Introduction
Osteosarcoma is the most common primary malignant
bone tumor, characterized by high metastatic potential.
Chemotherapy protocols, commencing in the mid-1970s, have increased
during the 5-year disease-free survival rate from approximately
15–20 to 60–70% (1,2). Ewing’s sarcoma is the second most
common malignant bone tumor in children. The inclusion of cytotoxic
polychemotherapy into multimodal treatment strategies has led to
marked prognostic improvements in patients with Ewing’s sarcoma
with 5-year survival rates reaching 75% for non-metastasized and
20–40% for primary metastasized Ewing’s sarcoma (3,4).
Over the last two decades, however, attempts to further intensify
therapy using conventional chemotherapeutic drugs, including
high-dose methotrexate, doxorubicin, cisplatin and high-dose
ifosfamide, have not delivered significantly improved results.
Furthermore, despite aggressive surgical and chemotherapy
approaches, patients with unresectable primary tumors or with
clinically evident metastases still had a poor prognosis. Thus, for
patients with refractory osteosarcoma and Ewing’s sarcoma more
beneficial prognostic factors and more effective therapeutic
modalities are needed.
Chondrosarcoma is the most common primary bone
sarcoma in adulthood, generally resistant to radiotherapy and
chemotherapy, thus surgical wide resection is the only curative
treatment. The rates of local recurrence, distant metastasis and
survival vary, while the histological grade of cartilage tumors is
currently considered to be the best tool for the assessment of
prognosis (5). However, the
histological determination of the tumor grade is basically
subjective and pathologists do not always agree on the extent or
level of cytological factors of chondrosarcoma cells (6). It is often difficult to distinguish a
low-grade chondrosarcoma and benign processes, such as enchondroma
and osteochondroma. To assess prognosis, more complex objective
methods have been sought.
Tumor growth and metastasis have been shown to
strongly depend on angiogenesis. Folkman et al(7) demonstrated the mechanisms and
relevance of angiogenesis in malignant tumors. Since then, several
reports demonstrated that angiogenesis assessed by microvessel
density (MVD) was correlated with patient prognosis in different
types of cancer (8), whereas data
regarding the relevance of angiogenesis and prognosis in malignant
bone tumors remain scarce and controversial. The aim of this study
was to examine MVD in representative malignant bone tumors, such as
osteosarcoma, chondrosarcoma and Ewing’s sarcoma, with a view to
clarify the role of angiogenesis in prognosis and refer to
therapeutic strategies.
Patients and methods
Patients
A total of 69 patients with malignant bone tumors,
including 44 osteosarcomas, 20 chondrosarcomas and 5 Ewing’s
sarcomas were reviewed retrospectively and treated at our hospital
between 1980 and 2007. Subsequent to approval of the present study
by the Institutional Review Board, clinical data from the patients’
medical charts were reviewed. Biopsy or pre-chemotherapy surgical
specimens were immunohistochemically stained.
Surgical resection specimens were used to evaluate
surgical margins and histologic response to pre-operative
chemotherapy. The surgical margins were classified using the method
described by Kawaguchi et al(9), whereby surgical margins are evaluated
according to the distance between the tumor’s margin and reactive
zone, and divided into four categories: curative, wide, marginal
and intralesional. The reactive zone is composed of hemorrhagic
tissue, scar tissue, degenerated muscle, edema or the tumor
capsule. If the surgical margin is >5 cm outside the reactive
zone, the margin is graded as curative, while it is referred to as
wide if the margin is <5 cm and outside the reactive zone. A
margin in the reactive zone is graded as marginal, while one
passing through a tumor as intralesional. Histologic necrosis
subsequent to pre-operative chemotherapy was determined according
to the Huvos grading system as described previously (10). Each case was defined as good (Huvos
grades III and IV) or poor responders (grades I and II).
Immunohistochemical staining
Each tissue block was cut into 6-μm sections,
transferred to MAS-coated glass slides (Matsunami, Osaka, Japan),
deparaffinized in xylene, rehydrated in a graded series of
decreasing ethanol concentrations, then rinsed in TBST (50 mM
Tris-HCl, pH 7.6, containing 0.3 M sodium chloride and 0.1%
Tween-20). Tissue sections were immersed in Target Retrieval
Solution (DakoCytomation Inc., Carpinteria, CA, USA), and subjected
to a hot water-bath for 20 min. Subsequent to antigen retrieval, a
cooling period of 20 min followed. CD34 antibody (Ready-to use
N-series Qbend 10; DakoCytomation, Inc.) was incubated with the
tissue sections for 30 min at room temperature in a moisture
chamber, then the polymer-peroxidase method was used
(EnVision®+/HRP; DakoCytomation, Inc.). The reaction products were
visualized by exposing the sections to 3,3′-diaminobenzidine.
Nuclei were lightly counterstained for ∼10 sec with Gill’s
formulation #2 hematoxylin. Non-specific reactivity was assessed by
omitting the primary antibody.
Microvessel counting
MVD values were calculated by two independent
observers, according to Weidner’s method (11). Briefly, in each tumor, hot-spot
areas exhibiting the highest vessel density were identified by
scanning tumor sections at low-power magnification (x40). The
maximum vessel density was determined from these hot-spot areas at
fields under high-power magnification (x400, HPF), and the mean of
the counts for the three fields was calculated. For a structure to
be defined a vessel, vessel lumens were not necessary. MVD was
classified as high when ≥39.7, 12.9 and 44.6 (the mean value)/HPF
in osteosarcomas, chondrosarcomas and Ewing’s sarcomas,
respectively.
Statistical analysis
Disease-free survival was defined as the interval
from diagnosis to relapse or the last follow-up. All survival
analyses were evaluated with the Kaplan-Meier method and the
log-rank test. Statistical significance of MVD among osteosarcomas,
chondrosarcomas and Ewing’s sarcomas was determined by the
Student’s t-test. The association of MVD with clinicopathological
parameters was analyzed, using the Student’s t-test. The analyses
were carried out with Excel Statistics 2008 (Social Survey Research
Information, Co., Ltd., Tokyo, Japan). P<0.05 was considered to
indicate a statistically significant difference.
Results
Diversity of angiogenesis in malignant
bone tumors
The mean ± standard deviation of MVD was 39.7±31.8,
12.9±32.2, and 44.6±33.8 in osteosarcomas, chondrosarcomas and
Ewing’s sarcomas, respectively. The MVD values of osteosarcomas and
Ewing’s sarcomas were significantly higher compared to
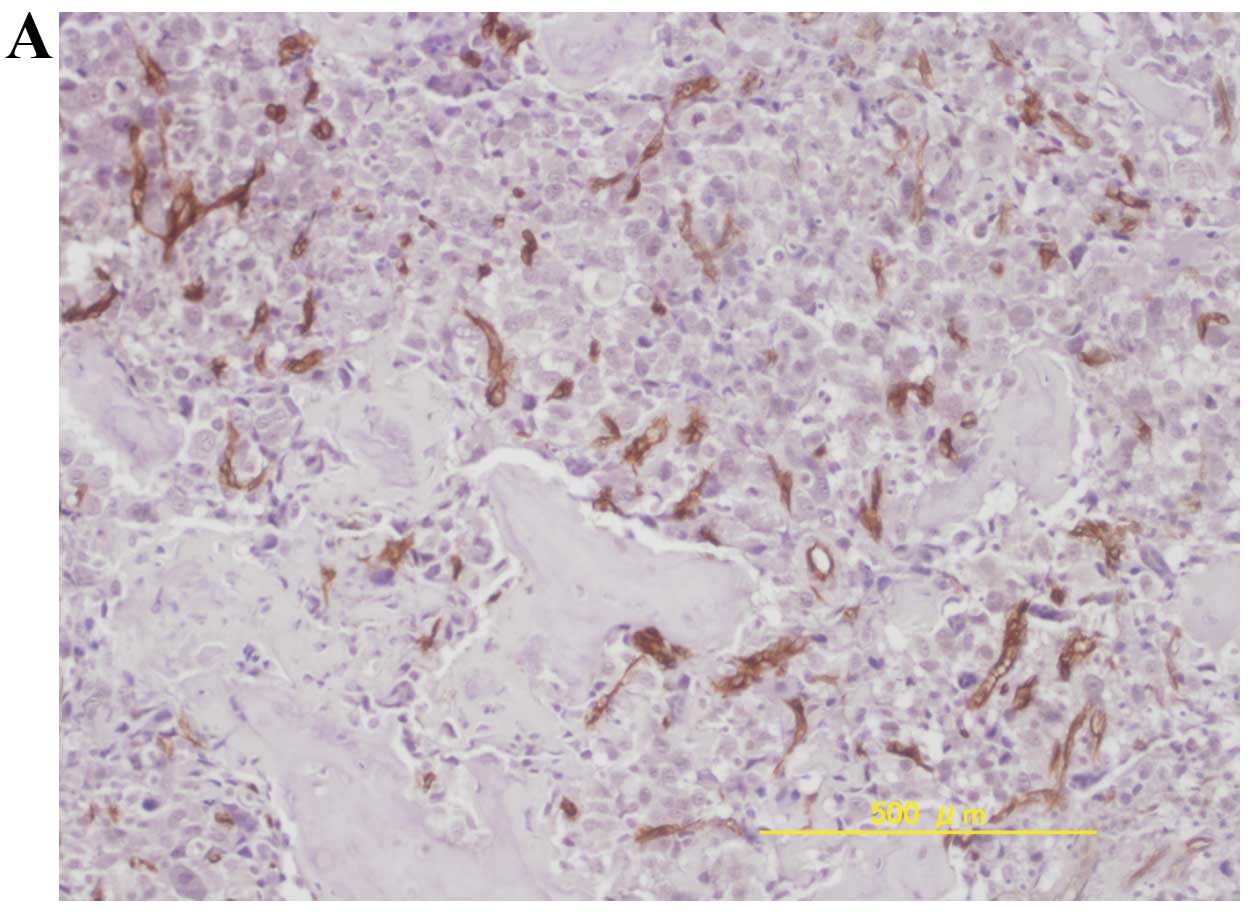
chondrosarcomas (P<0.05, Student’s t-test). Representative
immunohistochemical staining of high MVD in osteosarcomas,
chondrosarcomas and Ewing’s sarcomas is shown in Fig. 1.
Survival analysis in osteosarcomas
A total of 42 high-grade osteosarcomas and 2
low-grade osteosarcomas were reviewed. The median age at the time
of surgery was 21 years (range, 10–88). There were 20 males and 24
females. The tumor sites were the pelvis in 2 patients and the
extremities in 42 patients (27 femurs, 10 tibias or fibulas and 5
humeri). According to the 6th edition of the American Joint
Committee on Cancer (AJCC) Staging Manual, 2 tumors were stage IB,
10 were stage IIA, 23 were stage IIB and 2 were stage IV (7 missing
in tumor size). Between 1980 and 1997, 22 patients received
conventional chemotherapy, including high-dose methotrexate,
doxorubicin and cisplatin. The combinations of such agents were
made empirically. Since 1997, two established chemotherapy regimens
have been in use. NECO-95J and K2 protocols were applied in the
case of 12 patients between 1997 and 2002 and 8 patients between
2002 and 2006, respectively. Two patients (>70 years-old)
received no chemotherapy (12,13).
The operative treatments consisted of 26 limb salvage operations
and 18 ablations. The surgical margins of all limb salvage
operations were wide, while those of ablations were wide or
curative. Histologic subtypes comprised osteoblastic in 28 tumors,
fibroblastic in 6, chondroblastic in 3 and others in 7. There were
13 good and 22 poor responders to pre-operative chemotherapy (7
missing). The median follow-up was 5 years and 11 months (range,
2–211 months). There was 1 local recurrence and there were 19
distant metastases during patients’ clinical courses. Fifteen
patients were deceased, 27 demonstrated no evidence of disease,
while 2 were alive with signs of disease. The 5-year actual
disease-free survival rate was 54.6%.
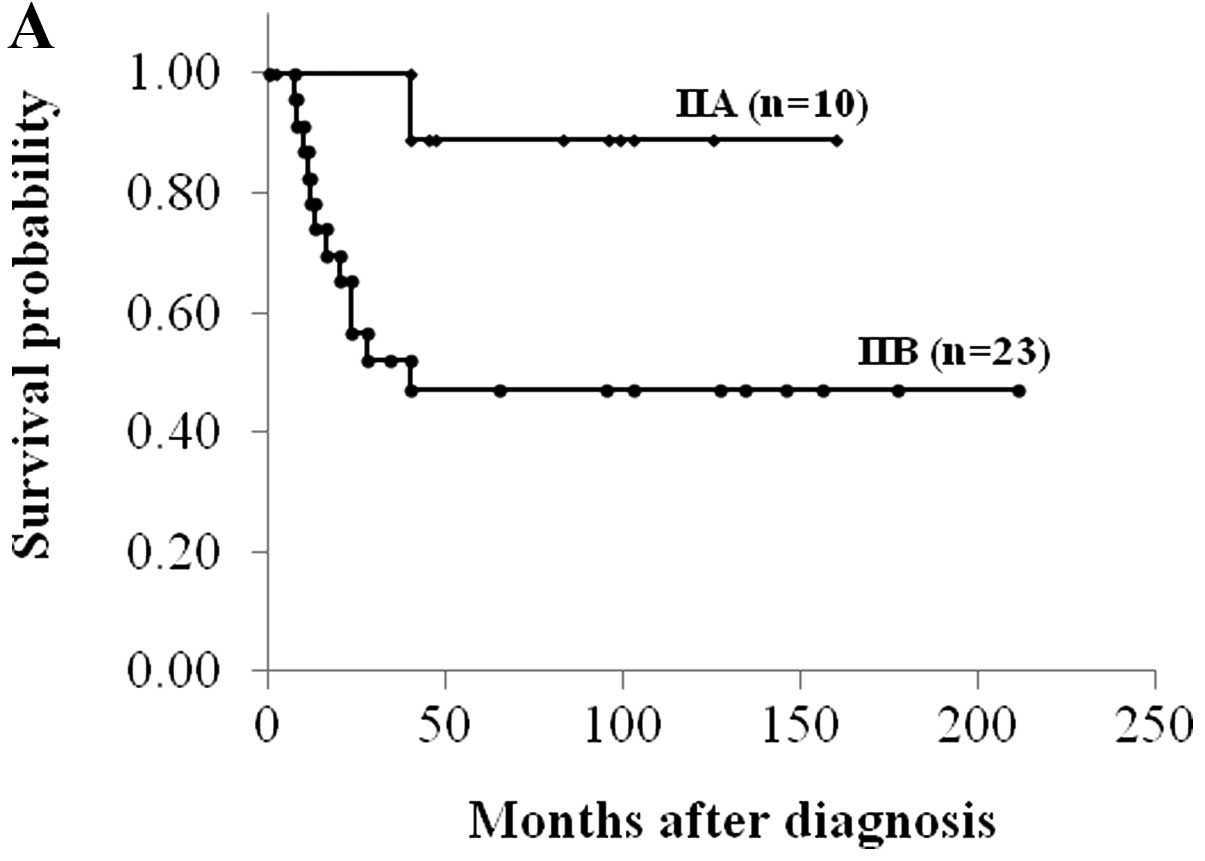
Sixteen of 42 (38.1%) osteosarcomas demonstrated
high MVD. High MVD, AJCC stage IIA and good histological response
to chemotherapy correlated significantly with better disease-free
survival (P<0.05, log-rank test) (Fig. 2 and Table I). There were statistically
significant associations of MVD with age and chemotherapy response
(P<0.05, Student’s t-test) (Table
II).
 | Table IPrognostic variables in osteosarcoma
as determined by univariate analysis of disease-free survival. |
Table I
Prognostic variables in osteosarcoma
as determined by univariate analysis of disease-free survival.
| Variable | 5-year DFS (n=44),
% | 95% CI | P-valuea |
|---|
| Age (years) | | | |
| <20 | 56 | 40–72 | 0.203 |
| ≥20 | 38 | 0–94 | |
| Gender | | | |
| Male | 60 | 40–80 | 0.697 |
| Female | 49 | 27–71 | |
| Tumor site | | | |
| Femur | 55 | 21–89 | 0.944 |
| Tibia or
fibula | 62 | 43–80 | |
| Surgical stage | | | |
| IIA | 89 | 68–100 | 0.035 |
| IIB | 47 | 26–68 | |
| Histology | | | |
| Osteoblastic | 52 | 32–71 | 0.758 |
| Fibroblastic | 60 | 17–100 | |
| Surgery | | | |
| Amputation | 47 | 23–71 | 0.529 |
| Limb salvage | 60 | 40–79 | |
| Response to
chemotherapy | | | |
| Poor | 40 | 20–61 | 0.026 |
| Good | 76 | 53–100 | |
| MVD | | | |
| Low | 38 | 19–56 | 0.009 |
| High | 87 | 69–100 | |
 | Table IIAssociation of MVD with
clinicopathological characteristics in 44 patients with
osteosarcoma. |
Table II
Association of MVD with
clinicopathological characteristics in 44 patients with
osteosarcoma.
| Variable | MVD | P-valuea |
|---|
| Age (years) | | |
| <20 | 46.4±33.7 | 0.007 |
| ≥20 | 19.6±10.2 | |
| Gender | | |
| Male | 42.6±33.8 | 0.293 |
| Female | 37.2±30.4 | |
| Tumor site | | |
| Femur | 42.0±32.0 | 0.309 |
| Tibia or
fibula | 36.1±30.9 | |
| Surgical stage | | |
| IIA | 44.1±25.5 | 0.226 |
| IIB | 35.2±32.6 | |
| Histology | | |
| Osteoblastic | 40.2±32.5 | 0.255 |
| Fibroblastic | 31.0±20.8 | |
| Surgery | | |
| Amputation | 35.0±32.9 | 0.213 |
| Limb salvage | 42.9±31.2 | |
| Response to
chemotherapy | | |
| Poor | 29.6±26.5 | 0.043 |
| Good | 48.3±35.7 | |
Survival analysis in chondrosarcomas
The patients with chondrosarcoma presented primary
lesions without any metastases. The median age at the time of
surgery was 42 years (range, 15–71). There were 8 males and 12
females. The tumor sites were 12 in the trunk (9 pelvises, 1 rib, 1
scapula and 1 vertebra) and 8 in the extremity (4 tibias, 3 humeri
and 1 femur). The median tumor size, defined as the maximum
diameter of the tumor at pathological analysis, was 11.7 cm (range,
4–27). Histological grades widely used were 14 in grade 1, 3 in
grade 2, and 3 in grade 3 (including one de-differentiated
chondrosarcoma and one mesenchymal chondrosarcoma). Sixteen
patients were treated with wide, 2 patients with marginal and 2
patients with intralesional excision. The median follow-up period
was 6 years and 6 months (range, 1–192 months). There was 1 local
recurrence and there were 3 lung metastases during the patients’
clinical courses. One patient was deceased due to the disease, 1 of
another cause, while 17 showed no evidence of disease and 1 was
alive with signs of the disease. The 5-year actual disease-free
survival rate was 81.3%.
Four of 20 (20%) chondrosarcomas showed high MVD.
Surgical margin (marginal and intralesional), MVD (high), tumor
size (≥8) and histological grade (grades 2 and 3) correlated
significantly with a shorter disease-free survival (P<0.05,
log-rank test) (Table III). There
were statistically significant associations of MVD with age and
histological grade (P<0.05, Student’s t-test) (Table IV).
 | Table IIIPrognostic variables in chondrosarcoma
as determined by univariate analysis of disease-free survival. |
Table III
Prognostic variables in chondrosarcoma
as determined by univariate analysis of disease-free survival.
| Variable | 5-year DFS (n=20),
% | 95% CI | P-valuea |
|---|
| Age (years) | | | |
| <40 | 71 | 38–100 | 0.468 |
| ≥40 | 89 | 68–100 | |
| Gender | | | |
| Male | 70 | 34–100 | 0.216 |
| Female | 89 | 68–100 | |
| Tumor size (cm) | | | |
| <8 | 100 | 0–100 | 0.020 |
| ≥8 | 66 | 34–98 | |
| Tumor site | | | |
| Extremity | 100 | 0–100 | 0.861 |
| Trunk | 66 | 34–98 | |
| Histological
grade | | | |
| 1 | 93 | 79–100 | 0.033 |
| 2 and 3 | 50 | 1–99 | |
| Surgical margin | | | |
| Wide | 84 | 64–100 | 0.017 |
| Intralesional and
marginal | 75 | 33–100 | |
| MVD | | | |
| Low | 82 | 68–100 | 0.025 |
| High | 67 | 13–100 | |
 | Table IVAssociation of MVD with
clinicopathological characteristics in 20 patients with
chondrosarcoma. |
Table IV
Association of MVD with
clinicopathological characteristics in 20 patients with
chondrosarcoma.
| Variable | MVD | P-valuea |
|---|
| Age (years) | | |
| <40 | 30.1±52.0 | 0.039 |
| ≥40 | 3.6±5.1 | |
| Gender | | |
| Male | 14.6±30.6 | 0.432 |
| Female | 11.9±34.3 | |
| Tumor size
(cm) | | |
| <8 | 1.9±3.0 | 0.087 |
| ≥8 | 21.9±42.1 | |
| Tumor site | | |
| Extremity | 11.4±27.3 | 0.428 |
| Trunk | 14.1±37.1 | |
| Histological
grade | | |
| 1 | 1.7±2.5 | 0.015 |
| 2 and 3 | 33.6±50.0 | |
| Surgical
margin | | |
| Wide | 14.5±36.0 | 0.337 |
| Intralesional and
marginal | 6.6±6.4 | |
Discussion
In this study, the diversity of angiogenesis in
malignant bone tumors was demonstrated. Osteosarcomas and Ewing’s
sarcomas were hypervascular, compared to the chondrosarcomas.
Although MVD in osteosarcomas and chondrosarcomas was a possible
prognostic marker, it had reverse effects on prognosis in
osteosarcomas and chondrosarcomas. High MVD correlated
significantly with a higher disease-free survival in osteosarcomas,
and a lower disease-free survival in chondrosarcomas.
Several studies have examined the clinical
significance of angiogenesis-related biomarkers in osteosarcomas.
Most of them, however, were studies with small series, thus the
role of angiogenesis remains to be clarified. Mikulić et
al(14) demonstrated that high
MVD was predictive of a greater probability of distant metastasis
and of shorter overall and disease-free survival. Kaya et
al(15) described vascular
endothelial growth factor (VEGF), a well-known proangiogenic
factor. Patients with a VEGF-positive tumor showed a shorter
disease-free, as well as an overall survival period. Numerous other
reports, however, failed to find a correlation between MVD or VEGF
and survival (16–18). Conversely, in their study Kreuter
et al examined 60 patients with high-grade central
osteosarcoma and found, that patients with a high MVD had
significantly longer overall and relapse-free survival period,
compared to patients with low MVD (19). A good response to chemotherapy was
significantly correlated with a higher MVD, to some extent possibly
due to the improved accessibility of chemotherapy to proliferating
osteosarcoma cells. Tumor microcirculation is an important factor
in drug delivery to cancer cells. The efficacy of drug delivery is
much higher in a tumor with a high degree of microvessels compared
to a tumor with low MVD, especially in a chemotherapy-sensitive
tumor, such as osteosarcoma. In contrast to other studies, patients
in the present study received a standardized treatment with
intensive pre- and post-operative chemotherapy, as well as
intensive chemotherapy, delivering results consistent with
Kreuter’s results, suggesting that hyper-vascularity might induce
good response to chemotherapy, thus leading to better prognosis.
The improvement of the distribution of hypovascular osteosarcoma is
required.
Angiogenesis is one of the probable elements
involved in the transformation and progression of neoplasia. It is
particularly significant in the progression of cartilage neoplasia,
since cartilage neoplasia has some phenotypic links to cartilage, a
vascular tissue. Several studies have demonstrated that cartilage
tumors have microvessels in the development and progression, while
the amount of vascularity correlates with tumor grade (20–22).
To further elucidate angiogenesis in cartilage tumors, Kalinski
et al(23) used
double-labeling immunohistochemistry, using von Willebrad factor
and MIB-1 aiming to show that the proliferating capillary index,
better characterizing the angiogenic properties of a distinct tumor
compared to MVD, was significantly higher in conventional
chondrosarcoma grades II and III compared to enchondroma,
chondrosarcoma grade I or de-differentiated chondrosarcoma.
Nakagawa et al(24)
demonstrated that there was a significant association of CD34 with
histological grades, although not with overall prognosis. In the
present study, high MVD was associated with histological grade and
predicted poor prognosis in chondrosarcoma. Surgical treatment with
wide resection was the mainstay of therapy for chondrosarcoma,
whereas chemotherapy and radiotherapy were negligible in their
treatment. At present, there is no widely adopted adjuvant therapy
(25). Results drawn from this
study seem to be of value for the development of antiangiogenic
chemotherapy, for patients with chondrosarcoma.
MVD was significantly associated with age in both
osteosarcomas and chondrosarcomas and was higher in patients aged
<20 years with osteosarcoma and patients aged <40 years with
chondrosarcoma. These interesting findings have never been
described before, however, the present study was limited to a small
sample size and the multivariate model did not converge. Therefore,
to confirm the conclusion, additional investigations involving a
larger number of patients is required.
In conclusion, the present study showed that there
was diversity of angiogenesis in malignant bone tumors.
Osteosarcomas and Ewing’s sarcomas were hypervascular, compared to
chondrosarcoma. In osteosarcomas, hypervascularity induced good
chemotherapy response, leading to better prognosis, while in
hypovascular osteosarcoma a better drug distribution in tumors is
needed. In chondrosarcomas, high MVD was associated with
histological grade and predicted poor prognosis, suggesting the
development of antiangiogenic chemotherapy for patients with
chondrosarcoma. These encouraging data should lead to additional
studies involving larger sample size, to exclude the possibility
that these divergences are caused by differences in patients’
characteristics and methodology in staining and analysis.
References
|
1.
|
Bacci G, Briccoli A, Ferrari S, et al:
Neoadjuvant chemotherapy for osteosarcoma of the extremity:
long-term results of the Rizzoli’s 4th protocol. Eur J Cancer.
37:2030–2039. 2001.
|
|
2.
|
Bielack SS, Kempf-Bielack B, Delling G, et
al: Prognostic factors in high-grade osteosarcoma of the
extremities or trunk: an analysis of 1,702 patients treated on
neoadjuvant cooperative osteosarcoma study group protocols. J Clin
Oncol. 20:776–790. 2002.
|
|
3.
|
Paulussen M, Fröhlich B and Jürgens H:
Ewing tumour: incidence, prognosis and treatment options. Paediatr
Drugs. 3:899–913. 2001.
|
|
4.
|
Paulussen M, Ahrens S, Dunst J, et al:
Localized Ewing tumor of bone: final results of the cooperative
Ewing’s Sarcoma Study CESS 86. J Clin Oncol. 19:1818–1829.
2001.
|
|
5.
|
Rizzo M, Ghert MA, Harrelson JM and Scully
SP: Chondrosarcoma of bone: analysis of 108 cases and evaluation
for predictors of outcome. Clin Orthop Relat Res. 391:224–233.
2001.
|
|
6.
|
Welkerling H, Kratz S, Ewerbeck V and
Delling G: A reproducible and simple grading system for classical
chondrosarcomas. Analysis of 35 chondrosarcomas and 16 enchondromas
with emphasis on recurrence rate and radiological and clinical
data. Virchows Arch. 443:725–733. 2003.
|
|
7.
|
Folkman J, Watson K, Ingber D and Hanahan
D: Induction of angiogenesis during the transition from hyperplasia
to neoplasia. Nature. 339:58–61. 1989.
|
|
8.
|
Hasan J, Byers R and Jayson GC:
Intra-tumoural microvessel density in human solid tumours. Br J
Cancer. 86:1566–1577. 2002.
|
|
9.
|
Kawaguchi N, Matumoto S and Manabe J: New
method of evaluating the surgical margin and safety margin for
musculoskeletal sarcoma, analysed on the basis of 457 surgical
cases. J Cancer Res Clin Oncol. 121:555–563. 1995.
|
|
10.
|
Huvos AG: Bone Tumors: Diagnosis,
Treatment, and Prognosis. 2nd edition. W.B. Saunders; Philadelphia:
pp. 85–155. 1991
|
|
11.
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumor angiogenesis and metastasis-correlation in invasive breast
carcinoma. N Engl J Med. 324:1–8. 1991.
|
|
12.
|
Iwamoto Y, Tanaka K, Isu K, et al:
Multiinstitutional phase II study of neoadjuvant chemotherapy for
osteosarcoma (NECO study) in Japan: NECO-93J and NECO-95J. J Orthop
Sci. 14:397–404. 2009.
|
|
13.
|
Kimura H, Tsuchiya H, Shirai T, et al:
Caffeine-potentiated chemotherapy for metastatic osteosarcoma. J
Orthop Sci. 14:556–565. 2009.
|
|
14.
|
Mikulić D, Ilić I, Cepulić M, et al: Tumor
angiogenesis and outcome in osteosarcoma. Pediatr Hematol Oncol.
21:611–619. 2004.
|
|
15.
|
Kaya M, Wada T, Nagoya S, Sasaki M,
Matsumura T and Yamashita T: The level of vascular endothelial
growth factor as a predictor of a poor prognosis in osteosarcoma. J
Bone Joint Surg Br. 91:784–788. 2009.
|
|
16.
|
Mantadakis E, Kim G, Reisch J, et al: Lack
of prognostic significance of intratumoral angiogenesis in
nonmetastatic osteosarcoma. J Pediatr Hematol Oncol. 23:286–289.
2001.
|
|
17.
|
Ek ET, Ojaimi J, Kitagawa Y and Choong PF:
Does the degree of intratumoural microvessel density and VEGF
expression have prognostic significance in osteosarcoma? Oncol Rep.
16:17–23. 2006.
|
|
18.
|
Ek ET, Ojaimi J, Kitagawa Y and Choong PF:
Outcome of patients with osteosarcoma over 40 years of age: is
angiogenesis a marker of survival? Int Semin Surg Oncol.
3:72006.
|
|
19.
|
Kreuter M, Bieker R, Bielack SS, et al:
Prognostic relevance of increased angiogenesis in osteosarcoma.
Clin Cancer Res. 10:8531–8537. 2004.
|
|
20.
|
Ayala G, Liu C, Nicosia R, Horowitz S and
Lackman R: Microvasculature and VEGF expression in cartilaginous
tumors. Hum Pathol. 31:341–346. 2000.
|
|
21.
|
McGough RL, Aswad BI and Terek RM:
Pathologic neovascularization in cartilage tumors. Clin Orthop
Relat Res. 397:76–82. 2002.
|
|
22.
|
McGough RL, Lin C, Meitner P, Aswad BI and
Terek RM: Angiogenic cytokines in cartilage tumors. Clin Orthop
Relat Res. 397:62–69. 2002.
|
|
23.
|
Kalinski T, Sel S, Kouznetsova I, Röpke M
and Roessner A: Heterogeneity of angiogenesis and blood vessel
maturation in cartilage tumors. Pathol Res Pract. 205:339–345.
2009.
|
|
24.
|
Nakagawa SA, Lopes A, Lopes de Carvalho A,
et al: Nitric oxide synthases, cyclooxygenase-2, nitrotyrosine, and
angiogenesis in chondrosarcoma and their relation to prognosis. J
Bone Joint Surg Am. 92:1738–1746. 2010.
|
|
25.
|
Riedel RF, Larrier N, Dodd L, Kirsch D,
Martinez S and Brigman BE: The clinical management of
chondrosarcoma. Curr Treat Options Oncol. 10:94–106. 2009.
|