whereas
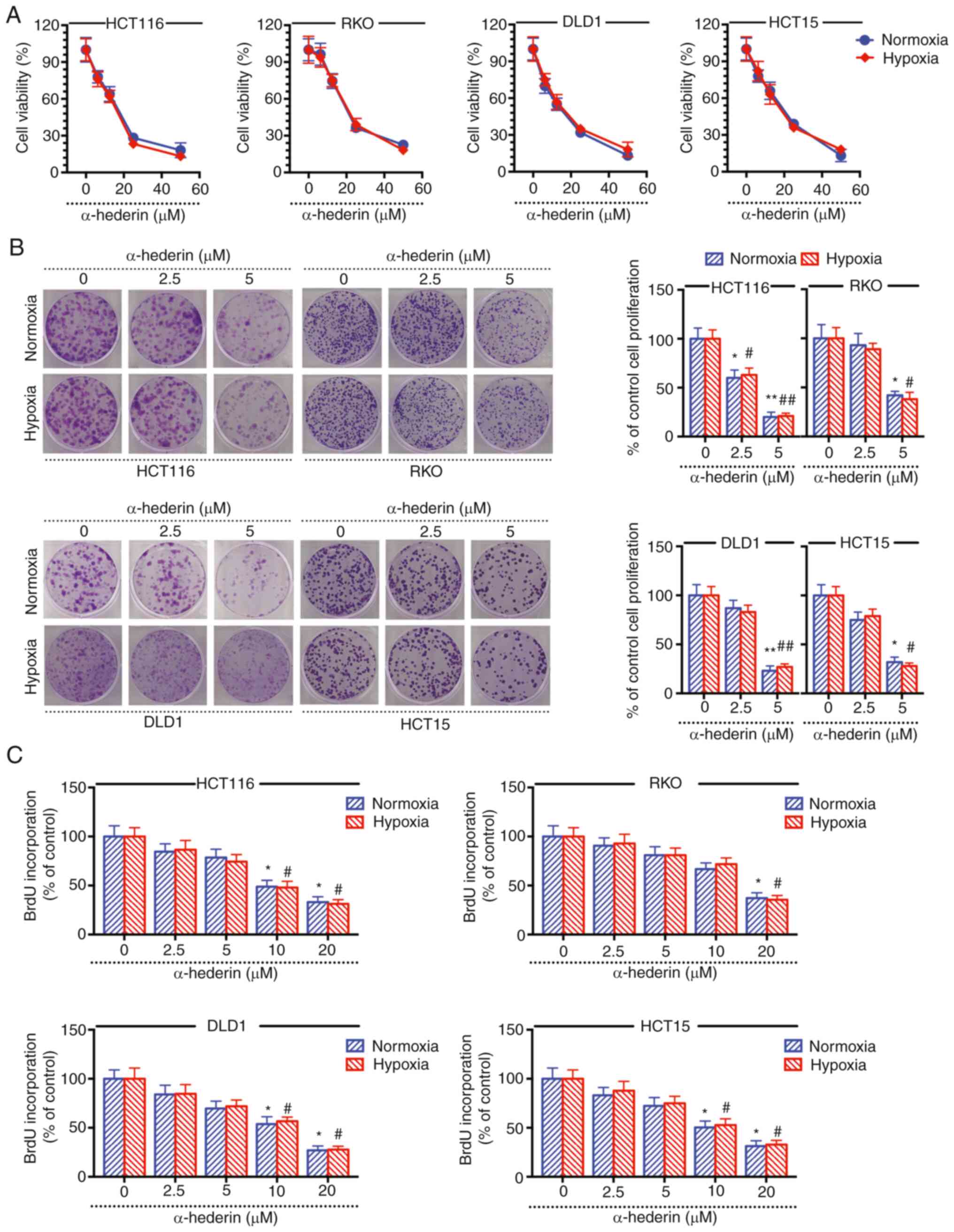
hypoxia did not cause CRC cell resistance to α-hederin (Fig. S1A and B). The results of the CCK-8
assay revealed that under normoxic and hypoxic conditions,
α-hederin inhibited the viability of CRC cells, suggesting that
α-hederin may overcome hypoxia-mediated resistance in CRC cells
(Fig. 1A). The cell colony
formation and BrdU assay results demonstrated that α-hederin
effectively inhibited CRC cell colony formation under normoxia and
hypoxia (Fig. 1B and C). Taken
together, these results suggested that α-hederin may inhibit the
viability and proliferation of CRC cells under hypoxia and that its
inhibitory effect is similar to that under normoxia.
Apoptotic pathway serves a key role in
the effects of α-hederin in overcoming hypoxia-mediated resistance
in CRC cells
The in vitro molecular mechanism by which
α-hederin overcomes hypoxia-mediated resistance in CRC cells was
subsequently assessed. The follow-up experiments were performed in
a representative sample of colorectal cancer cells (HCT116 and
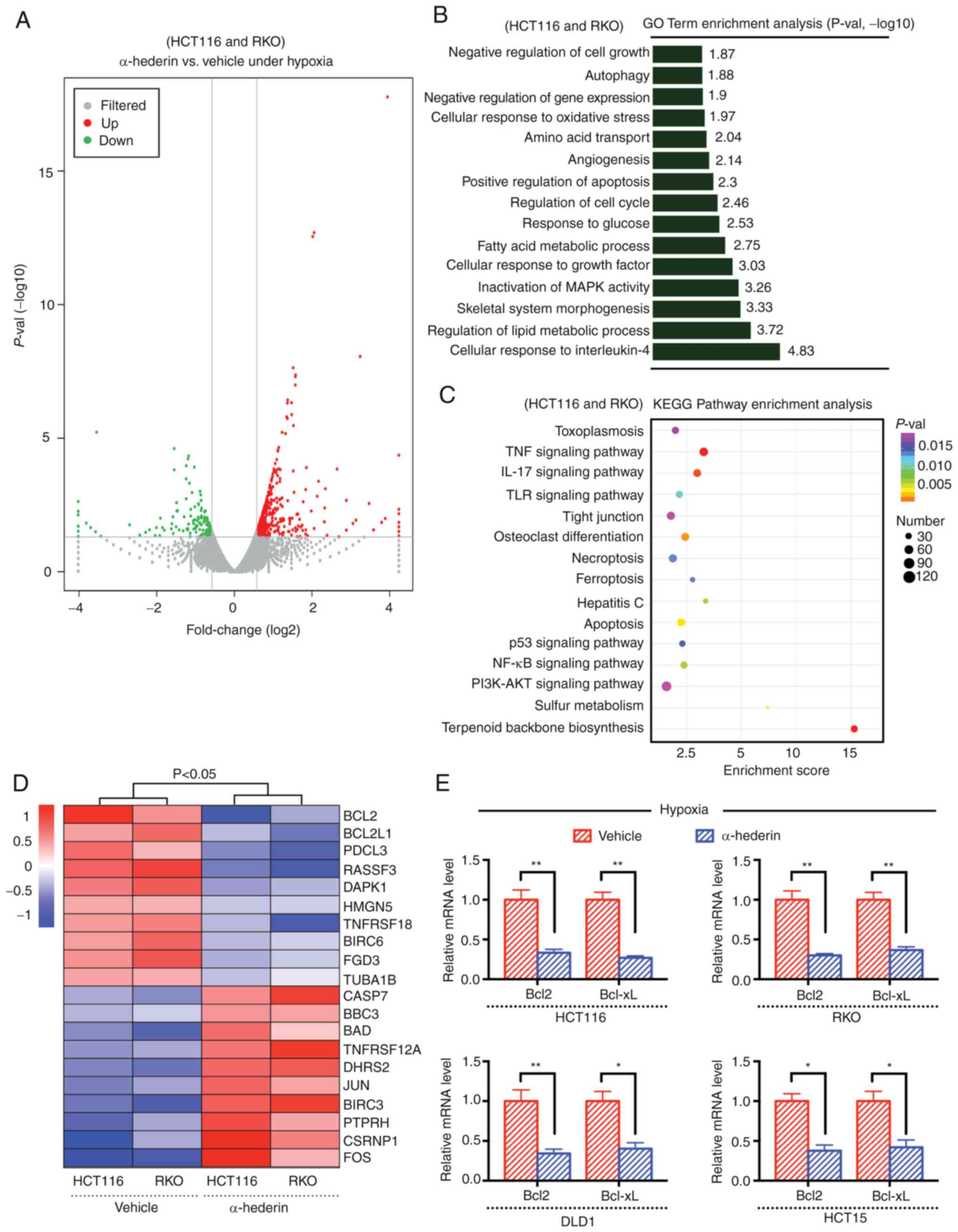
RKO). Transcriptome sequencing analysis revealed that
apoptosis-related pathways were downregulated in HCT116 cells under
hypoxia (Fig. S2A-C), and high
expression levels of Bcl2 (Fig.
S3A-C) and Bcl-xL (Fig. S3B and
C) was found in CRC cells under hypoxia, suggesting that they
may be key factors in the process of hypoxia-mediated resistance.
Firstly, the differentially expressed genes (including upregulated
and downregulated genes) between the hypoxia group and the drug
treatment group were visualized using a volcano plot (Fig. 2A), and GO and KEGG functional
enrichment analyses were subsequently performed (Fig. 2B and C). The results revealed that
the effect of α-hederin on CRC under hypoxia was mainly related to
the apoptosis pathway. In addition, gene expression profiling
showed that the Bcl2 transcription level changed most obviously
after α-hederin treatment of CRC cells under hypoxia (Fig. 2D), suggesting that α-hederin may
reduce the expression of the antiapoptotic gene Bcl2 to overcome
the resistance of CRC cells to hypoxia. RT-qPCR analysis further
confirmed that the expression levels of Bcl2 and Bcl-xL were
decreased following treatment of CRC cells with α-hederin under
hypoxia for 48 h (Fig. 2E). In
summary, these data indicated that the apoptotic pathway may serve
a key role in the mechanism by which α-hederin inhibits the
proliferation of CRC cells under hypoxic conditions.
α-hederin overcomes drug resistance in
CRC cells under hypoxic conditions by promoting apoptosis
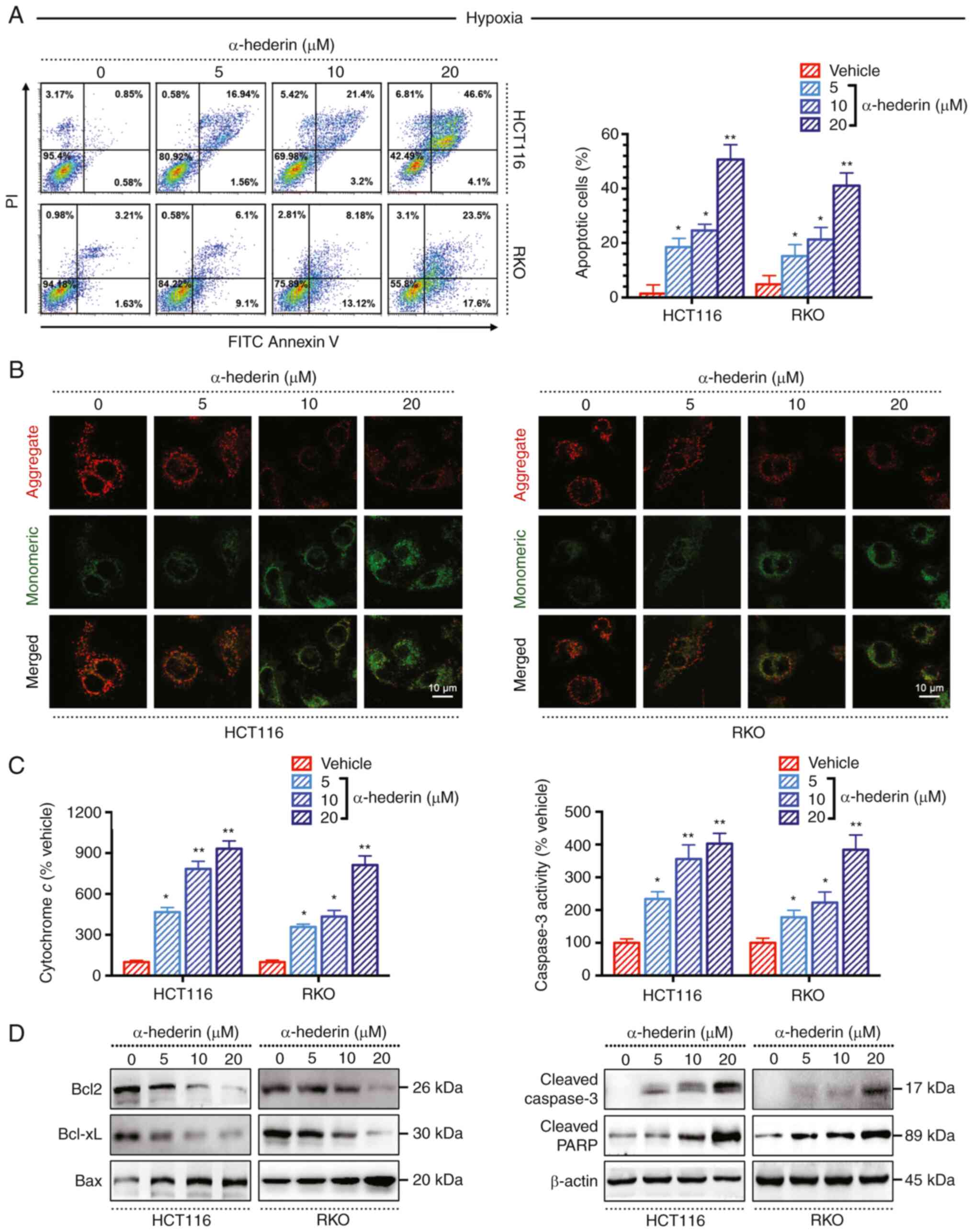
To confirm that α-hederin can overcome
hypoxia-mediated resistance in CRC by promoting apoptosis,
apoptosis assays were performed. Annexin V/propidium iodide
staining revealed that the apoptotic rate (early + late) of hypoxic
CRC cells was significantly increased following treatment with
α-hederin for 48 h in a dose-dependent manner (Fig. 3A). In addition, the JC-1
immunofluorescence assay demonstrated that with increasing drug
concentration, the intensity of green fluorescence changed from
weak to strong, indicating that apoptosis gradually increased
(Fig. 3B). Moreover, the
activities of apoptosis-related indicators, such as cytochrome
c and Caspase-3, were significantly increased in a
dose-dependent manner, as determined by ELISA (Fig. 3C). After α-hederin treatment of CRC
cells under hypoxic conditions, the expression levels of Bcl2 and
Bcl-xL were decreased, whereas the expression levels of Bax,
cleaved Caspase-3 and cleaved PARP were increased (Fig. 3D). Taken together, these results
suggested that α-hederin may inhibit the proliferation of CRC cells
under hypoxic conditions by promoting apoptosis.
AKT/Bcl2 signalling pathway is a key
mechanism by which α-hederin overcomes hypoxia-mediated resistance
in CRC cells
The present study also assessed whether Bcl-2 serves
a key role in the effects of α-hederin on overcoming
hypoxia-mediated resistance. A previous study indicated that HIF-1α
can affect Bcl2 expression (20).
The results of WB and RT-qPCR analyses revealed that there was no
change in the expression levels of HIF-1α in CRC cells treated with
α-hederin under hypoxic conditions, suggesting that α-hederin does
not overcome hypoxia-mediated resistance by inhibiting the
expression of HIF-1α (Fig. 4A and
B). Sequencing analysis results suggested that the AKT
signalling pathway was altered under hypoxia (Fig. S2C), and the levels of p-AKT and
p-STAT3 were increased in CRC cells under hypoxia (Fig. S3C). However, the results of WB
analysis showed that p-AKT levels were decreased in whole-cell
lysates following α-hederin treatment, whereas p-STAT3 levels were
not affected (Figs. 4A, S4A and B). In addition, RT-qPCR
demonstrated that the mRNA expression levels of Bcl2 and Bcl-xL
were decreased (Fig. 4B). The
successful overexpression of AKT in CRC cells was verified by
RT-qPCR and WB analyses (Fig. S4C and
D). Notably, when AKT expression was rescued in CRC cells, the
inhibitory effect of α-hederin on Bcl2 and Bcl-xL expression was
mitigated (Fig. 4C and D). In
addition, the role of AKT in the regulatory effects of α-hederin on
CRC cell apoptosis under hypoxic conditions was further explored.
The results revealed that the proapoptotic effect of α-hederin was
weakened in CRC cells overexpressing AKT (Fig. 4E-H); the apoptotic rate and the
levels of apoptosis-related indicators (cytochrome c and
Caspase-3) were decreased. These results indicated that the
AKT/Bcl2 signalling pathway may be the key mechanism by which
α-hederin inhibits CRC cell proliferation under hypoxic
conditions.
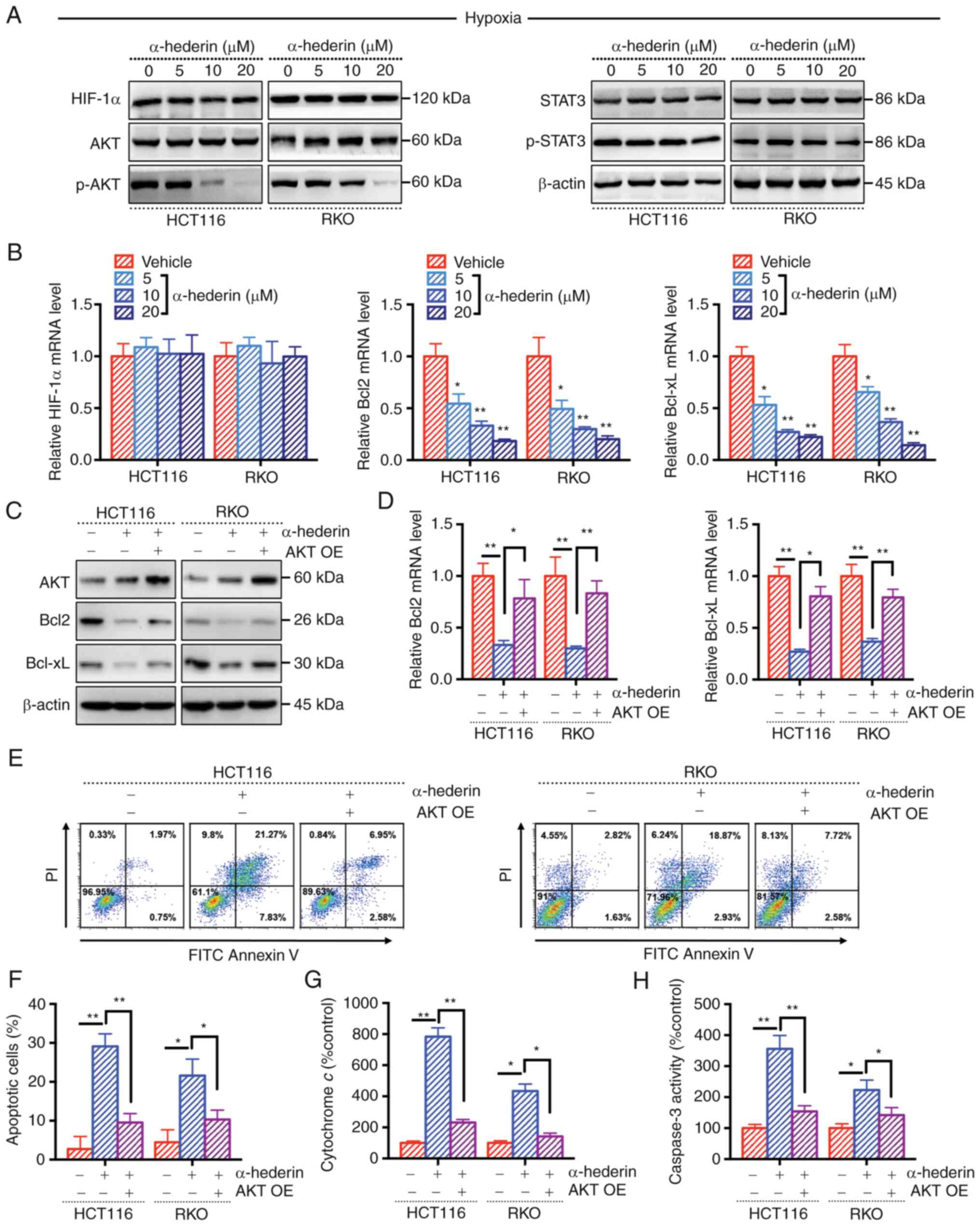 | Figure 4AKT/Bcl2 signalling pathway is a key
mechanism by which α-hederin overcomes hypoxia-mediated resistance
in CRC cells. (A) Treatment of hypoxic CRC cells with α-hederin
affected the expression of related proteins, as determined by
western blot analysis, β-actin blot is the loading control blot for
all of the proteins. (B) Reverse transcription-quantitative PCR was
used to measure the expression levels of HIF-1α, Bcl2 and Bcl-xL
after α-hederin treatment of hypoxic CRC cells. After OE of AKT,
the effect of α-hederin treatment on the (C) protein and (D) mRNA
expression levels of Bcl2 and Bcl-xL in hypoxic CRC cells was
observed. (E and F) After OE of AKT, the apoptosis of hypoxic CRC
cells treated with α-hederin was evaluated. After OE of AKT, the
levels of (G) cytochrome c and (H) Caspase 3 were observed
after α-hederin treatment of hypoxic CRC cells. Data are presented
as the mean ± SD. *P<0.05, **P<0.01 vs.
vehicle or as indicated. CRC, colorectal cancer; OE,
overexpression; p-, phosphorylated; PI, propidium iodide. |
α-hederin overcomes hypoxia-mediated
resistance in CRC via the AKT/Bcl2 pathway in vivo
To further explore the role of α-hederin in
overcoming hypoxia-mediated resistance in CRC in vivo, a
xenograft mouse model with HCT116 cells was established. The
results revealed that α-hederin inhibited tumour growth in both the
hypoxia and normoxia groups (Fig. 5A
and C). In addition, α-hederin reduced the expression levels of
Bcl2 and Bcl-xL in the tissues of mice in the hypoxia group
(Fig. 5D). Consistent with the
results of the previous cell experiments, the expression levels of
HIF-1α in the hypoxia group did not alter after α-hederin
treatment, whereas the levels of Ki67 (an indicator of cell
proliferation), Bcl2, Bcl-xL, and p-AKT were decreased, as shown by
IHC (Fig. 5B). In addition, the
TUNEL assay results showed that the green fluorescence intensity
was enhanced in response to α-hederin treatment, thus indicating
increased apoptosis (Fig. 5B).
Furthermore, the body weight of mice in different groups was not
significantly affected, indicating that the drug treatments exerted
no obvious toxicity (Fig. 5E).
There was no significant change in the biochemical indicators of
the orbital blood collected from the nude mice in each group
(Fig. 5F). H&E staining
confirmed that the organs were intact, without damage, and nuclei
were clearly visible, suggesting that α-hederin had no obvious
toxicity in vivo (Fig. 5G).
In summary, these data suggested that α-hederin overcomes
hypoxia-mediated resistance in CRC cells in vivo through the
AKT/Bcl2 signalling pathway.
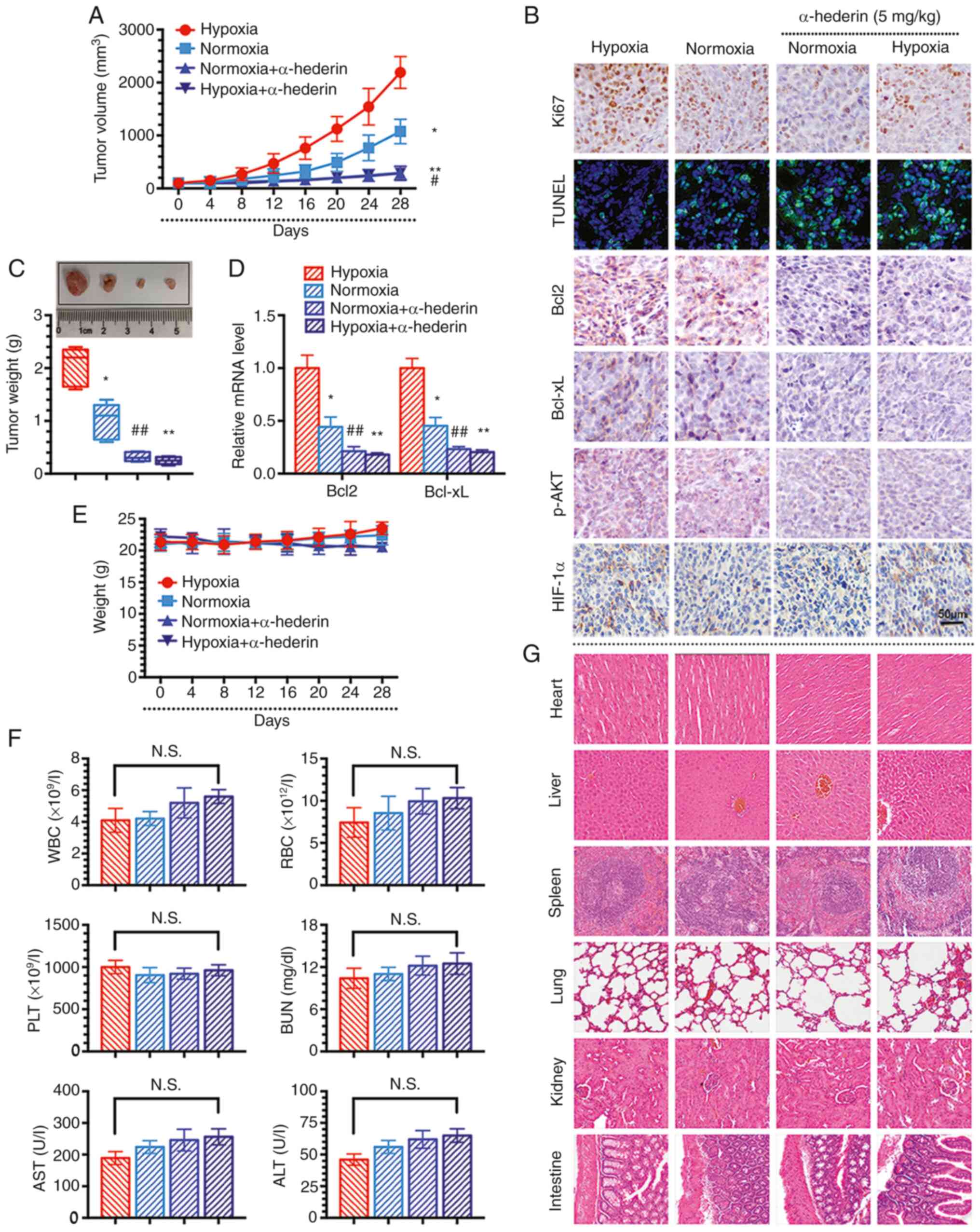 | Figure 5α-hederin overcomes hypoxia-mediated
resistance in colorectal cancer cells via the AKT/Bcl2 pathway
in vivo. (A) Xenograft tumour growth curves. (B)
Representative images of the immunohistochemical staining of Ki67,
Bcl2, Bcl-xL, p-AKT and HIF-1α in tissues and the evaluation of
apoptosis in each group by TUNEL. Scale bars, 50 µm. (C)
Images and weights of tumours. (D) Reverse
transcription-quantitative PCR was used to measure the expression
levels of Bcl2 and Bcl-xL in tissues. (E) Mouse weight curves after
treatment in the CRC xenograft model. (F) Detection of changes in
blood biochemical indicators in mice. (G) Haematoxylin and eosin
staining to observe organ damage (magnification, x100). Data are
presented as the mean ± SD. *P<0.05,
**P<0.01 vs. hypoxia; #P<0.05,
##P<0.01 vs. normoxia. ALT, alanine aminotransferase;
AST, aspartate aminotransferase; N.S., not significant; RBC, red
blood cell; PLT, platelets; WBC, white blood cell. |
α-hederin overcomes hypoxia-mediated
resistance by reducing Bcl2 and Bcl-xL expression in a PDX mouse
model
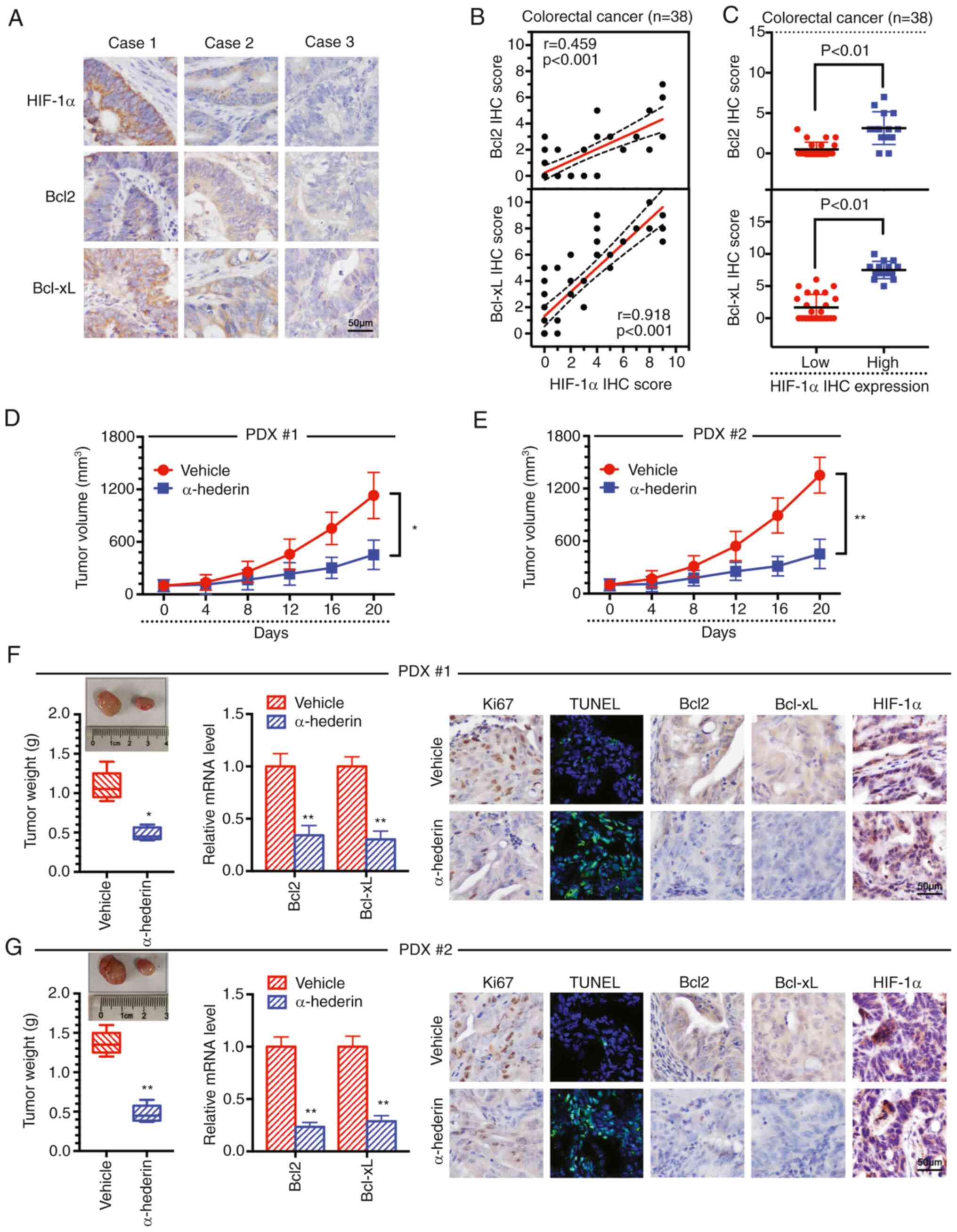
An important association has previously been
reported between HIF-1α and Bcl2 (21). Herein, the expression levels of
Bcl2 and Bcl-xL were observed to be positively correlated with the
expression levels of HIF-1α in the collected clinical tissue
samples, as demonstrated by IHC experiments, indicating that
apoptosis inhibition was positively correlated with hypoxia
(Fig. 6A-C). In addition, a PDX
model in mice was further established, and it was revealed that
α-hederin inhibited tumour growth (Fig. 6D-G). Furthermore, α-hederin reduced
the expression levels of Bcl2 and Bcl-xL in the tissues (Fig. 6F and G). There was no significant
change in the expression levels of HIF-1α following treatment with
α-hederin, whereas the expression levels of Ki67, Bcl2 and Bcl-xL
were decreased, as demonstrated by IHC experiments (Fig. 6F and G). In addition, the TUNEL
assay results showed that the fluorescence intensity gradually
increased, indicating that the degree of apoptosis in the tissue
increased after α-hederin treatment (Fig. 6F and G). These data suggested that
α-hederin overcomes hypoxia-mediated resistance in PDX mouse models
by reducing Bcl2 and Bcl-xL expression.
Discussion
The morbidity and mortality of patients with CRC
currently show an increasing trend (22). To date, chemotherapy is still
considered the mainstay of treatment for CRC; however, clinical
studies have revealed that a number of tumours are likely to
develop resistance to chemotherapy, ultimately leading to treatment
failure (23). Related research
has shown that the hypoxic microenvironment can promote tumour
resistance (24,25). One of the main mechanisms of tumour
resistance is the inhibition of apoptosis caused by overexpression
of anti-apoptotic proteins. Hypoxia-induced tumour resistance via
inhibition of the apoptotic signalling pathway has attracted
increasing attention (26).
Bcl2 family oncoproteins serve an important role in
regulating apoptosis. Specifically, abnormal Bcl2 activation is
associated with the occurrence and progression of various types of
cancer, and has become a key indicator for evaluating clinical
efficacy and prognosis (27-30).
In the hypoxic microenvironment, the expression of Bcl2 in tumours
can increase, whereas the expression of proapoptotic proteins
decreases. In the present study, the protein and mRNA expression
levels of anti-apoptotic genes were revealed to be significantly
increased under hypoxia. Notably, hypoxia activated and regulated
AKT phosphorylation to increase the expression of Bcl2 and exert an
anti-apoptotic effect. The sensitivity of malignant tumour cells to
apoptosis has been reported to be effectively increased by either
reducing Bcl2 protein levels or inhibiting Bcl2 activity (31).
A growing number of studies have elucidated the
effect of traditional Chinese medicines on inhibiting
hypoxia-mediated tumour development (32-34).
α-hederin, also referred to as koronaroside A or sapindoside A, is
a monodesmosidic triterpenoid saponin that widely exists throughout
the plant kingdom, including in Hedera and Nigella
species (35). Related studies
have reported that α-hederin possesses biological and
pharmacological properties. (36-38).
α-hederin has recently attracted attention for its marked
antitumour potential, as it exhibits cytotoxic activity against
various cancer cell lines by inhibiting cell proliferation and
promoting apoptosis in vitro and in vivo (39,40).
In the present study, obvious resistance to the chemotherapeutic
drugs OXA and 5-FU was observed under hypoxic conditions, and
α-hederin may overcome hypoxia-induced resistance in CRC cells.
Further studies have reported that α-hederin reduces
the phosphorylation of AKT and the expression of Bcl2 in CRC under
hypoxic conditions, suggesting that the regulation of AKT
phosphorylation can overcome hypoxia-mediated resistance in CRC
cells. In addition, the levels of cytochrome c and Caspase-3
were decreased in a dose-dependent manner after α-hederin treatment
in the present study, which also verified from another experimental
perspective that α-hederin can overcome hypoxia-mediated resistance
in CRC cells. Furthermore, it was demonstrated that α-hederin
decreased Bcl2 expression in CRC cells by inhibiting p-AKT in
rescue experiments. In vivo, α-hederin significantly
overcame hypoxia-mediated resistance in different mouse models via
the AKT/Bcl2 signalling pathway. In addition, in animal experiments
it was observed that the mice treated with α-hederin did not
exhibit toxic side effects. There was no marked change in the
biochemical indicators of the orbital blood of nude mice. In
addition, H&E staining confirmed that the organs were intact,
without damage, and nuclei were clearly visible, especially in the
intestinal tissue, suggesting that α-hederin had no obvious
toxicity in vivo. Upon reviewing the literature, no related
reports that indicated α-hederin affects normal colonic epithelial
cells were found. In addition, the expression levels of Bcl2 and
Bcl-xL were revealed to be positively correlated with the
expression levels of HIF-1α in clinical tissues, thus indicating
that there may be a positive correlation between hypoxia and
inhibition of apoptosis.
In conclusion, the present study identified that
α-hederin can overcome hypoxia-mediated resistance in CRC via the
AKT/Bcl2 pathway in vivo and in vitro. The results of
the present study may provide a reliable basis for the clinical
comprehensive treatment of CRC, particularly treatment with
adjuvant drugs that have α-hederin as the main active ingredient.
In the future, α-hederin may be used as a novel adjuvant for
reversing drug resistance in CRC.
Supplementary Data
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request. Sequencing data have been deposited in NCBI Sequence Read
Archive under the accession code PRJNA912906 (https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA912906).
Authors' contributions
WL and KX conceived and designed the study. JC, JX
and JY collected the data, and analysed and interpreted the data.
JC and JX drafted the article. WD, LJ, WW, XS, DZ, SL, PY, YZ and
YC and KY performed some of the experiments and aided in the
construction of the tumour model. SL, PY, YZ and YC critically
revised the article. All authors read and approved the final
manuscript. All authors confirmed the authenticity of all the raw
data.
Ethics approval and consent to
participate
All patient samples were obtained with oral informed
consent and the approval of the ethics committee of Putuo Hospital,
Shanghai University of Traditional Chinese Medicine (app roval no.
PTEC-A-2021-29-1). All animal experiments were conducted in
accordance with guidelines and protocols approved by the
institutional animal care and use committee of Putuo Hospital,
Shanghai University of Traditional Chinese Medicine (approval no.
AP202204011).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
This project was sponsored by the Natural Science Foundation of
Shanghai (grant no. 20ZR1450500), the Clinical Specialized Disease
Construction Project of Shanghai Putuo District Municipal Health
Commission (grant no. 2020tszb03), the Shanghai Rising-Star Program
(Sailing Special Project, grant no. 22YF1441400), the Shanghai Key
Medical Specialty Construction Project (grant no. ZK2019B18), the
Independent Innovation Project in Putuo District (grant no.
ptkwws201701), the Chengdu University of Traditional Chinese
Medicine 'Xinglin Scholars' Discipline Talents Research Promotion
Plan (grant no. YYZX2020120) and the budget project of Shanghai
University of Traditional Chinese Medicine (grant no.
2020LK072).
Abbreviations:
|
CRC
|
colorectal cancer
|
|
OXA
|
oxaliplatin
|
|
5-FU
|
5-fluorouracil
|
|
CCK-8
|
Cell Counting Kit-8
|
|
IHC
|
immunohistochemistry
|
|
OE
|
overexpression
|
|
qPCR
|
quantitative PCR
|
|
WB
|
western blot
|
References
|
1
|
de Oliveira MB, Koshkin V, Liu G and
Krylov SN: Analytical challenges in development of chemoresistance
predictors for precision oncology. Anal Chem. 92:12101–12110. 2020.
View Article : Google Scholar
|
|
2
|
Akman M, Belisario DC, Salaroglio IC,
Kopecka J, Donadelli M, De Smaele E and Riganti C: Hypoxia,
endoplasmic reticulum stress and chemoresistance: Dangerous
liaisons. J Exp Clin Cancer Res. 40:282021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McAleese CE, Choudhury C, Butcher NJ and
Minchin RF: Hypoxia-mediated drug resistance in breast cancers.
Cancer Lett. 502:189–199. 2021. View Article : Google Scholar
|
|
4
|
Dzhalilova DS and Makarova OV:
HIF-dependent mechanisms of relationship between hypoxia tolerance
and tumor development. Biochemistry (Mosc). 86:1163–1180. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang M, Liu Q, Li L, Ning J, Tu J, Lei X,
Mo Z and Tang S: Cytoplasmic M-CSF facilitates apoptosis resistance
by inhibiting the HIF-1α/BNIP3/Bax signalling pathway in MCF-7
cells. Oncol Rep. 41:1807–1816. 2019.
|
|
6
|
Xu D, Li DW, Xie J and Chen XW: Effect and
mechanism of survivin on hypoxia-induced multidrug resistance of
human laryngeal carcinoma cells. Biomed Res Int.
2019:56968012019.
|
|
7
|
Yun CW, Lee JH and Lee SH: Hypoxia-induced
PGC-1α regulates mitochondrial function and tumorigenesis of
colorectal cancer cells. Anticancer Res. 39:4865–4876. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y, Qi Z, Zhou M, Yang W, Hu R, Li G,
Ma X and Zhang Z: Stanniocalcin-1 promotes cell proliferation,
chemoresistance and metastasis in hypoxic gastric cancer cells via
Bcl-2. Oncol Rep. 41:1998–2008. 2019.
|
|
9
|
Zeng J, Huang T, Xue M, Chen J, Feng L, Du
R and Feng Y: Current knowledge and development of hederagenin as a
promising medicinal agent: A comprehensive review. RSC Adv.
8:24188–24202. 2018. View Article : Google Scholar
|
|
10
|
Wang J, Wu D, Zhang J, Liu H, Wu J and
Dong W: α-Hederin induces apoptosis of esophageal squamous cell
carcinoma via an oxidative and mitochondrial-dependent pathway. Dig
Dis Sci. 64:3528–3538. 2019. View Article : Google Scholar
|
|
11
|
Liu Y, Lei H, Ma J, Deng H, He P and Dong
W: α-Hederin increases the apoptosis of cisplatin-resistant gastric
cancer cells by activating mitochondrial pathway in vivo and vitro.
Onco Targets Ther. 12:8737–8750. 2019. View Article : Google Scholar
|
|
12
|
Sun J, Feng Y, Wang Y, Ji Q, Cai G, Shi L,
Wang Y, Huang Y, Zhang J and Li Q: α-hederin induces autophagic
cell death in colorectal cancer cells through reactive oxygen
species dependent AMPK/mTOR signaling pathway activation. Int J
Oncol. 54:1601–1612. 2019.PubMed/NCBI
|
|
13
|
Fang C, Liu Y, Chen L, Luo Y, Cui Y, Zhang
N, Liu P, Zhou M and Xie Y: α-Hederin inhibits the growth of lung
cancer A549 cells in vitro and in vivo by decreasing SIRT6
dependent glycolysis. Pharm Biol. 59:11–20. 2021. View Article : Google Scholar
|
|
14
|
Wang J, Deng H, Zhang J, Wu D, Li J, Ma J
and Dong W: α-Hederin induces the apoptosis of gastric cancer cells
accompanied by glutathione decrement and reactive oxygen species
generation via activating mitochondrial dependent pathway.
Phytother Res. 34:601–611. 2020. View Article : Google Scholar
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
16
|
Kim D, Langmead B and Salzberg SL: HISAT:
A fast spliced aligner with low memory requirements. Nat Methods.
12:357–360. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Anders S, Pyl PT and Huber W: HTSeq-a
Python framework to work with high-throughput sequencing data.
Bioinformatics. 31:166–169. 2015. View Article : Google Scholar
|
|
18
|
Roberts A, Pimentel H, Trapnell C and
Pachter L: Identification of novel transcripts in annotated genomes
using RNA-Seq. Bioinformatics. 27:2325–2329. 2011. View Article : Google Scholar
|
|
19
|
Zhan Y, Qiu Y, Wang H, Wang Z, Xu J, Fan
G, Xu J, Li W, Cao Y, Le VM, et al: Bufalin reverses multidrug
resistance by regulating stemness through the CD133/nuclear
factor-κB/MDR1 pathway in colorectal cancer. Cancer Sci.
111:1619–1630. 2020. View Article : Google Scholar
|
|
20
|
Zhao X, Liu L, Li R, Wei X, Luan W, Liu P
and Zhao J: Hypoxia-inducible factor 1-α (HIF-1α) induces apoptosis
of human uterosacral ligament fibroblasts through the death
receptor and mitochondrial pathways. Med Sci Monit. 24:8722–8733.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Valladares KJP, Balbinot KM, de Moraes AT,
Kataoka MSDS, Ramos AMPC, Ramos RTJ, da Silva ALDC, Mesquita RA,
Normando D, Júnior SM and de Jesus Viana Pinheiro J: HIF-1α is
associated with resistance to hypoxia-induced apoptosis in
ameloblastoma. Int J Dent. 2021:30603752021. View Article : Google Scholar
|
|
22
|
Ding Q, Kong X, Zhong W and Liu W: Fecal
biomarkers: Non-invasive diagnosis of colorectal cancer. Front
Oncol. 12:9719302022. View Article : Google Scholar
|
|
23
|
Zhang L, Lu Z and Zhao X: Targeting Bcl-2
for cancer therapy. Biochim Biophys Acta Rev Cancer.
1876:1885692021. View Article : Google Scholar
|
|
24
|
Belisario DC, Kopecka J, Pasino M, Akman
M, De Smaele E, Donadelli M and Riganti C: Hypoxia dictates
metabolic rewiring of tumors: Implications for Chemoresistance.
Cells. 9:25982020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu J, Gao L, Zhan N, Xu P, Yang J, Yuan
F, Xu Y, Cai Q, Geng R and Chen Q: Hypoxia induced ferritin light
chain (FTL) promoted epithelia mesenchymal transition and
chemoresistance of glioma. J Exp Clin Cancer Res. 39:1372020.
View Article : Google Scholar :
|
|
26
|
Cao Y, Jiang Z, Zeng Z, Liu Y, Gu Y, Ji Y,
Zhao Y and Li Y: Bcl-2 silencing attenuates hypoxia-induced
apoptosis resistance in pulmonary microvascular endothelial cells.
Apoptosis. 21:69–84. 2016. View Article : Google Scholar
|
|
27
|
Letai A, Sorcinelli MD, Beard C and
Korsmeyer SJ: Antiapoptotic BCL-2 is required for maintenance of a
model leukemia. Cancer Cell. 6:241–249. 2004. View Article : Google Scholar
|
|
28
|
Goff DJ, Recart AC, Sadarangani A, Chun
HJ, Barrett CL, Krajewska M, Leu H, Low-Marchelli J, Ma W, Shih AY,
et al: A Pan-BCL2 inhibitor renders bone-marrow-resident human
leukemia stem cells sensitive to tyrosine kinase inhibition. Cell
Stem Cell. 12:316–328. 2013. View Article : Google Scholar
|
|
29
|
Delbridge AR, Grabow S, Strasser A and
Vaux DL: Thirty years of BCL-2: Translating cell death discoveries
into novel cancer therapies. Nat Rev Cancer. 16:99–109. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee J, Sohn EJ, Yoon SW, Kim CG, Lee S,
Kim JY, Baek N and Kim SH: Anti-metastatic effect of
dehydrocorydaline on H1299 non-small cell lung carcinoma cells via
inhibition of matrix metalloproteinases and B cell lymphoma 2.
Phytother Res. 31:441–448. 2017. View Article : Google Scholar
|
|
31
|
Davids MS and Letai A: ABT-199: Taking
dead aim at BCL-2. Cancer. Cell. 23:139–141. 2013.
|
|
32
|
Li JR, Ren J, Yang SC, Han JQ, Guo YJ and
Ji ES: Effect of xiaotan huayu liqiao traditional Chinese medicine
compound on myocardial fibrosis in rats with chronic intermittent
hypoxia and its mechanism. Zhongguo Ying Yong Sheng Li Xue Za Zhi.
36:414–418. 2020.In Chinese.
|
|
33
|
Peng W and Zhang S, Zhang Z, Xu P, Mao D,
Huang S, Chen B, Zhang C and Zhang S: Jianpi Jiedu decoction, a
traditional Chinese medicine formula, inhibits tumorigenesis,
metastasis, and angiogenesis through the mTOR/HIF-1α/VEGF pathway.
J Ethnopharmacol. 224:140–148. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li RL, He LY, Zhang Q, Liu J, Lu F, Duan
HX, Fan LH, Peng W, Huang YL and Wu CJ: HIF-1α is a potential
molecular target for herbal medicine to treat diseases. Drug Des
Devel Ther. 14:4915–4949. 2020. View Article : Google Scholar
|
|
35
|
Rooney S and Ryan MF: Efects of α-hederin
and thymoquinone, constituents of Nigella sativa, on human cancer
cell lines. Anticancer Res. 25:2199–2204. 2005.PubMed/NCBI
|
|
36
|
Lorent JH, Léonard C, Abouzi M, Akabi F,
Quetin-Leclercq J and Mingeot-Leclercq MP: α-Hederin induces
apoptosis, membrane permeabilization and morphologic changes in two
cancer cell lines through a cholesterol-dependent mechanism. Planta
Med. 82:1532–1539. 2016. View Article : Google Scholar
|
|
37
|
Fallahi M, Keyhanmanesh R, Khamaneh AM,
Saadatlou MA, Saadat S and Ebrahimi H: Efect of alpha-hederin, the
active constituent of Nigella sativa, on miRNA126, IL-13 mRNA
levels and infammation of lungs in ovalbuminsensitized male rats.
Avicenna J Phytomed. 6:77–85. 2016.
|
|
38
|
Keyhanmanesh R, Saadat S, Mohammadi M,
Shahbazfar AA and Fallahi M: The protective efect of α-hederin, the
active constituent of nigella sativa, on lung infammation and blood
cytokines in ovalbumin sensitized guinea pigs. Phytother Res.
29:1761–1767. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li J, Wu DD, Zhang JX, Wang J, Ma JJ, Hu X
and Dong WG: Mitochondrial pathway mediated by reactive oxygen
species involvement in α-hederin-induced apoptosis in
hepatocellular carcinoma cells. World J Gastroenterol.
24:1901–1910. 2018. View Article : Google Scholar :
|
|
40
|
Cheng L, Xia TS, Wang YF, Zhou W, Liang
XQ, Xue JQ, Shi L, Wang Y, Ding Q and Wang M: The anticancer efect
and mechanism of α-hederin on breast cancer cells. Int J Oncol.
45:757–763. 2014. View Article : Google Scholar
|