Introduction
Colorectal cancer (CRC) is the most common
malignancy worldwide. Although surgery remains the mainstay of
treatment, the role of chemotherapy has become crucial over the
past 10 years. Modern chemotherapy, including molecular-targeted
agents, has increased the survival period of patients with
metastatic CRC to over 2 years (1–3).
Moreover, cytoreductive surgery for liver, lung and other
metastases has been widely used for therapy, although few patients
are suitable for metastasectomy. To prolong survival, multimodal
approaches, including surgery, radiotherapy and chemotherapy, alone
or in combination, have been suggested for recurrent and metastatic
CRC, while there have been efforts to increase the small proportion
of patients suitable for surgery. Thus, CRC patients have
experienced longer survival periods even in cases of incurable
metastases. Consequently, individuals with advanced CRC need to
maintain their compliance with cancer treatment to ensure quality
of life (QOL) for an extended period, especially in an outpatient
clinic.
Early palliative care for cancer patients has
attracted attention with the aim of achieving significant
improvements in clinical outcome. Palliative care is now believed
to be appropriately provided at any age and stage of a serious
illness, and is available together with curative treatment. A
number of studies have reported that the early integration of
palliative care for patients with metastatic non-small cell lung
cancer is a clinically meaningful and feasible care model,
affecting the survival period and QOL in a similar manner to that
of first-line chemotherapy in such patients (4–7).
Patients with metastatic or recurrent CRC who have
received therapeutic chemotherapy often experience multiple severe
symptoms, including various types of pain, due to several causes,
such as local recurrence, peritoneal and bone metastasis.
Therefore, early opioid use during CRC treatment has been widely
accepted worldwide. Early opioid use during chemotherapy
undoubtedly contributes to improvements in the QOL of CRC patients.
However, a limited number of studies have investigated the
oncologic significance of opioid use during an active treatment for
advanced CRC.
The aim of the present study was to clarify the
characteristics of CRC patients who were administered opioid to
maintain compliance with cancer treatment, and to evaluate the
usefulness of opioid in clinical management.
Patients and methods
Patient data
This was a retrospective study of the patients
(n=245) who received therapeutic chemotherapy for advanced or
recurrent CRC at the Department of Gastrointestinal Surgery, in the
Mie University Hospital (Mie, Japan), between March, 2000 and
December, 2011. One hundred and thirty-seven of these patients
(55.9%) were administered opioid-based pain control, while 117
patients were administered an opioid drug during chemotherapy.
Patients who were administered the opioid only in the setting of
terminal illness were excluded from the study. Various opioids were
administered to patients, and thus for the analyses the opioid dose
was converted into mg equivalent to intravenous morphine (8). Our Institutional Ethics Committee
approved the study, and written informed consent was obtained from
the patients for inclusion in the study.
Multimodal therapy
Patients with histologically proven unresectable
primary, synchronous metastatic and meta-chronous metastatic or
recurrent CRC were included in the present study. Patients who
underwent initial simultaneous primary tumor resection and
metastasectomy (e.g., lung and liver) were excluded. According to
our institutional policy, initial chemotherapy for the treatment of
metastatic CRC, including unresectable primary tumor, was
administered to the patients for 4–5 months (9). Cytoreductive therapy was defined as
surgery and/or radio-frequency ablation (RFA) therapy that reduced
tumor volume. The decision concerning whether or not to proceed
with multimodal therapy was determined based on patient response to
chemotherapy. Patients with a partial response or with a stable
disease after systemic chemotherapy were considered for
cytoreductive surgery and/or RFA. Multidisciplinary discussions
during chemotherapy determined the multimodal therapy for each
patient, including timing.
Chemotherapy
Over a period of 11 years, 245 consecutive patients
with CRC received triple-drug chemotherapy with 5-fluorouracil
(5-FU) and oxaliplatin or irinotecan (FOLFOX or FOLFIRI), with or
without bevacizumab or cetuximab. Between 2000 and 2005, the
Japanese social health insurance did not allow the use of
oxaliplatin in the treatment of CRC, and our first-line
chemotherapy for advanced CRC was 5-FU with or without irinotecan.
For patients with no extra-hepatic metastasis but with unresectable
liver metastasis, hepatic arterial infusion chemotherapy was used
with 5-FU, followed by secondary surgery (10). Drug approval in Japan is much
slower compared to the Western world. Since 2005, our first-line
chemotherapy for advanced or recurrent CRC has been FOLFOX or
FOLFIRI. The molecular-targeted agents bevacizumab, cetuximab and
panitumumab were approved for use in 2007, 2008 and 2010,
respectively. Since 2007, bevacizumab with FOLFOX or FOLFIRI have
been used as first-line chemotherapy for advanced or recurrent CRC.
Since 2008, cetuximab with or without irinotecan has also been used
as second- or third-line chemotherapy. Since 2010, cetuximab or
panitumumab with FOLFIRI or FOLFOX have been available in Japan as
first-line chemotherapy for patients with wild-type KRAS.
Radiotherapy with concurrent 5-FU-based chemotherapy was used to
improve the resectability of locally inoperable rectal cancer.
5-FU-based adjuvant chemotherapy was administered to patients with
complete secondary cytoreduction. In the case of patients with
incomplete cytoreduction, chemotherapy was reintroduced, depending
on their performance status (PS).
Oncologic emergencies
In this study, oncologic emergencies were defined as
events requiring urgent admission subsequent to chemotherapy in an
outpatient clinic. The emergencies resulted from cancer progression
or adverse effects due to cancer therapy.
Statistical analysis
JMP software, version 7 (SAS Institute, Inc., Cary,
NC, USA) was used to carry out the statistical analysis. Data were
presented as the mean ± standard error (SE). Contingency tables
were analyzed using Fisher’s exact test or the χ2 test
with Yate’s correction. Associations between continuous and
categorical variables were evaluated using the Mann-Whitney U test.
Survival curves were constructed according to the Kaplan-Meier
method, and differences were analyzed using the log-rank test. Each
significant predictor identified by the log-rank test was assessed
by multivariate analysis, using the logistic regression model.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patient data
A retrospective review of 245 patients with
unresectable primary, synchronous metastatic, and metachronous
metastatic or recurrent CRC, who were treated in our Department
(Department of Gastrointestinal Surgery, Mie University Hospital),
was carried out. There were 146 males (60%) and 99 females (40%),
with a mean age of 64 years (range, 29–85). Of these, 189 patients
(77%) had a PS of <2. The patients received therapeutic
chemotherapy, with 82/245 (33%) also receiving chemotherapy
subsequent to secondary cytoreductive surgery and/or RFA as
multimodal therapy. Of the 137 patients (56%) who were administered
an opioid drug for various types of pain due to cancer and/or
cancer treatment, 117 (48%) were prescribed opioids during
chemotherapy to maintain their compliance with cancer treatment,
including multimodal therapy. The median interval from the initial
chemotherapy to initiation of opioid administration was 14 months.
Maximum daily patient intravenous morphine doses converted from
oral morphine, oxycodone and fentanyl doses were: <9 mg in 16
patients; 10–29 mg in 29; 30–49 mg in 33 and >50 mg in 40
patients.
Correlation between clinical factors and
opioid use
Table I shows the
background characteristics of the 245 patients, who did/did not
receive opioid during cancer treatment. No statistically
significant associations were found between opioid use and gender,
PS, tumor state and site, carcino embryonic antigen (CEA) level
(<6 or ≥6 ng/ml), chemotherapy regimen, radiotherapy (yes/no)
and cytoreductive surgery and/or RFA (yes/no). A statistically
significant association was evident between opioid use and age
(<65 or ≥65 years; P= 0.0281), pathology (differentiated or
undifferentiated; P=0.0007) and the response rate to chemotherapy
(P=0.0056).
 | Table ICorrelation between clinical
background factors and opioid use. |
Table I
Correlation between clinical
background factors and opioid use.
| Variables | Total (n=245) | With opioid
(n=117) | Without opioid
(n=128) | P-value |
|---|
| Age (years) | | | | 0.0281 |
| <65 | 150 | 80 | 70 | |
| ≥65 | 95 | 37 | 58 | |
| Gender | | | | 0.2184 |
| Female | 99 | 52 | 47 | |
| Male | 146 | 65 | 81 | |
| PS | | | | 0.4918 |
| 0–1 | 189 | 88 | 101 | |
| 2–4 | 56 | 29 | 27 | |
| Tumor state | | | | 0.4819 |
| Synchronous | 143 | 71 | 72 | |
| Metachronous | 102 | 46 | 56 | |
| Tumor site | | | | 0.528 |
| Liver | 66 | 30 | 36 | |
| Lung | 44 | 18 | 26 | |
| Liver and lung | 13 | 5 | 8 | |
| Local
recurrence | 25 | 14 | 11 | |
| Unresectable
primary | 21 | 9 | 12 | |
| Lymph node | 13 | 8 | 5 | |
| Dissemination | 61 | 33 | 28 | |
| Others | 2 | 0 | 2 | |
| Pathology | | | | 0.0007 |
| Differentiated | 215 | 94 | 121 | |
|
Undifferentiated | 30 | 23 | 7 | |
| CEA (ng/ml) | | | | 0.137 |
| <6 | 171 | 87 | 84 | |
| ≥6 | 74 | 30 | 44 | |
| Chemotherapy | | | | 0.227 |
| 5-FU-based ±
CPT-11 | 116 | 57 | 59 | |
| FOLFOX/FOLFIRI | 36 | 21 | 15 | |
| Molecular
agents | 93 | 39 | 54 | |
| Response rate
(measurable) | | | | 0.0056 |
| CR | 6 | 1 | 5 | |
| PR | 81 | 38 | 43 | |
| SD | 111 | 62 | 49 | |
| PD | 33 | 15 | 18 | |
| Radiotherapy | | | | 0.407 |
| Yes | 45 | 24 | 21 | |
| No | 200 | 93 | 107 | |
| Secondary surgery
and/or RFA | | | | 0.5584 |
| Yes | 82 | 37 | 45 | |
| No | 163 | 80 | 83 | |
Effects of opioid use on cancer-specific
survival time
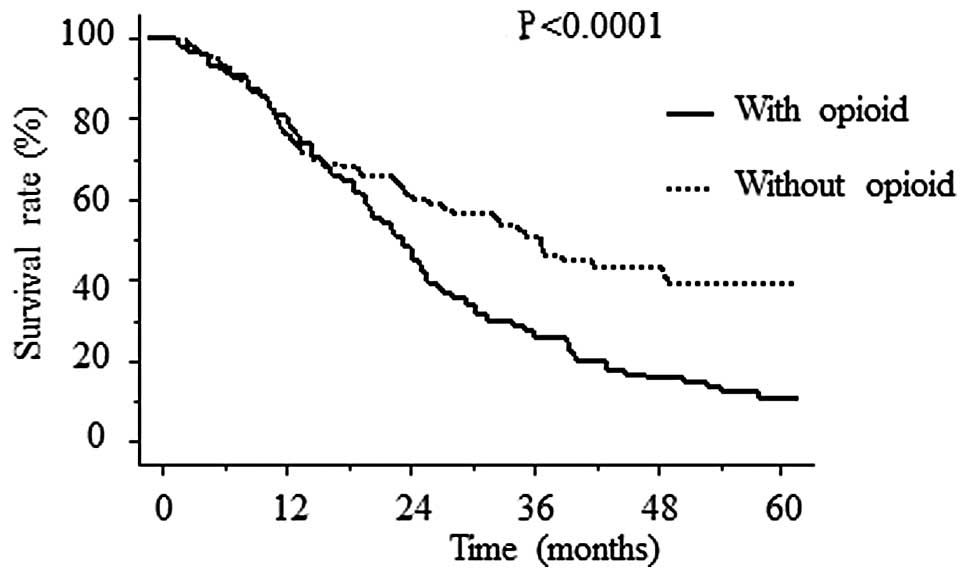
The Kaplan-Meier analysis demonstrated statistically
significant differences in survival based on opioid use (Fig. 1). The mean cancer-specific survival
period was 796±49 days with and 940±78 days without opioids
(P<0.0001). The prognostic significance of opioid use was
assessed, based on whether or not patients received multimodal
therapy.
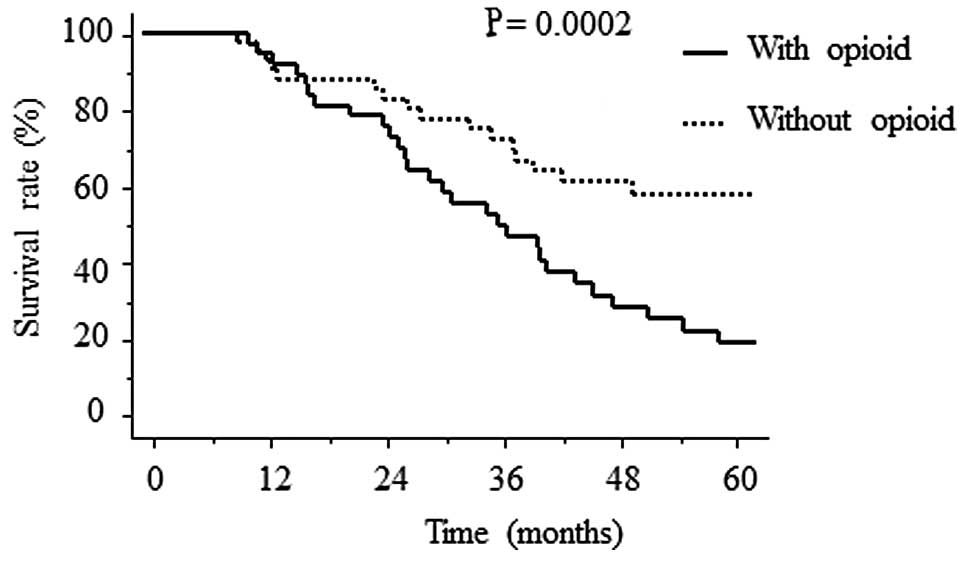
The Kaplan-Meier analysis demonstrated statistically
significant differences in the survival period in the patient
groups that were administered opioids and those that had not,
having undergone multimodal therapy (n=82; Fig. 2). The mean cancer-specific survival
period was 1140±95 days for patients administered with opioids and
1556±160 days for patients who had not received opioids (P=0.0002).
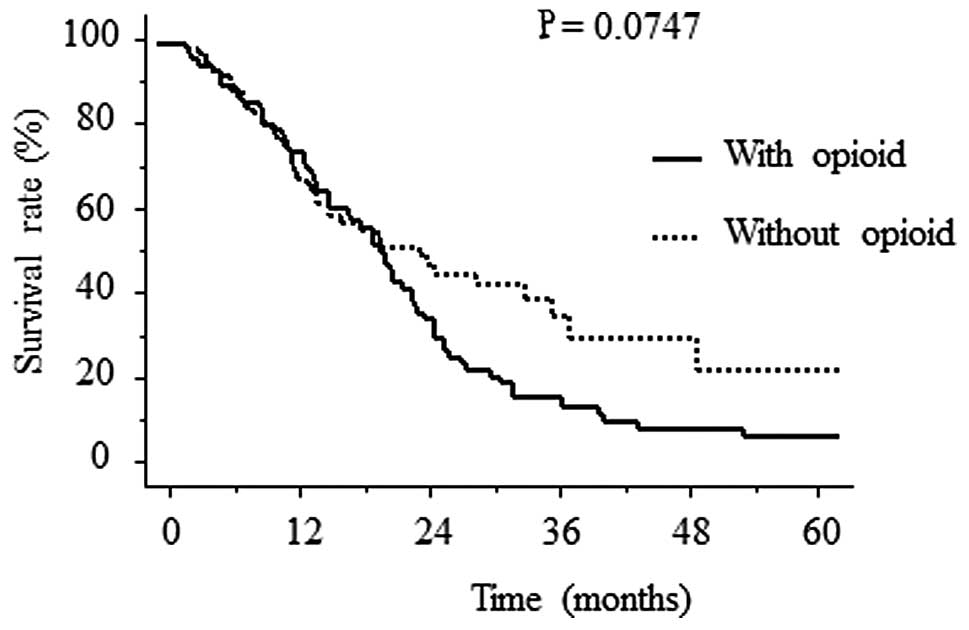
While differences in the survival period between the two groups of
patients having received chemotherapy alone (n=163) were not
statistically significant (P=0.0747), there was a tendency for the
mean cancer-specific survival period to be longer (636±46 days) for
the patients who had not received opioids, compared to those who
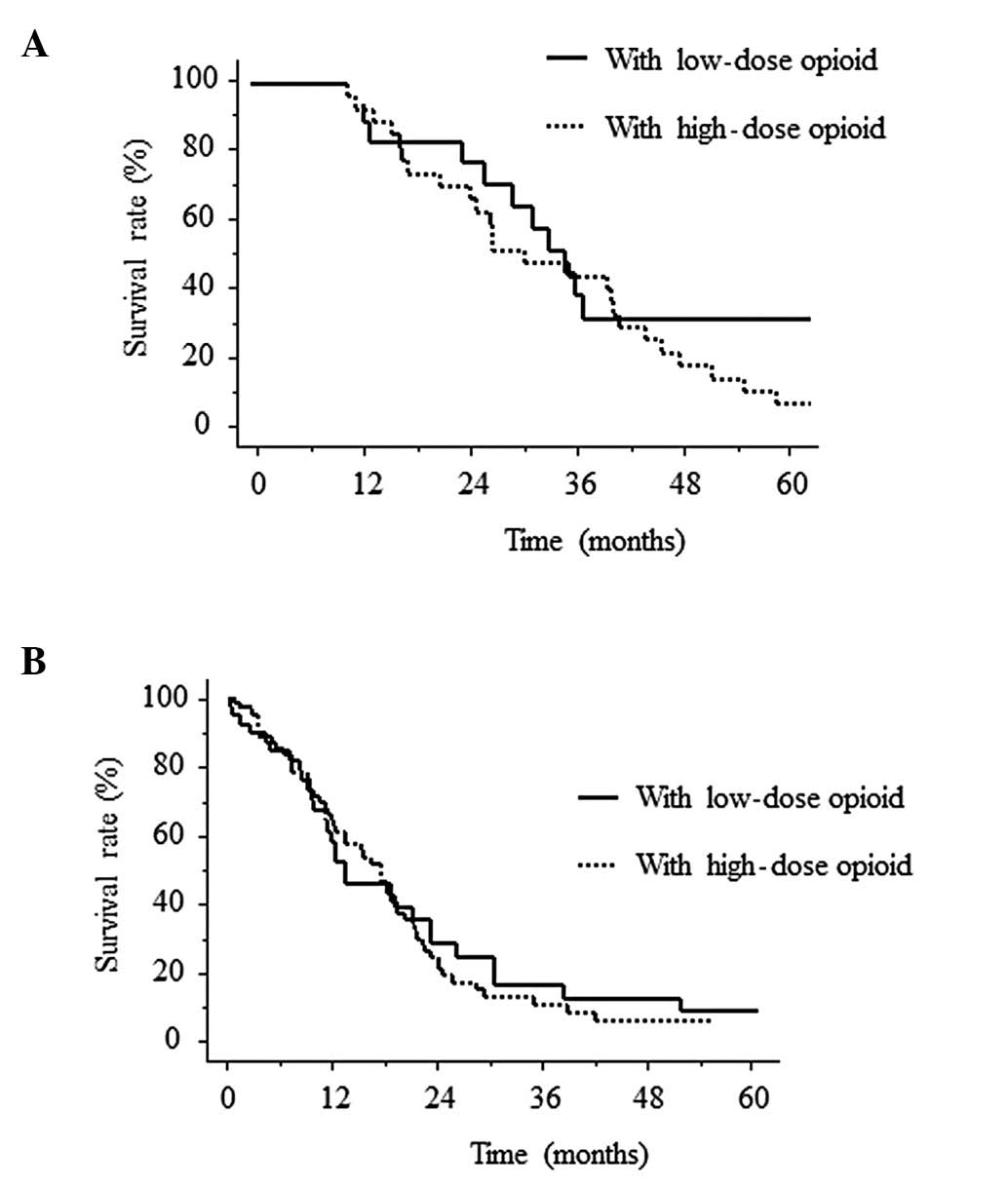
had received opioids (606±57 days; Fig. 3). No statistically significant
differences were found in the cancer-specific survival periods in
the patient groups that were administered low (<30 mg) and high
(>30 mg) doses of opioid subsequent to chemotherapy alone or
multimodal therapy (Fig. 4).
Multivariate analysis using the characteristics
listed in Table I demonstrated
that pathology (undifferentiated) [hazard ratio (HR), 4.300; 95%
confidence interval (CI), 1.754–10.539; P= 0.0014] and age <65
years (HR, 1.837; 95% CI, 1.072–3.148; P= 0.0268) were
independently associated with opioid use during chemotherapy
(Table II).
 | Table IIMultivariate analysis in relation to
opioid use during chemotherapy. |
Table II
Multivariate analysis in relation to
opioid use during chemotherapy.
| Variables | P-value | Hazard ratio | 95% CI |
|---|
| Age (<65 years vs.
≥65 years) | 0.0268 | 1.837 | 1.072–3.148 |
| Pathology
(undifferentiated vs. differentiated) | 0.0014 | 4.3 | 1.754–10.539 |
| Response rate (PD vs.
others) | 0.6072 | 1.229 | 0.560–2.699 |
Oncologic emergencies and opioid use
The patients had received chemotherapy mainly in an
outpatient clinic, while oncologic emergencies requiring admission
occurred in 35/245 (14%) patients. These emergencies resulted from
cancer progression (n=23) or adverse effects due to cancer therapy
(n=12). Oncologic emergencies due to cancer progression were
significantly correlated with opioid use (P=0.0002), although there
were no statistically significant differences between
cancer-specific survival and oncologic emergencies. Oncologic
emergencies also occurred during CRC therapy in patients receiving
opioid (Table III). Various
symptoms were confirmed, however, there was no mortality given the
adequate emergency management.
 | Table IIISymptoms of patients, who were
administered opioids, presenting with oncologic emergencies. |
Table III
Symptoms of patients, who were
administered opioids, presenting with oncologic emergencies.
| Symptoms | No. of patients | Percentage of
patients |
|---|
| Severe pain | 4 | 17 |
| Infectious
emergencies | | |
| Abscess | 2 | 9 |
| Pneumonia | 2 | 9 |
|
Cholangitis/jaundice | 3 | 13 |
| Renal failure
(hydronephrosis) | 2 | 9 |
| Neurogenic
emergencies | | |
| Spinal cord
compression | 2 | 9 |
| Brain
metastasis | 1 | 4 |
| Respiratory
emergencies | | |
| Bronchial
obstruction | 2 | 9 |
| Dyspnea (pleural
effusion) | 1 | 4 |
| Others | | |
| Bone fractures
(bone metastasis) | 1 | 4 |
| Small bowel
obstruction (peritoneal dissemination) | 3 | 13 |
| Total | 23 | 100 |
Discussion
This study clarified the characteristics of CRC
patients, who were administered opioid in combination with
therapeutic chemotherapy as outpatients. In order to maintain
compliance with cancer treatment including multimodal therapy, 48%
of the patients were prescribed opioids during chemotherapy.
Treatment of pain was the reason for opioid use. No significant
association was found between opioid use, gender, PS, tumor state,
tumor site, CEA level, chemotherapy regimen and the use of
multimodal therapy. However, age (<65 years), pathology
(undifferentiated) and poor response rate were significantly
associated with opioid use. Of these factors, age and pathology
were independent of opioid use during active cancer treatment. In
the current study, patients who were administered opioid due to
cancer pain had significantly poorer cancer-specific survival
compared to patients that were not. This reduction in survival time
was more significant in patients that had undergone multimodal
therapy compared to chemotherapy alone. This lower survival rate
may be correlated with patient characteristics, such as younger age
or undifferentiated pathology. The mean cancer-specific survival
times in the current study were 606±57 days (chemotherapy with
opioid), <636±46 days (chemotherapy without opioid), <1140±95
days (multimodal therapy with opioid) and <1556±160 days
(multimodal therapy without opioid). In addition, the median
survival time (MST) was 21.3 months following chemotherapy with
opioid and 24.7 months after chemotherapy without opioid. The MST
was similar to that achieved using modern chemotherapy, although
our study population comprised many patients who could not be
treated with molecular agents. Multimodal therapy was also
confirmed to increase patient survival time irrespective of opioid
use. Notably, based on these findings it is hard to conceive that
opioid use itself would have a negative effect on the survival time
of patients. Therefore, opioid use is assumed to be helpful in the
active treatment of cancer.
Emphasis has been placed on the role of opioids in
cancer recurrence and metastasis. Recent basic investigations have
demonstrated that the direct and indirect effects of
μ-opioids on cancer progression are correlated with the
immune function or angiogenesis (11–14).
Despite evidence from cell and epidemiologic animal studies, few
studies are available that indicate that opioids in clinical use
may adversely affect the prognosis of cancer patients. Conversely,
recent scientific attention has been focused on early palliative
care including opioid use for cancer therapy leading to significant
improvements in clinical outcome (4–7,15,16).
In a recent study, non-small cell lung cancer patients receiving
early palliative care have been reported to have less aggressive
care towards the end of their lives, while having a longer survival
period compared to patients receiving standard care (7). The same group also reported that
early palliative care of patients with non-small cell lung cancer
enabled optimization of the timing of the administration of the
final chemotherapy at the end of life (16). A recent phase II study of an
opioid-based pain control program for head and neck cancer patients
receiving chemoradiotherapy has also reported the use of a
systematic opioid-based pain control program to be likely to
improve compliance with chemoradiotherapy (17). It is conceivable that various
cancer therapies, including multimodal therapy, compensate for the
potential drawback in the use of opioid, with regard to its effects
on cancer progression by means of a basic mechanism. In the current
study, oncologic emergencies due to cancer progression
significantly correlated with opioid use, although no statistically
significant differences were observed between cancer-specific
survival and oncologic emergencies. This finding suggests that
opioid itself does not have a negative effect on survival, rather
CRC patients with cancer pain had a more elevated stage of the
disease compared to those without pain. Although the present study
was retrospective and small-scale, the results suggested an
oncologic significance of opioid use during active treatment for
advanced CRC.
In conclusion, together with improved chemotherapy
and multimodal therapy, marked advances in the management of
metastatic and recurrent CRC have enabled patients to experience a
prolonged survival period, and enhanced compliance with cancer
treatment and QOL for an extended period. Accordingly, opioid use
is highly recommended in CRC management to maintain compliance
during active cancer therapy. However, CRC patients receiving
opioid may have a progressive disease, and as a result clinicians
need to be aware of the fact that oncologic emergencies are likely
to arise during active CRC therapy.
References
|
1.
|
Hurwitz H, Fehrenbacher L, Novotny W, et
al: Bevacizumab plus irinotecan, fluorouracil, and leucovorin for
metastatic colorectal cancer. N Engl J Med. 350:2335–2342.
2004.
|
|
2.
|
Van Cutsem E, Köhne CH, Hitre E, et al:
Cetuximab and chemotherapy as initial treatment for metastatic
colorectal cancer. N Engl J Med. 360:1408–1417. 2009.
|
|
3.
|
Fuchs CS, Marshall J, Mitchell E, et al:
Randomized, controlled trial of irinotecan plus infusional, bolus,
or oral fluoropyrimidines in first-line treatment of metastatic
colorectal cancer: results from the BICC-C study. J Clin Oncol.
25:4779–4786. 2007.
|
|
4.
|
Brown J, Thorpe H, Napp V, et al:
Assessment of quality of life in the supportive care setting of the
big lung trial in non-small cell lung cancer. J Clin Oncol.
23:7417–7427. 2005.
|
|
5.
|
Non-small Cell Lung Cancer Collaborative
Group: Chemotherapy in non-small cell lung cancer: a meta-analysis
using updated data on individual patients from 52 randomised
clinical trials. BMJ. 311:899–909. 1995.
|
|
6.
|
Spiro SG, Rudd RM, Souhami RL, et al:
Chemotherapy versus supportive care in advanced non-small cell lung
cancer: improved survival without detriment to quality of life.
Thorax. 59:828–836. 2004.
|
|
7.
|
Temel JS, Greer JA, Muzikansky A, et al:
Early palliative care for patients with metastatic non-small cell
lung cancer. N Engl J Med. 363:733–742. 2010.
|
|
8.
|
American Pain Society: Principles of
Analgesic Use in the Treatment of Acute Pain and Cancer Pain. 5th
edition. American Pain Society; Glenview, IL: 2003
|
|
9.
|
Inoue Y, Hiro J, Toiyama Y, et al: Optimal
use of current chemotherapy in multimodality therapy for advanced
colorectal cancer. Oncol Lett. 3:363–368. 2012.
|
|
10.
|
Kusunoki M, Noda M, Yanagi H, et al:
Second-look hepatectomy after 5-FU arterial infusion in patients
with primary unresectable hepatic colorectal metastases. Int J
Oncol. 10:107–111. 1997.
|
|
11.
|
Lennon FE, Moss J and Singleton PA: The
μ-opioid receptor in cancer progression: is there a direct
effect? Anesthesiology. 116:940–945. 2012.
|
|
12.
|
Mathew B, Lennon FE, Siegler J, et al: The
novel role of the mu opioid receptor in lung cancer progression: a
laboratory investigation. Anesth Analg. 112:558–567. 2011.
|
|
13.
|
Nylund G, Pettersson A, Bengtsson C, et
al: Functional expression of mu-opioid receptors in the human colon
cancer cell line, HT-29, and their localization in human colon. Dig
Dis Sci. 53:461–466. 2008.
|
|
14.
|
Lennon FE, Mirzapoiazova T, Mambetsariev
B, et al: Over-expression of the μ-opioid receptor in human
non-small cell lung cancer promotes akt and mTOR activation, tumor
growth, and metastasis. Anesthesiology. 116:857–867. 2012.
|
|
15.
|
Smith TJ, Temin S, Alesi ER, et al:
American society of clinical oncology provisional clinical opinion:
the integration of palliative care into standard oncology care. J
Clin Oncol. 30:880–887. 2012.
|
|
16.
|
Greer JA, Pirl WF, Jackson VA, et al:
Effect of early palliative care on chemotherapy use and end-of-life
care in patients with metastatic non-small cell lung cancer. J Clin
Oncol. 30:394–400. 2012.
|
|
17.
|
Zenda S, Matsuura K, Tachibana H, et al:
Multicenter phase II study of an opioid-based pain control program
for head and neck cancer patients receiving chemoradiotherapy.
Radiother Oncol. 101:410–414. 2011.
|