Introduction
Hepatocellular carcinoma (HCC) is generally
considered to be chemoresistant, and the results of systemic
chemotherapy have been unsatisfactory (1). Sorafenib (Nexavar, Bayer Healthcare
Pharmaceuticals, Pittsburgh, PA, USA) is a multi-kinase inhibitor
that blocks tumor growth and cell proliferation, and was the first
systemic chemotherapeutic agent found to improve the survival time
of patients with advanced HCC, in the SHARP as well as Asia-Pacific
trials (2–5). However, findings of these trials
showed that several patients with advanced HCC remained refractory
to sorafenib, and the factors determining the patients benefiting
from sorafenib therapy remain unclear (2–5).
Although several studies have investigated the
prognostic factors in sorafenib treatment, no consensus factors
have yet been identified (6–12).
Pretreatment liver function parameters, such as Child-Pugh
classification, serum bilirubin, serum albumin (ALB) and serum
aminotransferase have been reported as prognostic factors in
sorafenib treatment (6–12).
Serum cholinesterase (ChE) in combination with ALB
is one of the main indices of the protein-synthetic ability of the
liver (13–19). Unlike ALB level, which is
influenced by various factors such as bleeding, inflammation,
chronic renal diseases or branched chain amino acid administration,
the serum ChE level simply reflects the background liver function
(13–19). Serum ChE level has been reported to
be an important prognostic factor in malignancies other than HCC,
including gastric and pancreatic cancers treated with systemic
chemotherapy (13–17). In addition, serum ChE level has
been reported as a prognostic factor in cholangiocellular carcinoma
patients treated with radiotherapy (18), patients with recurrent HCC after
hepatectomy (20), and in liver
transplantation recipients with chronic end-stage liver disease
(21). Serum ChE thus seems to be
significantly involved in the treatment of several malignancies
(22,23). However, to the best of our
knowledge, no studies have yet investigated the value of serum ChE
level as a predictive marker in sorafenib therapy for advanced HCC.
In the present study, we therefore focused on serum ChE level as an
index of liver function and investigated its significance in
advanced HCC patients treated with sorafenib.
Patients and methods
Patients
A total of 102 patients with unresectable HCC were
treated with sorafenib at the Department of Gastroenterology and
Hepatology (Osaka Red Cross Hospital, Osaka, Japan) between June,
2009 and February 2012. The indications for sorafenib therapy were:
unresectable advanced HCC determined by dynamic computed tomography
(CT) scan; Eastern Cooperative Oncology Group (ECOG) performance
status of 0–2; pretreatment Child-Pugh classification of A or B;
presence of extrahepatic metastases; refractory to previous HCC
therapies, such as transcatheter arterial chemoembolization (TACE);
unsuitability for TACE for anatomical reasons and absence of
uncontrollable ascites. Pretreatment serum ChE levels were not
measured in nine (8.8%) of the 102 patients, and the present study
population therefore consisted of 93 patients with measured
pretreatment serum ChE levels.
Study protocol
The median pretreatment serum ChE level was 138 IU/l
(range, 31–276 IU/l). Patients were therefore categorized into two
groups: group A with a pretreatment serum ChE level ≥140 IU/l
(n=46) and group B with a pretreatment serum ChE level <140 IU/l
(n=47). We retrospectively analyzed the correlation between overall
survival (OS), and pretreatment serum ChE level as well as other
pretreatment clinicopathological variables including age, gender,
cause of liver disease, Child-Pugh classification, pretreatment
tumor characteristics such as tumor node metastasis (TNM) stage,
Barcelona Clinic Liver Cancer (BCLC) stage, portal vein tumor
invasion and metastatic sites, tumor markers, laboratory data such
as total bilirubin (TBIL), ALB, aspartate aminotransferase (AST),
alanine aminotransferase (ALT), prothrombin time and serum
creatinine, as well as the presence of ascites. We also examined
the correlation between the development of severe liver damage
during sorafenib therapy, and pretreatment ChE level and the
above-mentioned clinicopathological variables. Severe liver damage
was defined as: liver dysfunction occurring within 3 months from
the administration of sorafenib, including elevated AST, ALT and
TBIL, and hepatic encephalopathy or liver failure of grade 3 or
higher based on the Common Terminology Criteria for Adverse Events
(CTCAE) version 4.0. In addition, we performed subgroup analyses on
patients with good liver function defined as Child-Pugh class A.
Written informed consent was obtained from the patients prior to
sorafenib therapy. This retrospective study protocol was in
compliance with the provisions of the Declaration of Helsinki.
Initial sorafenib dose and treatment
discontinuation
The initial sorafenib dose was determined according
to factors, such as patient body weight, body surface area, age,
comorbid diseases, performance status and liver function. The
initial sorafenib dose in this study ranged from 400 to 800 mg/day.
This took into account the fact that studies in several countries,
including Japan, have reported serious adverse events in several
advanced HCC patients administered an initial sorafenib dose of 800
mg/day, leading to treatment discontinuation. The initial sorafenib
dose was therefore determined taking this fact into consideration.
Sorafenib treatment was continued until disease progression,
unacceptable drug-related toxicity or the patient’s decision to
discontinue.
Statistical analysis
OS curves were generated using the Kaplan-Meier
method and compared using log-rank tests. OS was calculated from
the initial date of sorafenib treatment until death by any cause or
until the last follow-up. Serum ChE level and other
clinicopathological variables were analyzed using univariate and
multivariate analyses. Regarding OS, the Cox proportional hazard
model was used for multivariate analysis of factors considered
significant in univariate analysis. Associations between
pretreatment serum ChE level and additional clinicopathological
variables, and the development of liver damage during sorafenib
treatment were also examined using Fisher’s exact tests. Regarding
the development of liver damage, the variables found to be
significant in the univariate analysis were subjected to the
multivariate analysis using logistic regression analysis. Data were
presented as the median value (range). P<0.05 was considered to
indicate a statistically significant difference. Statistical
analyses were carried out using the SPSS software (SPSS for Windows
15.0, SPSS, Inc., Chicago, IL, USA).
Results
Patient characteristics
The baseline characteristics of the two groups are
shown in Table I. Group A
comprised 37 males and 9 females, with a median age of 70 years
(range, 46–86). Group B comprised 35 males and 12 females, with a
median age of 71 years (range, 47–89). With regard to tumor
characteristics, 22 patients in group A and 19 patients in group B
had extrahepatic metastases, while 29 and 27 patients were
classified with BCLC stage C disease in groups A and B,
respectively. Most patients had received previous therapies for
HCC: one or more sessions of TACE had been performed in 75
patients, radiofrequency ablation or percutaneous ethanol injection
therapy in 27, hepatectomy in 15 and palliative radiation therapy
in 16 patients. With regard to pretreatment liver function, 73
patients (78.5%) had Child-Pugh A and 20 (21.5%) had Child-Pugh B
function. The incidence of patients with Child-Pugh A status was
significantly higher in group A compared to group B (P=0.003).
 | Table IBaseline characteristics of patients
with advanced hepatocellular carcinoma. |
Table I
Baseline characteristics of patients
with advanced hepatocellular carcinoma.
| Group A (n=46) | Group B (n=47) | |
|---|
| Variable | N or median value
(range) | N or median value
(range) | P-value |
|---|
| Age (years) | 70 (46–86) | 71 (47–89) | 0.779a |
| Gender
(male/female) | 37/9 | 35/12 | 0.794b |
| Body surface area
(m2) | 1.59 (1.09–2.18) | 1.58 (1.38–1.89) | 0.852a |
| Etiology of liver
disease | | | |
| Hepatitis
B/hepatitis C/non-B non-C | 10/24/12 | 7/32/8 | 0.341b |
| TNM stage | | | |
| Stage
II/III/IVA/IVB | 3/14/7/22 | 1/18/9/19 | 0.822b |
| Site of metastases
(yes/no) | | | |
| Lung | 13/33 | 7/40 | 0.136b |
| Bone | 8/38 | 8/39 | 1.000b |
| Adrenal | 1/45 | 2/45 | 1.000b |
| Lymph node | 11/35 | 9/38 | 0.621b |
| Portal vein tumor
invasion (yes/no) | 8/38 | 10/37 | 0.794b |
| ECOG PS, 0/1/2 | 40/5/1 | 40/4/3 | 0.802b |
| Child-Pugh
classification, A/B | 41/5 | 29/18 | 0.003b |
| BCLC stage,
B/C | 17/29 | 20/27 | 0.673b |
| Pretreatment serum
AFP (ng/ml) | 476
(2.2–270,300) | 98
(2.9–688,400) | 0.251a |
| Pretreatment serum
DCP (mAU/ml) | 1935
(10–98,510) | 937
(11–421,210) | 0.462a |
| Previous therapies
for HCC (yes/no) | | | |
| TACE | 40/6 | 35/12 | 0.322b |
| RFA or PEIT | 15/31 | 12/35 | 0.649b |
| Surgery | 9/37 | 6/41 | 0.106b |
| Radiation | 5/41 | 11/36 | 0.089b |
| Initial sorafenib
dose (800/400 mg per day) | 13/33 | 13/34 | 1.000b |
| Treatment
response | | | |
|
CR/PR/SD/PD/NE | 0/9/9/14/14 | 1/2/11/10/23 | 0.410b |
Sorafenib was initiated at 800 mg/day in 26 patients
(28.0%) and at 400 mg/day in 67 patients (72.0%). With regard to
treatment response, complete response was obtained in 1 patient,
partial response (PR) in 10 patients, stable disease in 26 and
progressive disease in 25 patients, based on the modified Response
Evaluation Criteria in Solid Tumor (mRECIST) (24).
Predictive factors for OS and causes of
mortality
The median observation period for the analyzed cases
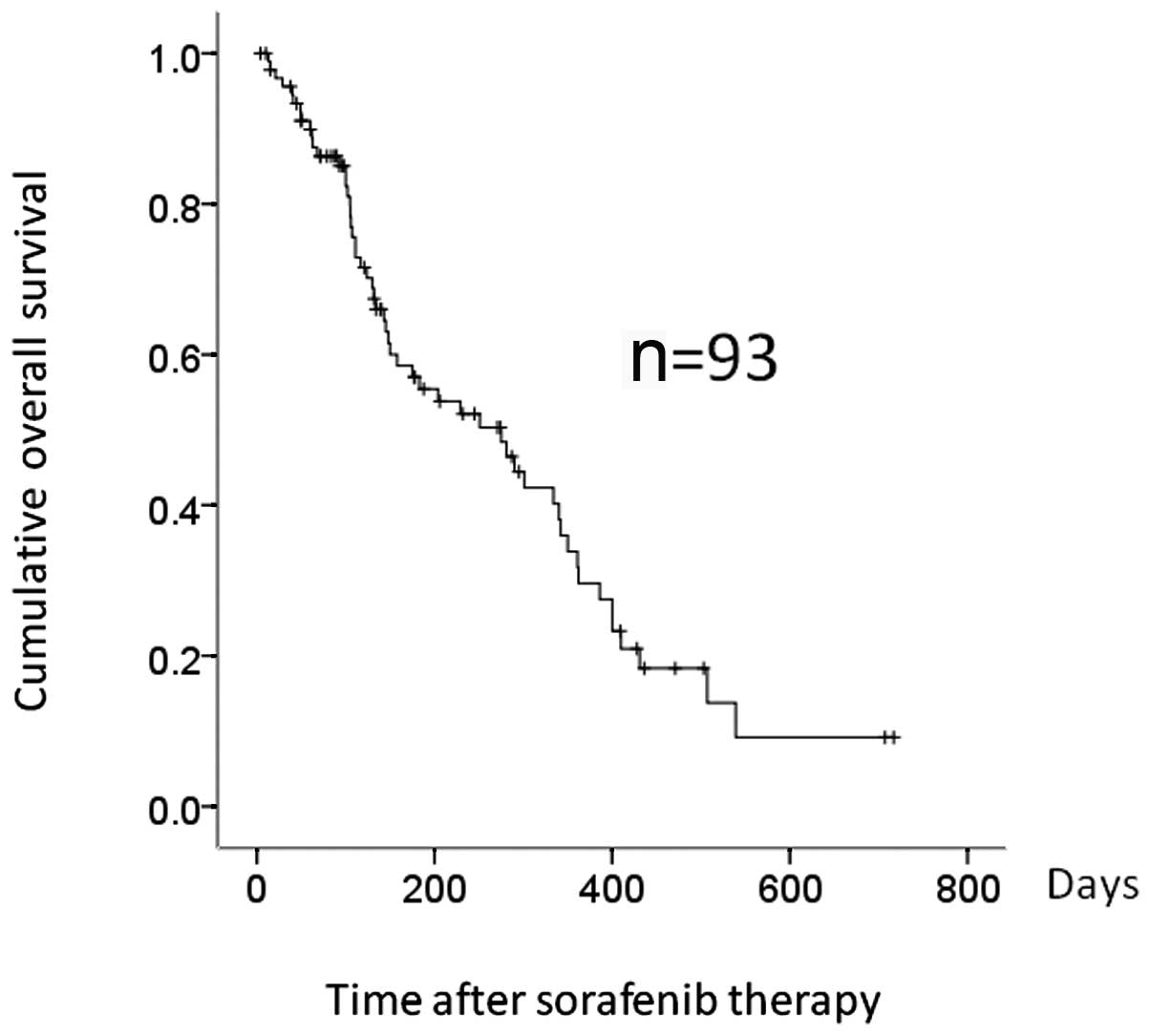
was 136 days (range, 3–716), while the median OS was 275 days
(Fig. 1). Fifty-three patients
(57.0%) succumbed to the disease during the observation period. The
causes of mortlity were HCC progression in 44 patients, liver
failure in 3 and miscellaneous causes in 6 patients. The median OS
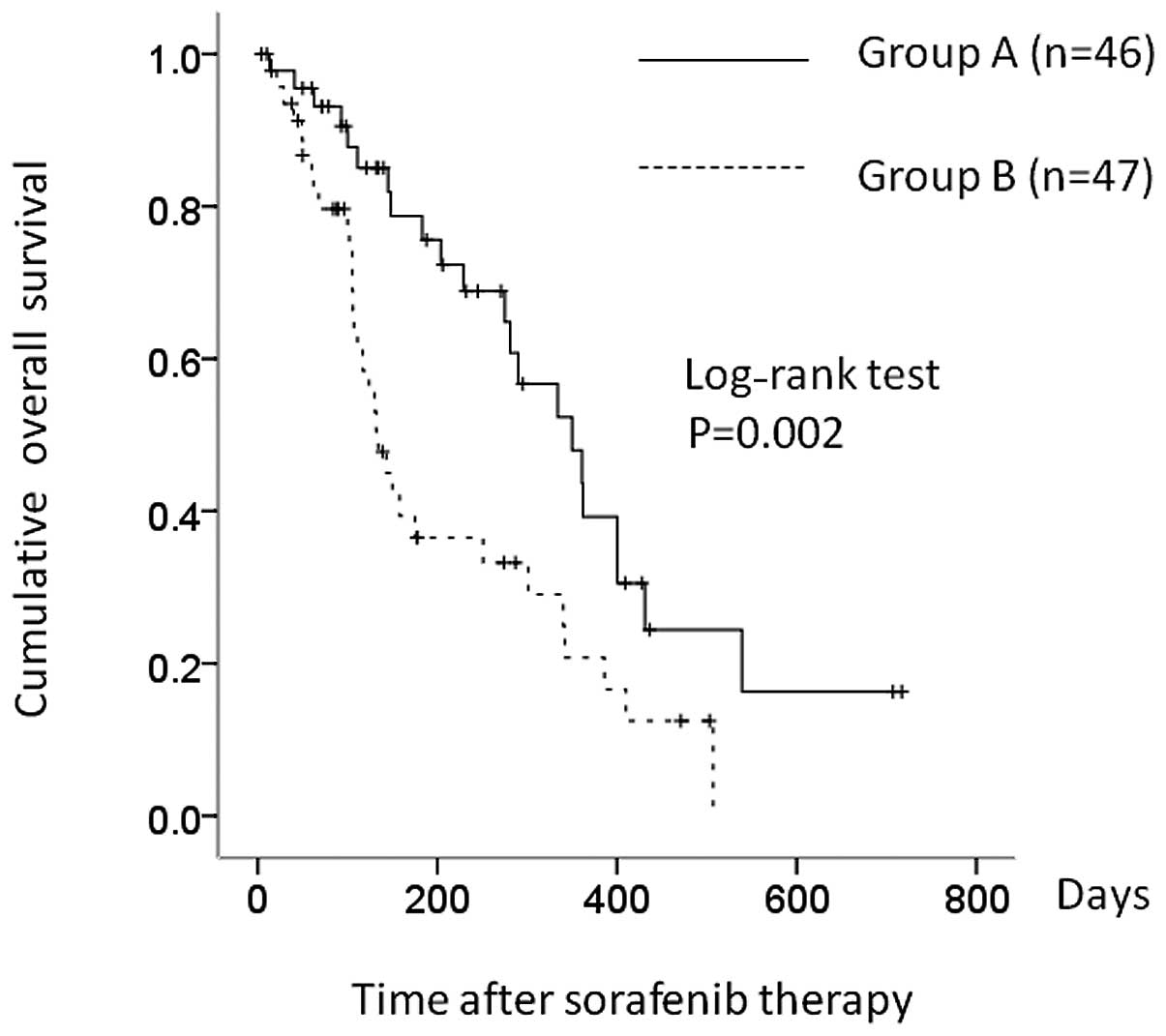
was 319 and 106 days for group A and B patients, respectively
(P=0.002) (Fig. 2).
Univariate analysis revealed the presence of bone
metastases (P= 0.005), ALB level ≥3.5 g/dl (P= 0.042), serum ChE
level ≥140 IU/l (P= 0.002) and the presence of ascites (P= 0.001)
to be significant independent factors linked to OS (Table II). However, multivariate analyses
of the four factors found to be significant by univariate analysis
revealed only bone metastases (P=0.018) and the presence of ascites
(P=0.011) to be significant independent factors linked to OS
(Table II). Serum ChE level tended
to correlate with OS, though the correlation was not significant in
multivariate analysis (P=0.068) (Table
II).
 | Table IIUnivariate and multivariate analyses
of factors contributing to overall survival in the cases
(n=93). |
Table II
Univariate and multivariate analyses
of factors contributing to overall survival in the cases
(n=93).
| | Univariate analysis
| Multivariate
analysis
|
|---|
| Variable | N | P-valuea | HR | 95% CI | P-valueb |
|---|
| Age ≥70 years
(yes/no) | 43/50 | 0.960 | | | |
| Gender
(male/female) | 76/17 | 0.308 | | | |
| HBsAg-positive
(yes/no) | 17/76 | 0.446 | | | |
| Child-Pugh
classification, A/B | 70/23 | 0.099 | | | |
| TNM stage, stage
III/stage IVA or IVB | 37/56 | 0.149 | | | |
| BCLC stage,
B/C | 37/56 | 0.375 | | | |
| Portal vein tumor
invasion (yes/no) | 18/75 | 0.377 | | | |
| Bone metastases
(presence/absence) | 16/77 | 0.005 | 2.45 | 1.168–5.172 | 0.018 |
| Serum AFP ≥320
ng/ml (yes/no) | 47/46 | 0.126 | | | |
| Serum DCP ≥1000
mAU/ml (yes/no) | 49/44 | 0.344 | | | |
| Total bilirubin ≥1
IU/l (yes/no) | 34/59 | 0.653 | | | |
| Serum albumin ≥3.5
g/dl (yes/no) | 37/56 | 0.042 | 1.15 | 0.560–2.353 | 0.705 |
| AST >50 IU/l
(yes/no) | 51/42 | 0.424 | | | |
| ALT >50 IU/l
(yes/no) | 30/63 | 0.671 | | | |
| Serum
cholinesterase ≥140 IU/l (yes/no) | 46/47 | 0.002 | 0.52 | 0.259–1.049 | 0.068 |
| Prothrombin time
≥70% (yes/no) | 82/11 | 0.192 | | | |
| Serum creatinine ≥1
IU/l (yes/no) | 25/68 | 0.643 | | | |
| Ascites
(presence/absence) | 20/73 | 0.001 | 2.07 | 1.180–3.628 | 0.011 |
Subgroup analyses in patients with
Child-Pugh A
A statistically significant difference was detected
between the two groups in terms of baseline Child-Pugh
classification. We therefore performed subgroup analyses based on
Child-Pugh status. We examined 70 patients with Child-Pugh class A
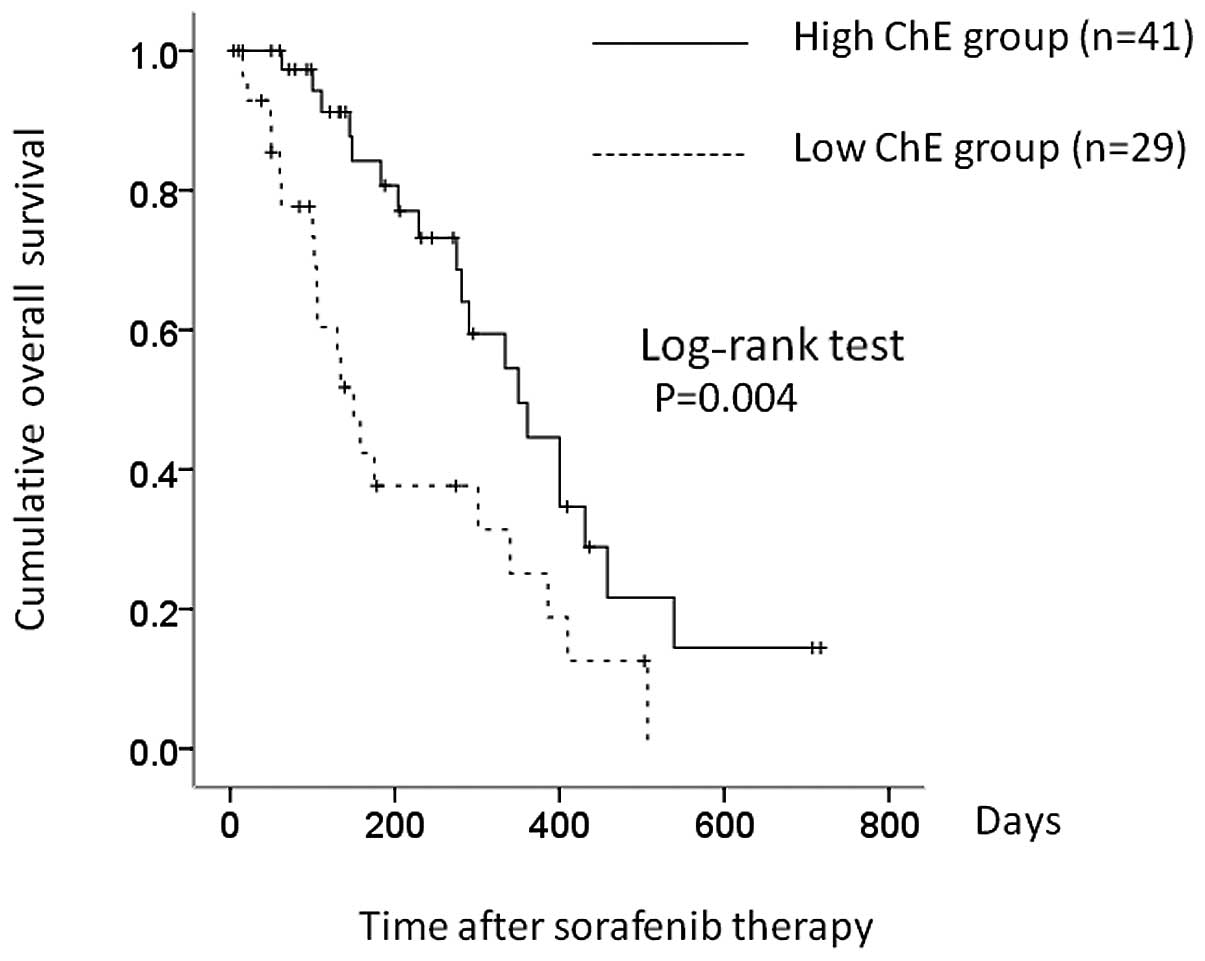
liver function, of whom 41 (58.6%) had higher serum ChE (≥140 IU/l)
and 29 (41.4%) had lower ChE (<140 IU/l). Median OS was 350 and
150 days in the higher and lower ChE groups, respectively (P=
0.004) (Fig. 3). Presence of bone
metastases (P= 0.010) and serum ChE level (P= 0.004) were markedly
associated with OS in univariate analyses (Table III), and were significant
independent factors linked to OS in Child-Pugh A patients,
according to multivariate analyses (Table III).
 | Table IIIUnivariate and multivariate analyses
of factors contributing to overall survival in patients with
Child-Pugh A (n=70). |
Table III
Univariate and multivariate analyses
of factors contributing to overall survival in patients with
Child-Pugh A (n=70).
| | Univariate analysis
| Multivariate
analysis
|
|---|
| Variable | n | P-valuea | HR | 95% CI | P-valueb |
|---|
| Age ≥70 years
(yes/no) | 35/35 | 0.833 | | | |
| Gender
(male/female) | 57/13 | 0.149 | | | |
| HBsAg positive
(yes/no) | 14/56 | 0.786 | | | |
| TNM stage, stage
III/stage IVA or IVB | 28/42 | 0.132 | | | |
| BCLC stage,
B/C | 28/42 | 0.163 | | | |
| Portal vein tumor
invasion (yes/no) | 12/58 | 0.175 | | | |
| Bone metastases
(presence/absence) | 11/59 | 0.010 | 3.367 | 0.121–0.730 | 0.008 |
| Serum AFP ≥320
ng/ml (yes/no) | 34/36 | 0.283 | | | |
| Serum DCP ≥1000
mAU/ml (yes/no) | 35/35 | 0.526 | | | |
| Total bilirubin ≥1
IU/l (yes/no) | 22/48 | 0.843 | | | |
| Serum albumin ≥3.5
g/dl (yes/no) | 34/36 | 0.087 | | | |
| AST ≥50 IU/l
(yes/no) | 36/34 | 0.324 | | | |
| ALT ≥50 IU/l
(yes/no) | 22/48 | 0.786 | | | |
| Serum
cholinesterase ≥140 IU/l (yes/no) | 41/29 | 0.004 | 2.612 | 1.174–5.810 | 0.019 |
| Prothrombin time
≥70% (yes/no) | 42/28 | 0.831 | | | |
| Serum creatinine ≥1
IU/l (yes/no) | 16/54 | 0.702 | | | |
Predictive factors for severe liver
damage
Twenty-two patients (23.7%) developed grade 3 or
higher liver dysfunction during sorafenib treatment, based on the
CTCAE version 4.0, resulting in the interruption or discontinuation
of sorafenib therapy. Most instances of severe liver dysfunction
occurred within 1 month from the initiation of sorafenib treatment.
Only two group A patients (4.3%) developed liver dysfunction,
compared to 20 group B patients (42.6%).
Results of the univariate analysis showed that TBIL
≥1 IU/l (P=0.041), ALB <3.5 g/dl (P=0.015), AST >50 IU/l
(P=0.045), ALT ≥50 IU/l (P=0.040), serum ChE level <140 IU/l
(P<0.001) and the presence of ascites (P=0.003) were
significantly associated with the development of liver dysfunction
(Table IV). However, results of
the multivariate analysis of these six factors found that only the
presence of ascites (P=0.030) and serum ChE <140 IU/l (P=0.002)
were significant independent factors associated with the
development of liver dysfunction during sorafenib therapy (Table IV).
 | Table IVUnivariate and multivariate analyses
of factors contributing to the development of liver damage in the
cases (n=93). |
Table IV
Univariate and multivariate analyses
of factors contributing to the development of liver damage in the
cases (n=93).
| | Univariate analysis
| Multivariate
analysis
|
|---|
| Variable | N | P-valuea | HR | 95% CI | P-valueb |
|---|
| Age ≥70 years
(yes/no) | 43/50 | 0.207 | | | |
| Gender
(male/female) | 76/17 | 0.631 | | | |
| HBsAg positive
(yes/no) | 17/76 | 0.174 | | | |
| TNM stage, stage
III/stage IVA or IVB | 37/56 | 0.191 | | | |
| BCLC stage,
B/C | 37/56 | 0.086 | | | |
| Portal vein tumor
invasion (yes/no) | 18/75 | 0.331 | | | |
| Bone metastases
(presence/absence) | 16/77 | 0.558 | | | |
| Serum AFP ≥320
ng/ml (yes/no) | 47/46 | 0.122 | | | |
| Serum DCP ≥1000
mAU/ml (yes/no) | 49/44 | 1.000 | | | |
| Total bilirubin ≥1
IU/l (yes/no) | 34/59 | 0.041 | 1.881 | 0.564–6.273 | 0.304 |
| Serum albumin ≥3.5
g/dl (yes/no) | 37/56 | 0.015 | 0.729 | 0.138–3.840 | 0.709 |
| AST ≥50 IU/l
(yes/no) | 51/42 | 0.045 | 0.824 | 0.179–3.798 | 0.804 |
| ALT ≥50 IU/l
(yes/no) | 30/63 | 0.04 | 4.269 | 0.933–19.545 | 0.061 |
| Serum
cholinesterase ≥140 IU/l | 46/47 | <0.001 | 0.061 | 0.010–0.373 | 0.002 |
| Prothrombin time
≥70% (yes/no) | 82/11 | 0.509 | | | |
| Serum creatinine ≥1
IU/l (yes/no) | 25/68 | 0.366 | | | |
| Ascites
(presence/absence) | 20/73 | 0.003 | 4.154 | 1.146–15.055 | 0.030 |
Subgroup analyses of severe liver damage
in patients with Child-Pugh A
The occurrence of severe liver damage in 70 patients
with good liver function (Child-Pugh A) was also examined.
Forty-one patients (58.6%) had a serum ChE level ≥140 IU/l, while
the other 29 patients (41.4%) had a serum ChE level <140 IU/l.
Only two patients (4.9%) with higher ChE developed severe liver
dysfunction, compared to 16 patients (55.2%) with lower serum
ChE.
Univariate analysis results showed TBIL ≥1 IU/l (P=
0.029), AST ≥50 IU/l (P=0.007), ALT ≥50 IU/l (P=0.029) and serum
ChE (P<0.001) to be significantly associated with the
development of severe liver dysfunction (Table V). Multivariate analysis of these
four factors showed that pre-treatment serum ChE level was the only
independent significant factor associated with the development of
severe liver dysfunction (P=0.016; HR=0.122; 95% CI, 0.022–0.676)
(Table V).
 | Table VUnivariate and multivariate analyses
of factors contributing to liver damage in patients with Child-Pugh
A (n=70). |
Table V
Univariate and multivariate analyses
of factors contributing to liver damage in patients with Child-Pugh
A (n=70).
| | Univariate analysis
| Multivariate
analysis
|
|---|
| Variable | N | P-valuea | HR | 95% CI | P-valueb |
|---|
| Age ≥70 years
(yes/no) | 35/35 | 0.500 | | | |
| Gender
(male/female) | 57/13 | 0.699 | | | |
| HBsAg positive
(yes/no) | 14/56 | 0.143 | | | |
| TNM stage, stage
III/stage IVA or IVB | 28/42 | 0.533 | | | |
| BCLC stage,
B/C | 28/42 | 0.533 | | | |
| Portal vein tumor
invasion (yes/no) | 12/58 | 0.605 | | | |
| Bone metastases
(presence/absence) | 11/59 | 0.533 | | | |
| Serum AFP ≥320
ng/ml (yes/no) | 34/36 | 0.077 | | | |
| Serum DCP ≥1000
mAU/ml (yes/no) | 35/35 | 0.752 | | | |
| Total bilirubin ≥1
IU/l (yes/no) | 22/48 | 0.029 | 1.496 | 0.293–7.635 | 0.628 |
| Serum albumin ≥3.5
g/dl (yes/no) | 34/36 | 0.190 | | | |
| AST ≥50 IU/l
(yes/no) | 36/34 | 0.007 | 2.330 | 0.268–20.247 | 0.443 |
| ALT ≥50 IU/l
(yes/no) | 22/48 | 0.029 | 2.104 | 0.374–11.851 | 0.399 |
| Serum
cholinesterase ≥140 IU/l | 41/29 | <0.001 | 0.122 | 0.022–0.676 | 0.016 |
| Prothrombin time
≥70% (yes/no) | 42/28 | 0.708 | | | |
| Serum creatinine ≥1
IU/l (yes/no) | 16/54 | 0.516 | | | |
Discussion
The positive results of sorafenib therapy identified
by the SHARP and Asia-Pacific trials have opened dimensions in the
treatment of advanced HCC (2,3).
However, in terms of tumor response rate, the efficacy of sorafenib
is limited compared to TACE (1).
The SHARP trial showed only 2% of PR in RECIST (25,26),
while the Asia-Pacific trial showed only 3% PR. By contrast,
Edeline et al (27)
reported that 12% of HCC patients treated with sorafenib achieved
PR using modified RECIST. Abbadessa et al (28) reported that HCC patients benefited
from the long-lasting effects of sorafenib. In addition, we have
previously treated a patient with advanced HCC with lung metastasis
who achieved CR with sorafenib (29). These results suggest that a number
of patients may achieve an objective response with sorafenib
therapy (27–30). However, characteristics of the
patients benefiting from, as well as features associated with
sorafenib-resistance have yet to be elucidated.
Llovet et al (7) examined plasma biomarkers as
predictors of outcome in HCC patients participating in the SHARP
trial and concluded that none of the tested biomarkers predicted a
response to sorafenib. Numerous studies have therefore investigated
biomarkers likely to predict prognosis in HCC patients treated with
sorafenib, although no consensus regarding prognostic factors has
been reached. Tsukui et al (11) reported that the Child-Pugh
classification was a significant prognostic factor, while Morimoto
et al (12) reported that
the Glasgow prognostic score, Japan integrated staging score and
performance status were independently associated with survival.
Regarding the clinical course, skin toxicity and reduction of serum
α-fetoprotein during sorafenib therapy were reported to be
associated with OS and time to progression (31,32).
However, to the best of our knowledge, no reports have investigated
the prognostic value of the serum ChE level.
The presence of ascites and bone metastases, and
serum albumin <3.5 g/dl and serum ChE level <140 IU/l were
found to be significant indicators of poor OS in the univariate
analysis. TNM and BCLC stage did not contribute to OS. These
findings are partially in agreement with those of Tsukui et
al (11), who reported that
background liver disease-derived factors, rather than tumor-derived
factors, were correlated with prognosis in advanced HCC patients
treated with sorafenib. By contrast, results of the multivariate
analysis showed that the presence of bone metastases was a
significant adverse prognostic factor in the analyzed patients as
well as in Child-Pugh A patients. Advanced HCC patients with bone
metastasis frequently present with severe pain and other symptoms
associated with poor quality of life (33), thus these factors are likely to be
associated with poor prognosis.
In general, patients with pretreatment liver
function damage, such as Child-Pugh B, elevated AST or ALT,
hypoalbuminemia and the presence of ascites are considered to be
intolerant to sorafenib therapy (1–5).
Results of the multivariate analysis demonstrated serum ChE level
to be the strongest predictive factor for severe liver dysfunction.
Under conditions of poor hepatic functional reserve, reflected by
lower serum ChE levels, hepatocytes may easily be damaged by
systemic chemotherapy, leading to severe liver damage. Our findings
suggest that the serum ChE level, as well as the above-mentioned
factors, are important predictors correlated with the development
of severe liver dysfunction. Consequently, clinicians should take
serum ChE levels into consideration prior to initiation of
sorafenib therapy.
The present study found that pretreatment serum ChE
<140 IU/l was a significant poor prognostic factor in the
multivariate analysis in the subgroup of patients with good liver
function of Child-Pugh class A. Moreover, patients with
pretreatment serum ChE <140 IU/l were more likely to develop
severe liver dysfunction during sorafenib treatment compared to
those with pretreatment serum ChE >140 IU/l. These findings
suggest that even patients with good pretreatment liver function of
Child-Pugh class A should be treated with sorafenib with caution if
they have low serum ChE levels, and a favorable clinical outcome in
these patients is less likely as a result of the high frequency of
discontinuation of sorafenib therapy.
There were several limitations to the present study.
First, this was a retrospective, single-center study. Second, the
number of patients analyzed was relatively small. Third, the
initial sorafenib dose varied in individual patients, which could
have led to bias. Fourth, nine patients whose pretreatment ChE
levels were not tested were excluded from the study, also
potentially leading to bias. A larger prospective study is
therefore needed to clarify the prognostic factors in advanced HCC
patients treated with sorafenib. Nevertheless, the findings of this
study have demonstrated that lower pretreatment serum ChE levels
were a significant risk factor for poor prognosis and liver
dysfunction, even in patients with pretreatment Child-Pugh A liver
function, suggesting that patients with lower serum ChE levels
should be treated with caution.
In conclusion, serum ChE level may be a reliable
prognostic marker for the treatment of patients with advanced HCC
in the sorafenib era. Clinicians should therefore be aware of serum
ChE levels, as well as various other clinical findings, prior to
initiation of sorafenib therapy.
Acknowledgements
The authors would like to thank Haruko
Takada for the data collection.
References
|
1
|
Nishikawa H, Osaki Y, Kita R and Kimura T:
Hepatic arterial infusion chemotherapy for advanced hepatocellular
carcinoma in Japan. Cancers. 4:165–183. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR,
Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici
M, Voliotis D and Bruix J; SHARP Investigators Study Group:
Sorafenib in advanced hepatocellular carcinoma. N Engl J Med.
359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abou-Alfa GK, Schwartz L, Ricci S, Amadori
D, Santoro A, Figer A, De Greve J, Douillard JY, Lathia C, Schwartz
B, Taylor I, Moscovici M and Saltz LB: Phase II study of sorafenib
in patients with advanced hepatocellular carcinoma. J Clin Oncol.
24:4293–4300. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J,
Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D and Guan Z:
Efficacy and safety of sorafenib in patients in the Asia-Pacific
region with advanced hepatocellular carcinoma: a phase III
randomised, double-blind, placebo-controlled trial. Lancet Oncol.
10:25–34. 2009. View Article : Google Scholar
|
|
5
|
Wilhelm SM, Carter C, Tang L, Wilkie D,
McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, Cao Y,
Shujath J, Gawlak S, Eveleigh D, Rowley B, Liu L, Adnane L, Lynch
M, Auclair D, Taylor I, Gedrich R, Voznesensky A, Riedl B, Post LE,
Bollag G and Trail PA: BAY 43-9006 exhibits broad spectrum oral
antitumor activity and targets the RAF/MEK/ERK pathway and receptor
tyrosine kinases involved in tumor progression and angiogenesis.
Cancer Res. 64:7099–7109. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baek KK, Kim JH, Uhm JE, Park SH, Lee J,
Parl JO, Park YS, Kang WK and Lim HY: Prognostic factors in
patients with advanced hepatocellular carcinoma treated with
sorafenib: a retrospective comparison with previously known
prognostic models. Oncology. 80:167–174. 2011. View Article : Google Scholar
|
|
7
|
Llovet JM, Peña CE, Lathia CD, Shan M,
Meinhardt G and Bruix J; Investigators SHARP Study Group: Plasma
biomarkers as predictors of outcome in patients with advanced
hepatocellular carcinoma. Clin Cancer Res. 18:2290–2300. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Raoul JL, Bruix J, Greten TF, Sherman M,
Mazzaferro V, Hilgard P, Scherubl H, Scheulen ME, Germanidis G,
Dominguez S, Ricci S, Nadel A, Moscovici M, Voliotis D and Llovet
JM: Relationship between baseline hepatic status and outcome, and
effect of sorafenib on liver function: SHARP trial subanalyses. J
Hepatol. 56:1080–1088. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng AL, Guan Z, Chen Z, Tsao CJ, Qin S,
Kim JS, Yang TS, Tak WY, Pan H, Yu S, Xu J, Fang F, Zou J, Lentini
G, Voliotis D and Kang YK: Efficacy and safety of sorafenib in
patients with advanced hepatocellular carcinoma according to
baseline status: subset analyses of the phase III sorafenib
Asia-Pacific trial. Eur J Cancer. 48:1456–1465. 2012. View Article : Google Scholar
|
|
10
|
Ezzoukhry Z, Louandre C, Trécherel E,
Godin C, Chauffert B, Dupont S, Diouf M, Barbare JC, Mazière JC and
Galmiche A: EGFR activation is a potential determinant of primary
resistance of hepatocellular carcinoma cells to sorafenib. Int J
Cancer. View Article : Google Scholar
|
|
11
|
Tsukui Y, Mochizuki H, Hoshino Y, Kawakami
S, Kuno T, Fukasawa Y, Iwamoto F, Hirose S, Yoshida T, Hosoda K,
Suzuki Y, Hosoda K, Kojima Y, Hirose Y, Shindou K, Matsuda M,
Yagawa S, Tawara A, Kobayashi M, Konishi T, Yamazaki T, Takahashi
S, Fujii H, Enomoto N and Omata M: Factors contributing to the
overall survival in patients with hepatocellular carcinoma treated
by sorafenib. Hepatogastroenterology. View
Article : Google Scholar
|
|
12
|
Morimoto M, Numata K, Moriya S, Kondo M,
Nozaki A, Morioka Y, Maeda S and Tanaka K: Inflammation-based
prognostic score for hepatocellular carcinoma patients on sorafenib
treatment. Anticancer Res. 32:619–623. 2012.PubMed/NCBI
|
|
13
|
Mohri Y, Tanaka K, Ohi M, Yokoe T, Miki C
and Kusunoki M: Prognostic significance of host- and tumor-related
factors in patients with gastric cancer. World J Surg. 34:285–290.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Battisti V, Bagatini MD, Maders LD, Chiesa
J, Santos KF, Gonçalves JF, Abdalla FH, Battisti IE, Schetinger MR
and Morsch VM: Cholinesterase activities and biochemical
determinations in patients with prostate cancer: influence of
Gleason score, treatment and bone metastasis. Biomed Pharmacother.
66:249–255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mitsunaga S, Kinoshita T, Hasebe T,
Nakagohri T, Konishi M, Takahashi S, Gotohda N and Ochiai A: Low
serum level of cholinesterase at recurrence of pancreatic cancer is
a poor prognostic factor and relates to systemic disorder and nerve
plexus invasion. Pancreas. 36:241–248. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Morera Ocón FJ, Ripoll Orts F,
García-Granero Ximénez M, Pastor MJ and Bernal Sprekelsen JC:
Decrease of serum cholinesterase in colorectal cancer. Med Clin
(Barc). 129:729–730. 2007.(In Spanish).
|
|
17
|
Chougule A, Hussain S and Agarwal DP:
Prognostic and diagnostic value of serum pseudocholinesterase,
serum aspartate transaminase, and serum alinine transaminase in
malignancies treated by radiotherapy. J Cancer Res Ther. 4:21–25.
2008. View Article : Google Scholar
|
|
18
|
Hamamoto Y, Niino K, Ishiyama H and Hosoya
T: Impact of pretreatment cholinesterase level on survival of
inoperable intrahepatic or hepatic-hilar carcinomas treated with
three-dimensional conformal radiotherapy. Radiat Med. 22:316–323.
2004.
|
|
19
|
Nishikawa H, Osaki Y, Inuzuka T, Takeda H,
Nakajima J, Matsuda F, Henmi S, Sakamoto A, Ishikawa T, Saito S,
Kita R and Kimura T: Branched-chain amino acid treatment before
transcatheter arterial chemoembolization for hepatocellular
carcinoma. World J Gastroenterol. 18:1379–1384. 2012. View Article : Google Scholar
|
|
20
|
Kaibori M, Matsui K, Saito T and Kamiyama
Y: Risk factors for early death due to recurrence after resection
of large hepatocellular carcinomas. Hepatogastroenterology.
55:2151–2156. 2008.PubMed/NCBI
|
|
21
|
Weismüller TJ, Prokein J, Becker T,
Barg-Hock H, Klempnauer J, Manns MP and Strassburg CP: Prediction
of survival after liver transplantation by pre-transplant
parameters. Scand J Gastroenterol. 43:736–746. 2008.PubMed/NCBI
|
|
22
|
Motta M, Giugno I, Ruello P, Pistone G, Di
Fazio I and Malaguarnera M: Lipoprotein (a) behaviour in patients
with hepatocellular carcinoma. Minerva Med. 92:301–305.
2001.PubMed/NCBI
|
|
23
|
Fernández Prieto RM, Ramallo Bravo A,
Carmona Carmona G and Carrasco Jiménez MS: Update on the current
role of plasma cholinesterase. Rev Esp Anestesiol Reanim.
58:508–516. 2011.(In Spanish).
|
|
24
|
Lencioni R and Llovet JM: Modified RECIST
(mRECIST) assessment for hepatocellular carcinoma. Seminars in
Liver Dis. 30:52–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors European
Organization for Research and Treatment of Cancer, National Cancer
Institute of the United States, National Cancer Institute of
Canada. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar
|
|
26
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D and
Verweij J: New response evaluation criteria in solid tumors:
revised RECIST guideline (ver.1.1). Eur J Cancer. 45:228–247. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Edeline J, Boucher E, Rolland Y, Vauléon
E, Pracht M, Perrin C, Le Roux C and Raoul JL: Comparison of tumor
response by Response Evaluation Criteria in Solid Tumors (RECIST)
and modified RECIST in patients treated with sorafenib for
hepatocellular carcinoma. Cancer. 118:147–156. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Abbadessa G, Rimassa L, Pressiani T,
Carrillo-Infante C, Cucchi E and Santoro A: Optimized management of
advanced hepatocellular carcinoma: four long-lasting responses to
sorafenib. World J Gastroenterol. 17:2450–2453. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Inuzuka T, Nishikawa H, Sekikawa A, Takeda
H, Henmi S, Sakamoto A, Saito S, Kita R, Kimura T, Osaki Y and Kudo
M: Complete response of advanced hepatocellular carcinoma with
multiple lung metastases treated with sorafenib: case report.
Oncology. 81:152–157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sacco R, Bargellini I, Gianluigi G,
Bertini M, Bozzi E, Altomare E, Battaglia V, Romano A, Bertoni M,
Capria A, Bresci G and Bartolozzi C: Complete response for advanced
liver cancer during sorafenib therapy: case report. BMC
Gastroenterol. 11:42011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shao YY, Lin ZZ, Hsu C, Shen YC, Hsu CH
and Cheng AL: Early alpha-fetoprotein response predicts treatment
efficacy of antiangiogenic systemic therapy in patients with
advanced hepatocellular carcinoma. Cancer. 116:4590–4596. 2010.
View Article : Google Scholar
|
|
32
|
Vincenzi B, Santini D, Russo A, Addeo R,
Giuliani F, Montella L, Rizzo S, Venditti O, Frezza AM, Caraglia M,
Colucci G, Del Prete S and Tonini G: Early skin toxicity as a
predictive factor for tumor control in hepatocellular carcinoma
patients treated with sorafenib. Oncologist. 15:85–92. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xiang ZL, Zeng ZC, Tang ZY, Fan J, He J,
Zeng HY and Zhu XD: Potential prognostic biomarkers for bone
metastasis from hepatocellular carcinoma. Oncologist. 16:1028–1039.
2011. View Article : Google Scholar : PubMed/NCBI
|