Introduction
Treatment outcomes of lung cancer are relatively
poor and have not improved significantly in the past 20 years. In
addition to common unresectable cases, resectable cases require
systemic chemotherapy at a certain stage. Although improvement in
the outcomes of chemotherapy in lung cancer has been gradually
observed, they remain unsatisfactory in circumstances whereby
medication efficacy is only 2–3%. Therefore, gene therapy is
clinically expected as the treatment strategy is different from
that of the existing chemotherapy or radiation therapy.
Subcutaneous implantation is widely practiced since cancer growth
is easily observed. However, studies have shown that the biological
behavior of cancer cells is influenced by the implantation site
(1), and that the orthotopic
implantation of human tumor cells into relevant organs of nude mice
provides an in vivo model to study the biology and therapy
of these cells (1,2).
On the other hand, chemokines are a family of small
cytokines that primarily induce the directed migration of
hematopoietic cells when bound to their seven-transmembrane, G
protein-coupled receptors (3,4).
Chemokines are attractive candidates for immune cell-based
approaches to cancer gene therapy, as they function as
chemoattractants for several immune effector cell types. CX3CL1
(also known as fractalkine) is a unique chemokine that functions as
an adhesion and chemotactic molecule towards its receptor
CX3CR1-expressing cells. Although chemokines activate adhesion
molecules, such as integrins, to bind to target cells or the
cellular matrix, the interaction of transmembrane CX3CL1-CX3CR1
strongly induces cell-to-cell contact in an adhesion
molecule-independent manner (5,6).
This study was conducted to examine the efficacy of mouse CX3CL1
for gene therapies by employing an orthotopic transplantation model
of mouse lung cancer cells, a model reflecting cancer growth in the
lung (7).
Materials and methods
Cell culture and transfection
Mouse lung cancer cells, Lewis lung carcinoma (LLC),
were maintained in EMEM containing 10% fetal bovine serum (FBS), 2
mM L-glutamine, 100 U/ml penicillin and 100 μg/ml
streptomycin. Cultures were kept at 37°C in a humidified atmosphere
of 5% CO2/95% air. LLC cells were transfected by
Nucleofector™ (Amaxa Biosystems, Gaithersburg, MD, USA). Expression
vectors (pIRES2-EGFP vector; Takara Bio, Inc., Shiga, Japan) for
mouse membrane-bound CX3CL1, pIRES2-EGFP-CX3CL1 were used. DNA was
adjusted to 1 μg with empty vectors. Subsequent to
transfection, EGFP-positive LLC cells were sorted using FACSCalibur
(BD Biosciences, Franklin Lakes, NJ, USA) and cultured at 37°C with
the antibiotic G148 (Invitrogen, Carlsbad, CA, USA). CX3CL1-stable
expression cells (LLC-CX3CL1) and control cells (LLC-mock) were
also cultured. CX3CR1-stable expression cells (L-CX3CR1) were
maintained in RPMI-1640 supplemented with 10% FBS and
antibiotics.
Animals
Specific pathogen-free C57BL/6 mice (7-week-old
females) were purchased from Japan SLC, Inc. (Hamamatsu, Japan).
The mice were maintained in the Laboratory for Animal Experiments
of the Institute of Natural Medicine, Toyama Medical and
Pharmaceutical University, Japan, under laminar air flow
conditions. This study was conducted in accordance with the
standards established by the Guidelines for the Care and Use of
Laboratory Animals of the Toyama University.
Reverse transcription-polymerase chain
reaction (RT-PCR)
RT-PCR was performed as previously described
(8). Briefly, total RNA was
extracted using an RNeasy mini kit (Qiagen, Hilden, Germany),
according to the manufacturer's instructions. First-strand cDNA was
prepared from an RNA template (2 μg) using an oligo(dT)18
primer and SuperScript II reverse transcriptase (Invitrogen,
Carlsbad, CA, USA). Reverse transcription was performed at 42°C for
50 min and then at 70°C for 15 min. PCR amplification was performed
using the Takara Ex Taq HS PCR kit (Takara Bio, Inc.) under the
following conditions: 26 cycles of denaturation at 94°C for 30 sec,
annealing at 60°C for 60 sec, and extension at 72°C for 60 sec. The
primers were verified to yield the expected products under the
indicated conditions. PCR products were electro-phoresed on 1.5%
agarose gels and stained with ethidium bromide. The primer
sequences were: GAPDH, sense: 5′-TGAAGGTCGGAGTCAACGGATTTGGT-3′ and
anti-sense: 5′-CATGTGGGCCATGAGGTCCACCAC-3′; mouse CX3CL1, sense:
5′-GAGCATTGGAAGTTTTGAGG-3′ and antisens:
5′-GGTTGAAGGTGAAGTAGTGGA-3′.
Fluorescence-activated cell sorting
(FACS) analysis
LLC-CX3CL1 or LLC-mock cells were fixed with 4%
labeled with paraformaldehyde, and incubated with anti-mouse CX3CL1
(provided by the Kan Research Institute, Kobe, Japan). Subsequent
to incubation, the cells were washed three times with PBS, then
stained with PE anti-Armenian and Syrian hamster immunoglobulin G
(IgG) as the second antibody. FITC-labeled cells were then analyzed
by flow cyto-metric analysis using FACSCalibur (BD
Biosciences).
Migration assay
The migration assay was performed in Transwell cell
culture chambers (Corning Incorporated Life Sciences, Tewksbury,
MA, USA), as reported previously, with some modifications (9). Polyvinylpyrrolidone-free
polycarbonate (PVFP) filters (8.0 μm pore size; Nuclepore,
Pleasanton, CA, USA) were pre-coated with 1 μg fibronectin
(Asahi Technoglass Co., Tokyo, Japan) on the lower compartment.
LLC-CX3CL1 or LLC-mock cell suspension [1×105 cells/100
μl in EMEM with 0.1% bovine serum albumin (BSA)] was added
to the upper compartment and incubated for 4 h at 37°C. Cells that
invaded the lower surface were counted under a microscope in five
pre-determined fields at ×400 magnification.
Adhesion assay
The adhesion assay was performed as described
previously, with some modifications (10). Triplicate wells of 96-well plates
were coated with 1 μg fibronectin. LLC-CX3CL1 or LLC-mock
cells (1×104 cells/100 μl in EMEM with 0.1% BSA)
were incubated for 25 min at 37°C. Cells that adhered to the well
were counted under the microscope in five pre-determined fields at
×400 magnification.
ELISA
Conditioned media of LLC-CX3CL1 or LLC-mock cells
were collected in 1.5 ml tubes, centrifuged at 2,000 rpm for 5 min
to remove cell debris, and kept at −80°C until assay. Assays were
performed in a mouse CX3CL1 ELISA system (R&D Systems Inc.,
Minneapolis, MN, USA).
Chemotaxis assay
Chemotaxis assay was performed as described
previously, with some modifications (11). LLC-CX3CL1 or LLC-mock cells were
cultured in EMEM with 0.1% BSA for 24 h and conditioned media were
collected. L-CX3CR1 cells labeled with DiA (Invitrogen) were
applied to the upper wells of Transwell cell culture chambers of 8
μm pore size (Corning Incorporated Life Sciences) in 100
μl assay buffer (RPMI-1640 with 10 mM HEPES, pH 7.4, 1%
BSA). Aliquots of conditioned media were pre-treated with or
without anti-mouse CX3CL1 or control IgG for 30 min at 37°C and
then applied to the lower wells in a volume of 350 μl. After
4 h at 37°C, cells that migrated into lower wells were lysed with
0.2% Triton X-100 in 20% ethanol and quantitated by fluorescence
intensity measurement (excitation, 595 nm; emission, 485 nm).
Orthotopic implantation
The orthotopic intrapulmonary implantation of LLC
cells was performed as described previously, with some
modifications (7). Briefly,
log-phase cell cultures of LLC-CX3CL1 or LLC-mock cells were
suspended at a cell density of 2.5×105 cells/ml in PBS
containing 500 μg/ml Matrigel. Animals (n=6) were
anesthetized and a small skin incision was made to the left chest
wall (∼5 mm in length). A 29-gauge needle attached to a 0.5-ml
insulin syringe was inserted directly through the intercostal space
into the lung to a depth of 3 mm. Cells (5×105) were
re-suspended in 20 μl of PBS containing 10 μg
Matrigel, and injected into the lung parenchyma. To deplete the NK
cells in vivo, mice (n=6) were intraperitoneally injected
with 200 μl anti-asialo GM1 antibody twice a week.
Statistical analysis
The significance of differences between groups was
determined by applying the two-tailed Student's t-test. P<0.05
were considered to indicate a statistically significant difference.
The means and SDs were calculated for all variables
Results
Stable expression of mouse CX3CL1 in
mouse lung cancer cells
We initially intended to obtain CX3CL1
stable-expression polyclonal LLC cells lines (LLC-CX3CL1) by
combining antibiotic selection and flow cytometric sorting to
obtain an index of the EGFP expression (Fig. 1A). LLC-CX3CL1 was found to express
mRNA of mouse CX3CL1 (Fig. 1B).
Moreover, as CX3CL1 has two phenotypes, flow cytometry and western
blotting confirmed a higher expression of membrane-bound and
soluble CX3CL1 in LLC-CX3CL1 compared to LLC-mock cells (Fig. 1C and D). Gene introduction into
cells often generates various changes in the characteristics
compared to the control cells. No changes were observed regarding
the cell proliferation, adhesion and migration towards the
fibronectin of LLC-CX3CL1, when compared with CX3CL1-mock cells
(data not shown).
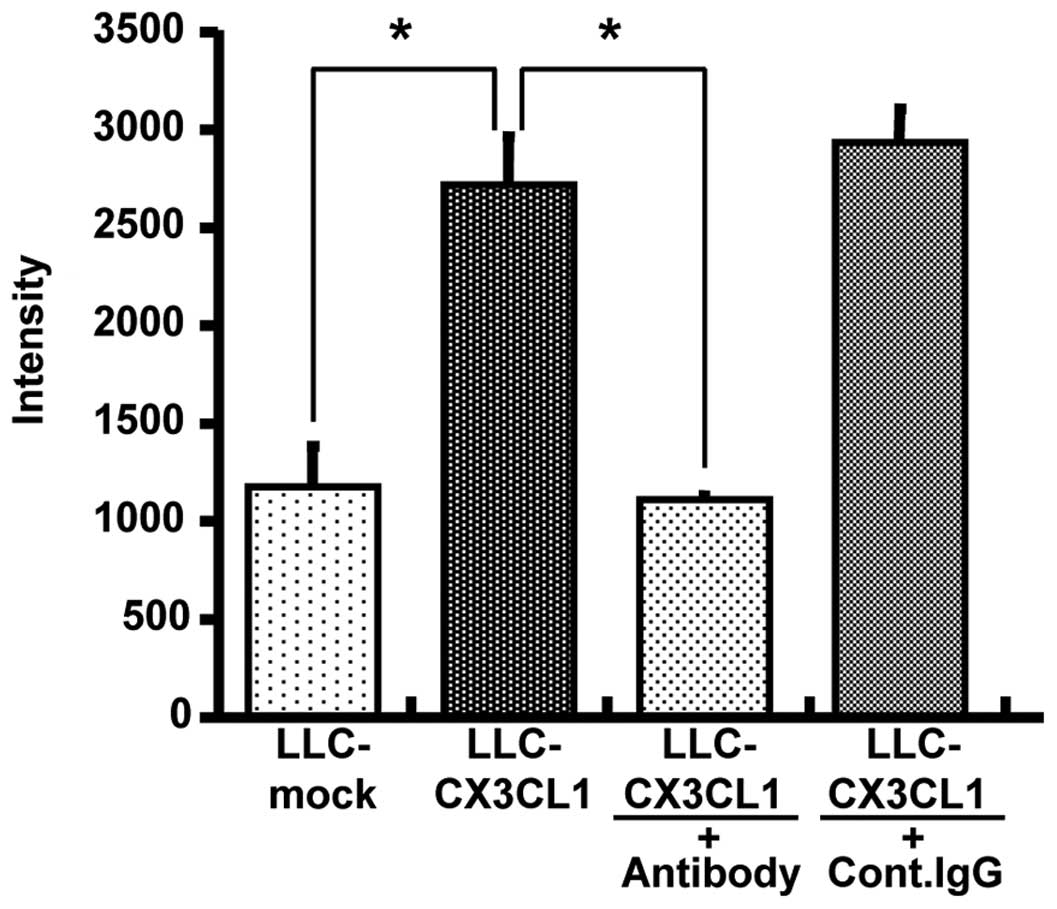
Chemotactic activity of
LLC-CX3CL1-generated CX3CL1
Given that LLC-CX3CL1 cells were stably expressed in
mouse membrane-bound and soluble CX3CL1, and their properties were
not different from those of CX3CL1-mock cells, polyclonal LLC cell
line-generated CX3CL1 was verified to be biologically active. As a
result of migration assay-induced chemotactic activity using
L-CX3CR1 cells in culture supernatants, the supernatant of
LLC-CX3CL1 significantly induced L-CX3CR1 as well as anti-mouse
CX3CL1 cell migration, although the control IgG antibody did not
completely neutralize chemotactic activity (Fig. 2).
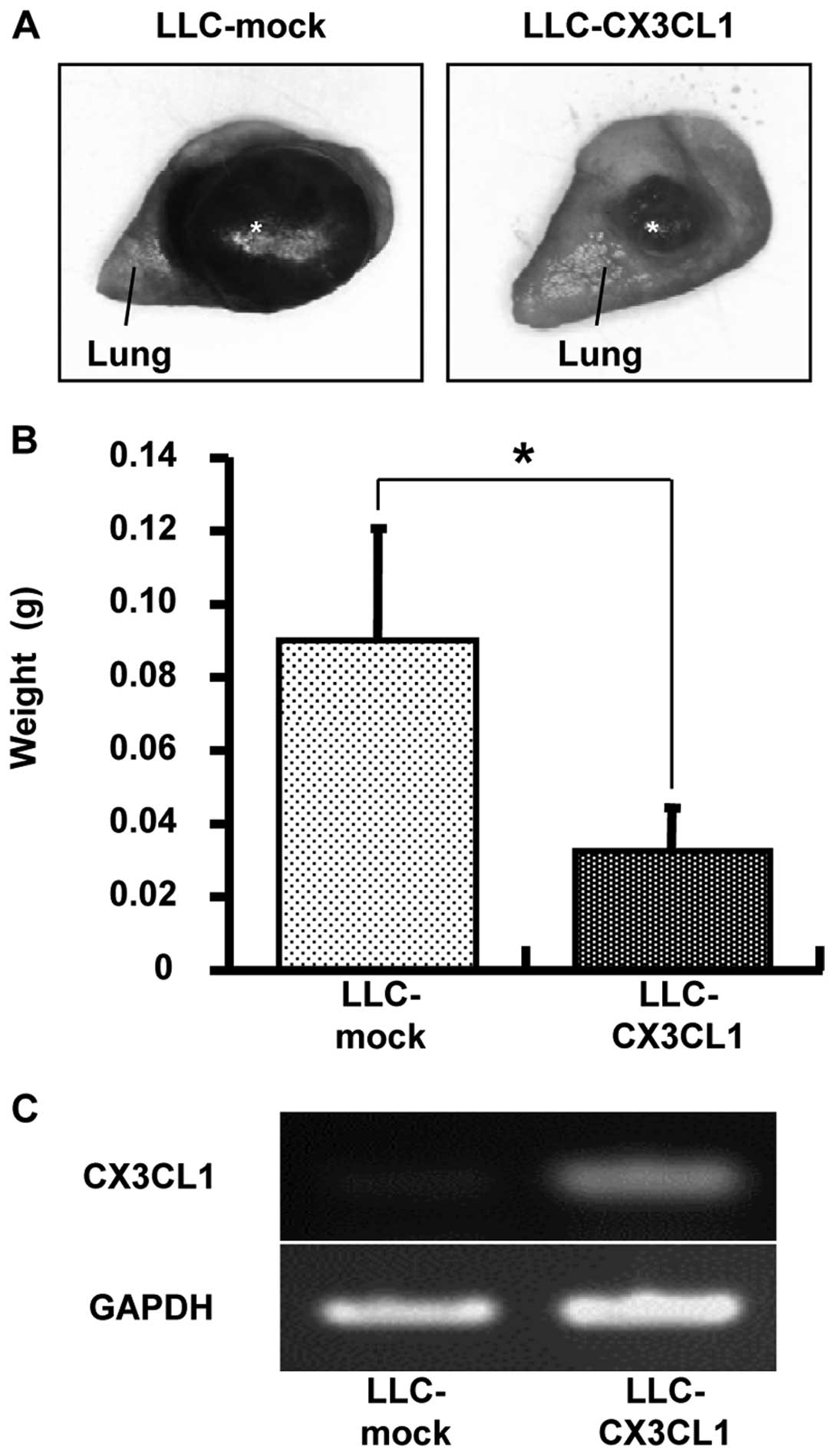
Antitumor effect of CX3CL1 in the
orthotopic transplantation of lung cancer models
The effect of CX3CL1-induced tumor growth inhibition
was examined using orthotopic transplantation models. Seventeen
days after LLC/CX3CL1 and LLC-mock were transplanted into the left
lung of a mouse, the weight of the solid tumor was examined. The
result showed a 65% inhibition of tumor growth compared to LLC-mock
(Fig. 3).
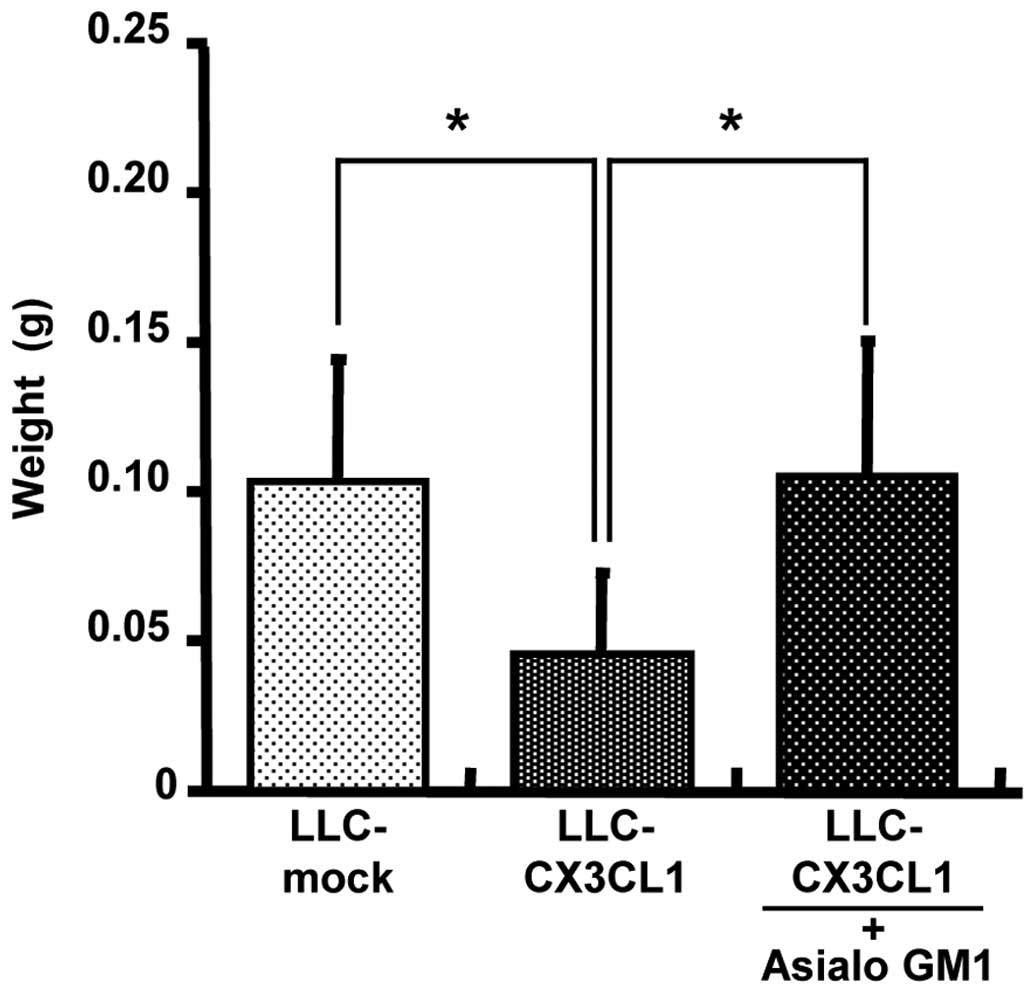
Elucidation of CX3CL1-induced antitumor
effects by immune cells
Anti-asialo GM1 antibody was used for NK cell
depletion in vivo. Initially, FACS analysis was used to
determine whether NK1.1-positive NK cells are eliminated by
administering 200 μg anti-asialo GM1 antibody via the tail
vein to a C57BL/6 mouse twice with in a distance of 2 days (data
not shown). In the non-treated group, LLC/CX3CL1 growth was
significantly inhibited compared to the LLC-mock in the orthotopic
intrapulmonary implantation of LLC cells (Fig. 4). In the depletion groups, the
inhibition of LLC/CX3CL1 growth was suppressed. By contrast, the
growth suppressive effects of CX3CL1 against LLC were affected by
neither CD4 nor CD8 depletion (data not shown). These results
suggest that NK cells contributed to the marked antitumor effect of
CX3CL1.
Discussion
Chemokines are molecules attractive for cancer gene
therapy (12), a method
categorized as a cell-based cancer immuno-therapy due to the
involvement of chemokine-mediated immune cell chemotaxis, as well
as the angiostatic activity of some chemokines (13). CX3CL1 is a type I transmembrane
ligand expressed in activated vascular endothelial and dendritic
cells (14-16). The secreted CX3CL1 is chemotactic
for chemokine receptor, CX3CR1-expressing cells. The membrane form
adheres to chemo-attracted cells, resulting in the interaction of
dendritic cells, monocytes, T cells and natural killer cells (NK
cells) locally (5,6). Initially, the chemotactic activity of
CX3CL1 secreted by LLC-CX3CL1 stably expressing CX3CL1 was
confirmed (Fig. 2). The secreted
form of CX3CL1 is produced by a disintegrin-like metalloproteinase
(ADAM)-mediated shedding of the extra-cellular chemokine domain,
acting as a chemoattractant of mainly NK cells through CX3CR1
(17,18).
In this experiment, pIRES2-EGFP-encoded mouse CX3CL1
was used as the full-length transmembrane type, resulting in an
efficient production of soluble CX3CL1 through cleavage at the
membrane site by the ADAM family. Previous studies have showed that
the biological behavior of cancer cells is influenced by the
implantation organ (1), while
properties of the immune system are also known to be different in
each organ. A useful model for a solitary pulmonary tumor has
previously been established by the intrapulmonary implantation of
LLC cells in syngeneic immunocompetent mice (7,19-21).
This model has the advantage of being simple and easy regarding the
implantation procedure with a small skin incision at a
pre-determined site followed by direct puncturing through the
intercostal space to the lung parenchyma, without thoracotomy or
intubation. Marked lung cancer regression was observed in
LLC-CX3CL1 when compared to tumors comprising control cells
(LLC-mock) (Fig. 3). Given the
lack of cell functions, as well as the cell proliferation, adhesion
and migration of LLC/CX3CL1 and LLC-mock (data not shown), these
results suggest that the growth inhibition effect in vivo
respresents the antitumor effect depending on CX3CL1. CX3CL1
secreted from LLC/CX3CL1 in vitro generates the migration of
CX3CR1-positive lymphocytes (Fig.
2). Therefore, to investigate the immune cell functions
involved in the antitumor effects of CX3CL1, an in vivo
depletion analysis was carried out using specific antibodies
against CX3CR1-positive immune cells (NK, CD8+ T and
CD4+ T cells), in the orthotopic intrapulmonary
implantation of LLC cells. The CX3CL1-dependent antitumor effect
was derived from NK cell activities (Fig. 4).
In clinical studies, leukocyte accumulation in
cancers directed by cancer cell-derived chemokines are crucial in
cancer progression and metastasis. Chemokine expression was
detected in several cancers, while cancer cell-derived chemokines
were responsible for the infiltration of various types of
leukocytes, mainly macrophages, into these cancers (22,23).
CCL5 [also termed regulated on activation, normal T-cell expressed
and secreted, (RANTES)] and CCL2 [also known as monocyte
chemotactic protein-1, (MCP-1)] are chemokines frequently observed
in cancer. In breast cancer, a lower CCL2 expression was correlated
with longer relapse-free survival and decreased tumor-associated
macrophage (TAM) (24), while a
higher level of CCL5 expression was associated with an increase of
TAM and lymph node metastasis (25). In contrast to CCL2 and CCL5, a
high-level expression of chemokine CXCL16 by tumor cells has
recently been reported to correlate with a good prognosis and
increased CD8+ T as well as CD4+ T cells in
CRC (26). In addition, CX3CL1 is
correlated with a better prognosis and an increased number of
CX3CR1-positive CD8+ T and NK cells migrated into
primary cancer in several cancers, such as CRC (27) or gastric adenocarcinoma (28). Although these clinical studies do
not comprise cancer gene therapy, they indicate that the
accumulation of CX3CR1-positive immune cells in primary cancer
results in antitumor activity. Therefore, the finding that a cancer
gene therapy strategy supports the accumulation of NK cells by
CX3CL1, might be an effective therapeutic approach towards lung
cancer, although the correlation of lung cancer-derived CX3CL1,
migrated NK cells and good prognosis need to be further
investigated.
Acknowledgements
The authors would like to thank Drs
Takashi Nakayama and Osamu Yoshie, (Department of Microbiology and
SORST, Kinki University School of Medicine, Osaka, Japan) for their
helpful advice and technical support. This study was supported by a
Grant-in-Aid for Young Scientists (B) (no. 15790089), Grants-in-Aid
for Cancer Research (nos. 16022224 and 16023225), the 21st Century
COE Program from the Ministry of Education, Culture, Sports,
Science and Technology and a grant for Cooperative Link of Unique
Science and Technology for Economy Revitalization (CLUSTER) from
the Ministry of Education, Culture, Sports, Science and Technology
of Japan.
References
|
1.
|
Fidler IJ: Rationale and methods for the
use of nude mice to study the biology and therapy of human cancer
metastasis. Cancer Metastasis Rev. 5:29–49. 1986.
|
|
2.
|
McLemore TL, Egglestone LC, Shoemaker RH,
Abbott BJ, Bohlman ME, Liu MC, Fine DL, Mayo JG and Boyd MR:
Comparison of intrapulmonary, percutaneous, intrathoracic, and
subcutaneous models for the propagation of human pulmonary and
non-pulmonary cancer cell lines in athymic nude mice. Cancer Res.
48:2880–2886. 1988.
|
|
3.
|
Yoshie O, Imai T and Nomiyama H: Novel
lymphocyte-specific CC chemokines and their receptors. J Leukoc
Biol. 62:634–644. 1997.
|
|
4.
|
Yoshie O, Imai T and Nomiyama H:
Chemokines in immunity. Adv Immunol. 78:57–110. 2001.
|
|
5.
|
Imai T, Hieshima K, Haskell C, Baba M,
Nagira M, Nishimura M, Kakizaki M, Takagi S, Nomiyama H, Schall TJ
and Yoshie O: Identification and molecular characterization of
fractalkine receptor CX3CR1, which mediates both leukocyte
migration and adhesion. Cell. 91:521–530. 1997.
|
|
6.
|
Umehara H, Bloom E, Okazaki T, Domae N and
Imai T: Fractalkine and vascular injury. Trends Immunol.
22:602–607. 2001.
|
|
7.
|
Doki Y, Murakami K, Yamaura T, Sugiyama S,
Misaki T and Saiki I: Mediastinal lymph node metastasis model by
orthotopic intrapulmonary implantation of Lewis lung carcinoma
cells in mice. Br J Cancer. 79:1121–1126. 1999.
|
|
8.
|
Nakamura ES, Koizumi K, Kobayashi M and
Saiki I: Inhibition of lymphangiogenesis-related properties of
murine lymphatic endothelial cells and lymph node metastasis of
lung cancer by the matrix metalloproteinase inhibitor MMI270.
Cancer Sci. 95:25–31. 2004.
|
|
9.
|
Koizumi K, Kozawa Y, Ohashi Y, Nakamura
ES, Aozuka Y, Sakurai H, Ichiki K, Doki Y, Misaki T and Saiki I:
CCL21 promotes the migration and adhesion of highly lymph node
meta-static human non-small cell lung cancer Lu-99 in vitro.
Oncol Rep. 17:1511–1516. 2007.
|
|
10.
|
Senda K, Koizumi K, Prangsaengtong O,
Minami T, Suzuki S, Takasaki I, Tabuchi Y, Sakurai H, Doki Y,
Misaki T and Saiki I: Inducible capillary formation in lymphatic
endothelial cells by blocking lipid phosphate phosphatase-3
activity. Lymphat Res Biol. 7:69–74. 2009.
|
|
11.
|
Cho S, Koizumi K, Takeno N, Kato S, Yamada
M, Hashimoto I, Sakurai H, Tsukada K and Saiki I: Antitumor effect
of combining CC chemokine 22 (CCL22) and an anti-CD25 antibody on
myeloma cells implanted subcutaneously into mice. Mol Med Rep.
2:773–777. 2009.
|
|
12.
|
Chada S, Ramesh R and Mhashilkar AM:
Cytokine- and chemokine-based gene therapy for cancer. Curr Opin
Mol Ther. 5:463–474. 2003.
|
|
13.
|
D'Elios MM, Del Prete G and Amedei A: New
frontiers in cell-based immunotherapy of cancer. Expert Opin Ther
Pat. 19:623–641. 2009.
|
|
14.
|
Bazan JF, Bacon KB, Hardiman G, Wang W,
Soo K, Rossi D, Greaves DR, Zrotnik A and Schall TJ: A new class of
membrane-bound chemokine with a CX3C motif. Nature. 385:640–644.
1997.
|
|
15.
|
Imaizumi T, Yoshida H and Satoh K:
Regulation of CX3CL1/fractalkine expression in endothelial cells. J
Atheroscler Thromb. 11:15–21. 2004.
|
|
16.
|
Kanazawa N, Nakamura T, Tashiro K,
Muramatsu M, Morita K, Yoneda K, Inaba K, Imamura S and Honjo T:
Fractalkine and macrophage-derived chemokine: T cell-attracting
chemokines expressed in T cell area dendritic cells. Eur J Immunol.
29:1925–1932. 1999.
|
|
17.
|
Hundhausen C, Misztela D, Berkhout TA,
Broadway N, Saftig P, Reiss K, Hartmann D, Fahrenholz F, Postina R,
Matthews V, et al: The disintegrin-like metalloproteinase ADAM10 is
involved in constitutive cleavage of CX3CL1 (fractalkine) and
regulates CX3CL1-mediated cell-cell adhesion. Blood. 15:1186–1195.
2003.
|
|
18.
|
Garton KJ, Gough PJ, Blobel CP, Murphy G,
Greaves DR, Dempsey PJ and Raines EW: Tumor necrosis
factor-alpha-converting enzyme (ADAM17) mediates the cleavage and
shedding of fractalkine (CX3CL1). J Biol Chem. 12:37993–38001.
2001.
|
|
19.
|
Yamaura T, Doki Y, Murakami K and Saiki I:
Model for mediastinal lymph node metastasis produced by orthotopic
intra-pulmonary implantation of lung cancer cells in mice. Hum
Cell. 12:197–204. 1999.
|
|
20.
|
Yamaura T, Murakami K, Doki Y, Sugiyama S,
Misaki T, Yamada Y and Saiki I: Solitary lung tumors and their
spontaneous metastasis in athymic nude mice orthotopically
implanted with human non-small cell lung cancer. Neoplasia.
2:315–324. 2000.
|
|
21.
|
Ichiki K, Mitani N, Doki Y, Hara H, Misaki
T and Saiki I: Regulation of activator protein-1 activity in the
mediastinal lymph node metastasis of lung cancer. Clin Exp
Metastasis. 18:539–545. 2000.
|
|
22.
|
Vicari AP and Caux C: Chemokines in
cancer. Cytokine Growth Factor Rev. 13:143–154. 2002.
|
|
23.
|
Koizumi K, Hojo S, Akashi T, Yasumoto K
and Saiki I: Chemokine receptors in cancer metastasis and cancer
cell-derived chemokines in host immune response. Cancer Sci.
98:1652–1658. 2007.
|
|
24.
|
Ueno T, Toi M, Saji H, Muta M, Bando H,
Kuroi K, Koike M, Inadera H and Matsushima K: Significance of
macrophage chemoattractant protein-1 in macrophage recruitment,
angiogenesis, and survival in human breast cancer. Clin Cancer Res.
6:3282–3289. 2000.
|
|
25.
|
Luboshits G, Shina S, Kaplan O, Engelberg
S, Nass D, Lifshitz-Mercer B, Chaitchik S, Keydar I and Ben-Baruch
A: Elevated expression of the CC chemokine regulated on activation,
normal T cell expressed and secreted (RANTES) in advanced breast
carcinoma. Cancer Res. 59:4681–4687. 1999.
|
|
26.
|
Hojo S, Koizumi K, Tsuneyama K, Arita Y,
Cui Z, Shinohara K, Minami T, Hashimoto I, Nakayama T, Sakurai H,
et al: High-level expression of chemokine CXCL16 by tumor cells
correlates with a good prognosis and increased tumor-infiltrating
lymphocytes in colorectal cancer. Cancer Res. 15:4725–4731.
2007.
|
|
27.
|
Ohta M, Tanaka F, Yamaguchi H, Sadanaga N,
Inoue H and Mori M: The high expression of Fractalkine results in a
better prognosis for colorectal cancer patients. Int J Oncol.
26:41–47. 2005.
|
|
28.
|
Hyakudomi M, Matsubara T, Hyakudomi R,
Yamamoto T, Kinugasa S, Yamanoi A, Maruyama R and Tanaka T:
Increased expression of fractalkine is correlated with a better
prognosis and an increased number of both CD8+ T cells
and natural killer cells in gastric adenocarcinoma. Ann Surg Oncol.
15:1775–1782. 2008.
|