Introduction
Prostate cancers have recently increased with regard
to the number of patients and the mortality rate (1). L-type amino acid transporter 1 (LAT1)
is a Na+-independent amino acid transporter responsible
for system L, a major nutrient transport system for large neutral
amino acids (2,3). Previous studies have shown that LAT1
is often excessively expressed in primary human neoplasms of
various organs and is involved in tumor cell proliferation due to
its function in transporting essential amino acids (4–6).
Previous studies have demonstrated the upregulation of LAT1
immunohistochemically in prostate cancers (7), as well as in squamous cell carcinomas
(8,9), gliomas (10), urothelial carcinomas (11), non-small cell lung cancers
(12,13), breast cancers (14) and pancreatic cancers (15). In their previous study, Sakata
et al (7) suggested that
elevated LAT1 expression in prostate cancer core needle biopsy
samples may be considered a novel biomarker for high-grade
malignancy, but did not clarify the correlation between LAT1
expression and Gleason score, the most commonly used tool for
grading the malignancy of prostate cancers. In the present study,
to examine the associations between LAT1 expression and certain
clinicopathological features, and to further evaluate the
significance of LAT1 expression in prostate cancer, we
retrospectively reviewed a total of 54 patients with surgically
resected primary prostatic adenocarcinoma, and conducted
immunohistological examinations for LAT1, Ki-67 labeling index
(LI), CD34-positive microvessel density (MVD) and vascular
endothelial growth factor (VEGF). Age, preoperative serum
concentration of prostate-specific antigen (PSA), pathological
stage (pStage) of cancer and Gleason score were also assessed for
each patient.
Materials and methods
Patients
Fifty-four patients with primary prostatic
adenocarcinoma who underwent radical prostatectomy in the Dokkyo
Medical University Hospital (Tochigi, Japan) between January, 1999
and March, 2010 with no neoadjuvant therapies were evaluated in
this study. Using the surgically resected tumor specimen of each
patient, we determined the pStage using the TNM Classification of
Malignant Tumours Seventh Edition of the International Union
Against Cancer (UICC TNM 2009) (16) and the Gleason score, according to
the guidelines of the 2005 International Society of Urological
Pathology consensus conference (17). The study protocol was approved by
our institutional review board.
Immunohistochemical staining
We performed immunohistochemical staining for LAT1
(anti-human monoclonal mouse antibody 4A2, 2 μg/ml; kindly
provided by Dr H. Endou, J-Pharma Co., Ltd., Tokyo, Japan), Ki-67
(monoclonal mouse antibody MIB-1, 1:50; Dako Cytomation,
Carpinteria, CA, USA), CD34 (monoclonal mouse antibody NU-4A1,
ready-to-use; Nichirei Biosciences, Tokyo, Japan) and VEGF
(monoclonal mouse antibody R11, 1:100; Immuno-Biological
Laboratories Co., Ltd., Fujioka, Japan) using 4-μm
formalin-fixed paraffin-embedded tissue sections from the
surgically resected tumor samples of the 54 patients.
Deparaffinization of the sections was performed by passage through
xylene, while endogenous peroxidase activity was blocked with 1%
H2O2 in methanol for 30 min and antigen
retrieval was performed with microwave pretreatment in citrate
buffer if required (LAT1: 0.01 mol/l, pH 6.0, 95°C, 20 min; Ki-67
and CD34: 0.01 mol/l, pH 9.0, 95°C, 10 min; VEGF: no antigen
retrieval techniques required). After rinsing in 0.01 mol/l
phosphate-buffered saline (PBS), pH 7.4, and treatment with a
protein blocking agent (Ultra Tech HRP Streptavidin-Biotin
Detection System; Beckman Coulter, Fullerton, CA, USA) for 10 min,
the sections were incubated with each primary antibody for 2 h in
humidity chambers at room temperature. After a thorough wash with
PBS, the sections were incubated with a biotinylated secondary
antibody (Ultra Tech HRP Streptavidin-Biotin Detection System) for
10 min, followed by a streptavidin-peroxidase reagent (Ultra Tech
HRP Streptavidin-Biotin Detection System) for an additional 10 min.
3,3′-Diaminobenzidine tetrahydrochloride (Nichirei Biosciences,
Inc.) was used for visualization. The sections were then lightly
counterstained with hematoxylin.
Immunohistochemical evaluation
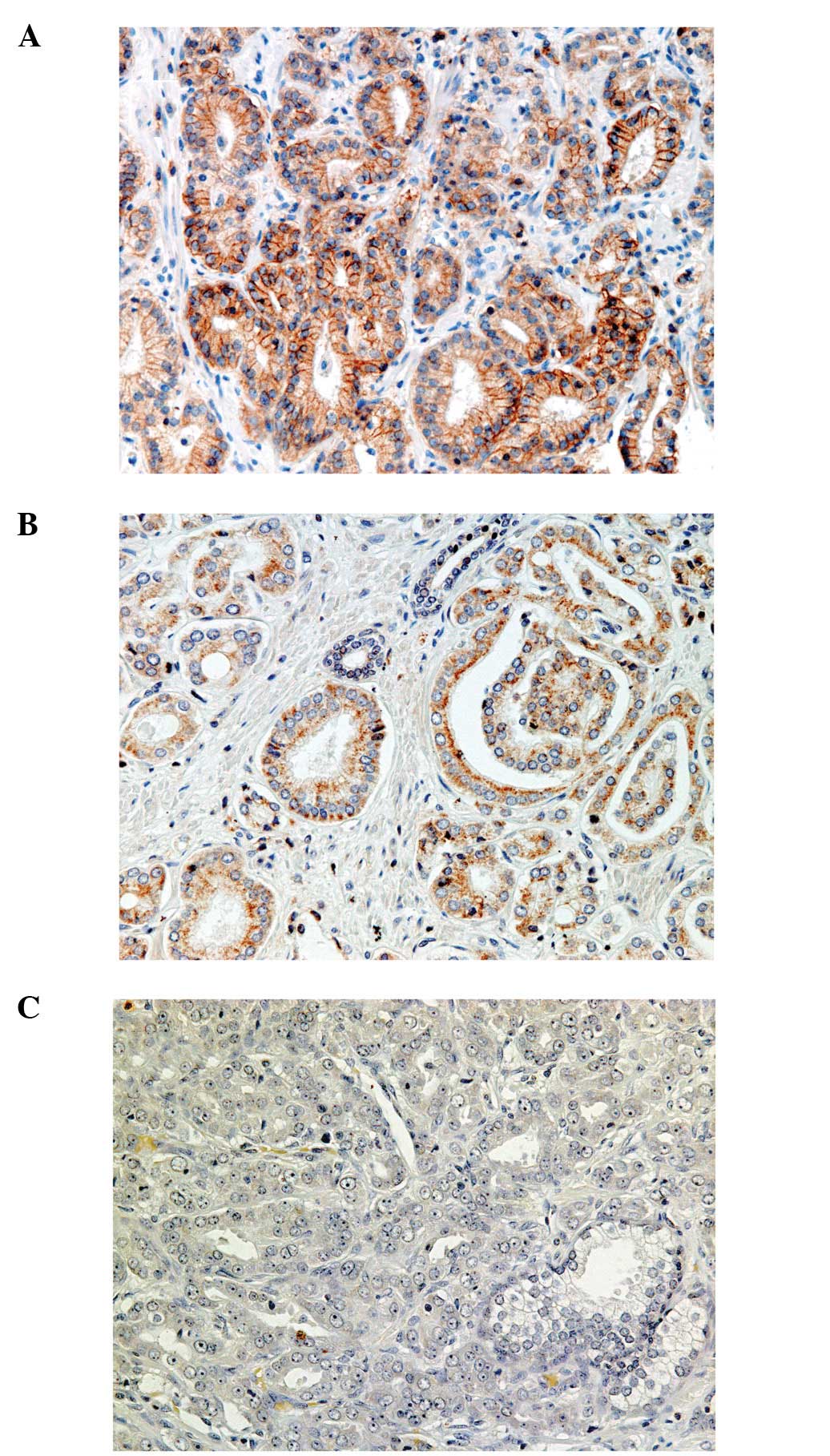
LAT1 expression was considered positive only when
the cell membranes of cancer cells showed obvious immunoreactivity
(Fig. 1), since Sakata et
al (7) demonstrated that LAT1
was localized on the cell membranes of cancer cells. Western blot
analysis and absorption testing performed in a previously published
study (7) clearly showed the
specificity of monoclonal antibody 4A2 for the N-terminal of the
LAT1 peptide, and the good stability of 4A2 for
immunohistochemistry on formalin-fixed and paraffin-embedded tissue
sections (7).
We counted ∼1,000 nuclei in a highly cellular area
of each Ki-67-immunoreactive section of the 54 cases and defined
positive cancer cells as those with nuclear immunoreactivity of any
intensity. We assessed the proliferative activity as the percentage
of immunopositive nuclei in the 1,000 cancer cells, representing
the so-called Ki-67 LI (18).
On the basis of the criteria of Weidner et al
(19), we assessed the potential
for invasion as MVD in the tumor areas showing the highest number
of discrete microvessels positively stained for CD34. Any
brown-stained endothelial cell or cell cluster that was clearly
separated from adjacent microvessels, tumor cells and other
connective tissue elements was considered to be a single countable
microvessel. The number of CD34-positive vessels in a ×400 field
(0.26 mm2 field area) was counted at four selected
hotspots and the MVD was defined as the mean count of
microvessels/0.26 mm2 field area.
VEGF expression was assessed according to the
percentage of immunoreactive cells in a total of 1,000 neoplastic
cells (quantitative analysis) (20).
The Gleason score determination and
immunohistochemical evaluation of each antibody were separately
performed by two board-certified pathologists. In cases of
discrepancy, the sections were simultaneously inspected by the two
pathologists until they reached agreement for the final
assessment.
Statistical analysis
The Mann-Whitney U test was used to assess the
association between two factors comprising LAT1 expression, Gleason
score and other clinicopathological characteristics listed in
Table I. P<0.05 was considered
to indicate a statistically significant difference. Statistical
analysis was performed using StatView version 4.54 for the Windows
software (SAS Institute Inc., Cary, NC, USA).
 | Table IPatient characteristics (n=54). |
Table I
Patient characteristics (n=54).
| No. | LAT1 | Gleason score | pStage | PSA (ng/ml) | Age (years) | Ki-67 LI (%) | CD34 MVD | VEGF (%) |
|---|
| 1 | Positive | 5+4=9 | 4 | 6.5 | 69 | 9.1 | 12 | 100 |
| 2 | Positive | 4+5=9 | 3 | 6.6 | 74 | 1.8 | 40 | 28 |
| 3 | Positive | 4+5=9 | 3 | 6.5 | 64 | 0.1 | 18 | 0 |
| 4 | Positive | 4+4=8 | 3 | 8.9 | 64 | 0 | 30 | 0 |
| 5 | Positive | 3+5=8 | 3 | 6.7 | 64 | 3.6 | 33 | 73 |
| 6 | Positive | 3+5=8 | 3 | 5.9 | 61 | 0.1 | 38 | 0 |
| 7 | Positive | 4+3=7 | 3 | 15.6 | 66 | 0 | 28 | 100 |
| 8 | Positive | 4+3=7 | 3 | 11.5 | 72 | 2.2 | 62 | 0 |
| 9 | Positive | 4+3=7 | 3 | 8.0 | 64 | 1.5 | 19 | 100 |
| 10 | Positive | 4+3=7 | 2 | 12.9 | 69 | 5.9 | 24 | 100 |
| 11 | Positive | 4+3=7 | 2 | 10.4 | 61 | 0.4 | 28 | 8 |
| 12 | Positive | 4+3=7 | 2 | 4.2 | 72 | 6.3 | 38 | 100 |
| 13 | Positive | 3+4=7 | 2 | 5.1 | 60 | 0.2 | 28 | 42 |
| 14 | Negative | 4+5=9 | 3 | 4.9 | 72 | 0.1 | 58 | 0 |
| 15 | Negative | 4+5=9 | 2 | 12.1 | 68 | 0.1 | 33 | 85 |
| 16 | Negative | 4+4=8 | 3 | 35.8 | 77 | 6.7 | 24 | 35 |
| 17 | Negative | 4+4=8 | 2 | 9.4 | 69 | 2.8 | 18 | 83 |
| 18 | Negative | 3+5=8 | 3 | 6.2 | 60 | 4.6 | 18 | 100 |
| 19 | Negative | 4+3=7 | 3 | 17.0 | 51 | 0.9 | 20 | 60 |
| 20 | Negative | 4+3=7 | 3 | 13.3 | 73 | 1.9 | 33 | 55 |
| 21 | Negative | 4+3=7 | 3 | 13.1 | 63 | 2.1 | 24 | 0 |
| 22 | Negative | 4+3=7 | 3 | 5.7 | 64 | 3.8 | 16 | 75 |
| 23 | Negative | 4+3=7 | 3 | 5.1 | 57 | 3.1 | 32 | 0 |
| 24 | Negative | 4+3=7 | 3 | 3.7 | 61 | 0.2 | 20 | 11 |
| 25 | Negative | 4+3=7 | 2 | 11.8 | 71 | 2.5 | 28 | 100 |
| 26 | Negative | 4+3=7 | 2 | 10.5 | 67 | 0.6 | 29 | 0 |
| 27 | Negative | 4+3=7 | 2 | 6.2 | 59 | 0 | 30 | 100 |
| 28 | Negative | 3+4=7 | 3 | 5.9 | 65 | 0.4 | 15 | 0 |
| 29 | Negative | 3+4=7 | 3 | 2.3 | 61 | 0.7 | 31 | 0 |
| 30 | Negative | 3+4=7 | 2 | 15.2 | 62 | 0 | 31 | 100 |
| 31 | Negative | 3+4=7 | 2 | 8.9 | 51 | 0.2 | 21 | 0 |
| 32 | Negative | 3+4=7 | 2 | 8.8 | 75 | 1.1 | 16 | 63 |
| 33 | Negative | 3+4=7 | 2 | 8.6 | 73 | 5.4 | 33 | 44 |
| 34 | Negative | 3+4=7 | 2 | 8.6 | 58 | 0.5 | 18 | 100 |
| 35 | Negative | 3+4=7 | 2 | 8.5 | 73 | 0.1 | 30 | 0 |
| 36 | Negative | 3+4=7 | 2 | 7.0 | 54 | 2 | 32 | 100 |
| 37 | Negative | 3+4=7 | 2 | 5.5 | 66 | 0.1 | 21 | 12 |
| 38 | Negative | 3+4=7 | 2 | 4.2 | 72 | 1 | 57 | 0 |
| 39 | Negative | 3+4=7 | 1 | 5.3 | 75 | 0.8 | 17 | 0 |
| 40 | Negative | 3+3=6 | 3 | 11.5 | 64 | 0 | 16 | 47 |
| 41 | Negative | 3+3=6 | 3 | 11.0 | 65 | 0.2 | 27 | 15 |
| 42 | Negative | 3+3=6 | 3 | 10.3 | 59 | 0.1 | 16 | 0 |
| 43 | Negative | 3+3=6 | 3 | 7.2 | 66 | 0.1 | 18 | 75 |
| 44 | Negative | 3+3=6 | 3 | 1.7 | 74 | 8.2 | 13 | 88 |
| 45 | Negative | 3+3=6 | 2 | 24.5 | 68 | 1.4 | 17 | 67 |
| 46 | Negative | 3+3=6 | 2 | 5.8 | 66 | 3.4 | 21 | 67 |
| 47 | Negative | 3+3=6 | 2 | 5.7 | 74 | 1 | 22 | 44 |
| 48 | Negative | 3+3=6 | 2 | 4.5 | 63 | 0 | 31 | 27 |
| 49 | Negative | 3+3=6 | 2 | 4.1 | 73 | 3.6 | 17 | 0 |
| 50 | Negative | 3+3=6 | 2 | 4.1 | 72 | 0.5 | 30 | 10 |
| 51 | Negative | 3+3=6 | 2 | 3.8 | 68 | 0.7 | 39 | 0 |
| 52 | Negative | 3+3=6 | 2 | 3.8 | 61 | 0.1 | 25 | 0 |
| 53 | Negative | 3+3=6 | 1 | 8.1 | 61 | 0.4 | 15 | 0 |
| 54 | Negative | 3+3=6 | 1 | 4.0 | 63 | 0.1 | 14 | 0 |
Results
Patient characteristics
Characteristics of the 54 patients enrolled in the
present study are listed in Table
I. Patient age ranged from 51 to 77 years (median, 65.5 years),
and the median value was considered to be the cut-off point. For
the preoperative serum PSA concentration, the range was 1.7 to 35.8
ng/ml (median, 6.9 ng/ml). We determined the cut-off point for
serum PSA at 10.0 ng/ml, according to the D’Amico risk assessment
(21). We found that 16 (30%) of
54 patients had higher PSA concentrations compared to the cut-off
value. The pStage distribution by the TNM classification was as
follows: pStage I or II, n=29; pStage III or IV, n=25. Since the
Gleason score is well-known to be markedly correlated with lethal
risk for patients following treatment, we divided the 54 patients
into two groups, comprising a high (Gleason score of 4+3=7, ≥8) and
a low GS group (Gleason score 3+4=7, ≤6), according to the findings
of previously published studies (21–23).
Overall, 26 patients were in the high and 28 patients in the low GS
group.
Immunohistochemical analysis
Of the 54 patients, 13 (24%) exhibited positive LAT1
immunoreactivity. Expression of LAT1 was not observed in
non-neoplastic prostate epithelium. The median values of Ki-67 LI,
MVD determined by CD34-positivity, and VEGF expression were 0.7%
(range, 0.0–9.1), 24.5 (range, 12–62) and 31.5% (range, 0.0–100),
respectively. The cut-off point of Ki-67 LI was set at 1.0%, based
on the findings of a previously published study by Laitinen et
al (24). For MVD, by
CD34-positivity, and VEGF expression, each median value was chosen
as the cut-off point. Of the 54 patients, expression higher than
the cut-off value was respectively identified in 22 (41%) patients
for Ki-67 LI, 27 (50%) patients for MVD by CD34-positivity and 27
(50%) patients for VEGF expression.
Associations between LAT1 expression and
other factors
The associations between LAT1 expression and other
factors are shown in Table II.
Overall, 12 of 13 LAT1-positive cases belonged to the high GS
group, and a significant correlation was clearly observed between
LAT1 expression and Gleason score (P<0.01). LAT1 expression
showed a tendency for more occasional expression in cases with a
higher pStage (P=0.059), but was not markedly associated with age,
serum PSA, Ki-67 LI, MVD by CD34-positivity or VEGF expression.
 | Table IIAssociations between LAT1 expression
and clinicopathological parameters in patients with prostate
cancer. |
Table II
Associations between LAT1 expression
and clinicopathological parameters in patients with prostate
cancer.
| Variables | LAT1-positive | LAT1-negative | P-value |
|---|
| Gleason score | | | |
| ≥8 and 4+3=7 | 12 | 14 | <0.01 |
| ≤6 and 3+4=7 | 1 | 27 | |
| Pathological
stage | | | |
| ≥III | 9 | 16 | 0.059 |
| ≤II | 4 | 25 | |
| Age (years)
(cut-off: 65.5 years) | | | |
| ≥66 | 6 | 21 | 0.75 |
| ≤65 | 7 | 20 | |
| Median/range | 64/60–74 | 66/51–77 | |
| Serum PSA (ng/ml)
(cut-off: 10 ng/ml) | | | |
| >10 | 4 | 12 | 0.92 |
| ≤10 | 9 | 29 | |
| Median/range | 6.7/4.2–15.6 | 7.0/1.7–35.8 | |
| Ki-67 labeling
index (%) (cut-off: 1.0%) | | | |
| >1.0 | 7 | 15 | 0.27 |
| ≤1.0 | 6 | 26 | |
| Median/range | 1.5/0.0–9.1 | 0.7/0.0–8.2 | |
| MVD by
CD34-positivity (cut-off: MVD 24.5) | | | |
| ≥MVD 25 | 9 | 18 | 0.12 |
| ≤MVD 24 | 4 | 23 | |
| Median/range | 28/12–62 | 22/13–58 | |
| VEGF (%) (cut-off:
31.5%) | | | |
| ≥32 | 7 | 20 | 0.75 |
| ≤31 | 6 | 21 | |
|
Median/range | 42/0.0–100 | 27/0.0–100 | |
In the 13 cases with positive LAT1 immunoreactivity,
the positively stained cancer cells were mainly distributed on
invasive borders, rather than inside tumor cell clusters. The
predominant histological forms of the prostate cancers at the
LAT1-positive areas varied among the 13 cases with no statistically
significant difference (trabecular pattern in two cases, fused
glands in nine cases, and single-shaped glands in two cases).
Associations between Gleason score and
other factors
The associations between Gleason score and other
factors are shown in Table III.
The Gleason score was significantly correlated with LAT1 expression
(P<0.01), pStage (P<0.01), and Ki-67 LI (P=0.016). The serum
PSA concentration tended to be higher in the patients in the high
GS group (P=0.051). No statistically significant correlations were
detected between Gleason score and age, or MVD by CD34-positivity
or VEGF expression.
 | Table IIIAssociations between Gleason score
and clinicopathological parameters in patients with prostate
cancer. |
Table III
Associations between Gleason score
and clinicopathological parameters in patients with prostate
cancer.
| Variables | GS≥8 or 4+3=7 | GS≤6 or 3+4=7 | P-value |
|---|
| LAT1 | | | |
| Positive | 12 | 1 | <0.01 |
| Negative | 14 | 27 | |
| Pathological
stage | | | |
| ≥III | 18 | 7 | <0.01 |
| ≤II | 8 | 21 | |
| Age (years)
(cut-off: 65.5 years) | | | |
| ≥66 | 13 | 14 | 1.00 |
| ≤65 | 13 | 14 | |
| Median/range | 65.0/51–77 | 65.5/51–75 | |
| Serum PSA (ng/ml)
(cut-off: 10 ng/ml) | | | |
| >10 | 11 | 5 | 0.051 |
| ≤10 | 15 | 23 | |
| Median/range | 8.5/3.7–35.8 | 5.9/1.7–24.5 | |
| Ki-67 labeling
index (%) (cut-off: 1.0%) | | | |
| >1.0 | 15 | 7 | 0.016 |
| ≤1.0 | 11 | 21 | |
| Median/range | 1.85/0.0–9.1 | 0.45/0.0–8.2 | |
| MVD by
CD34-positivity (cut-off: MVD 24.5) | | | |
| ≥MVD 25 | 15 | 12 | 0.28 |
| ≤MVD 24 | 11 | 16 | |
| Median/range | 28/12–62 | 21/13–57 | |
| VEGF (%) (cut-off:
31.5%) | | | |
| ≥32 | 15 | 12 | 0.28 |
| ≤31 | 11 | 16 | |
| Median/range | 57.5/0.0–100 | 13.5/0.0–100 | |
Discussion
LAT1 is a Na+-independent amino acid
transporter with 12 putative membrane-spanning domains (25,26),
and its activity is essential for tumor cell proliferation in
various organs including the prostate (7–15).
Sakata et al (7) suggested
that LAT1 expression in a prostate cancer may be regarded as a
strongly significant prognostic factor for the patient and that an
immunoreactive LAT1 expression test has potential as a biomarker
indicating high-grade malignancy of prostate cancers. When prostate
cancers are assessed for their degree of malignancy, the Gleason
score is the most commonly used parameter worldwide. However, a
great discrepancy remains, since no statistically significant
correlation between immuno-reactive LAT1 expression and Gleason
score has been detected in studies conducted by Sakata et al
(7). Gleason scores were grouped
using individual scores from 5 to 10, while urologists and
pathologists usually divide the Gleason scores into two or three
groups: low and high GS groups, or low, middle, and high GS groups
(21–23). We estimated that this could be the
reason why Sakata et al (7)
did not demonstrate a significant association between LAT1
expression and Gleason score. In the present study, we have clearly
demonstrated this significant correlation, after adequately
grouping the Gleason scores into two groups. To the best of our
knowledge, the present study is the first to markedly correlate
immunoreactive LAT1 expression and the monoclonal antibody 4A2 with
the Gleason score, showing that LAT1 is reliable as a biomarker for
high-grade prostate cancers.
The Gleason score was also significantly correlated
with the pStage of the patients and Ki-67 LI on tissue sections and
an almost significant association was found between serum PSA and
Gleason score, while LAT1 expression did not show statistically
significant correlations with any of the pStage, Ki-67 LI or serum
PSA. Age and MVD by CD34-positivity and VEGF expression showed no
statistically significant correlations with LAT1 expression or
Gleason score, although Kaira et al (20) reported statistically significant
correlations between LAT1 and MVD by CD34-positivity and VEGF
expression for primary lung adenocarcinomas. This discrepancy may
occur as prostate cancers usually exhibit a slower growth compared
to lung cancers. In other studies, lung adenocarcinomas and breast
cancers showed much more intense and clearer immunoreactivity on
the cell membranes (14,20) compared to the prostate
adenocarcinomas discussed in this study. This discrepancy may also
occur due to the relatively gentle character of prostate cancers.
However, a pale stain in the tissue sections prepared, may have
resulted from inferiority in certain technical skills.
The LAT1-positive cancer cells appeared to be mainly
distributed on invasive borders, rather than inside tumor cell
clusters, suggesting that LAT1 is involved in the infiltration of
tumor cells into interstitial tissues.
We conclude that LAT1 expression in prostate cancer
tissue sections is a promising biomarker for high-grade malignancy.
However, the reliability of LAT1 is not likely_to exceed that of
the Gleason score. Although the Gleason grading system is more
reliable compared to the LAT1 immunoreactivity, LAT1 may
nevertheless be as useful as the Gleason score, since testing for
immunoreactive LAT1 expression is relatively simple and the results
are easy to interpret, while the Gleason grading system requires a
sufficient number of skilled pathologists for proper application in
a population.
Acknowledgements
The authors are deeply grateful to Dr
Hitoshi Endou (J-Pharma Co., Ltd.) for providing the anti-LAT1
antibody. The authors are also greatly indebted to Mr. S Suzuki and
Mr. H Kojima (Dokkyo Medical University, Tochigi, Japan) for their
excellent technical assistance.
References
|
1
|
Matsuda T and Saika K: Comparison of time
trends in prostate cancer incidence (1973–2002) in Asia, from
cancer incidence in five continents, Vols IV–IX. Jpn J Clin Oncol.
39:468–469. 2009.
|
|
2
|
Kanai Y, Segawa H, Miyamoto K, Uchino H,
Takeda E and Endou H: Expression cloning and characterization of a
transporter for large neutral amino acids activated by the heavy
chain of 4F2 antigen (CD98). J Biol Chem. 273:23629–23632. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meier C, Ristic Z, Klauser S and Verrey F:
Activation of system L heterodimeric amino acid exchangers by
intracellular substrates. EMBO J. 21:580–589. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yanagida O, Kanai Y, Chairoungdua A, et
al: Human L-type amino acid transporter 1 (LAT1): characterization
of function and expression in tumor cell lines. Biochim Biophys
Acta. 1514:291–302. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fuchs BC and Bode BP: Amino acid
transporters ASCT2 and LAT1 in cancer: partners in crime? Semin
Cancer Biol. 15:254–266. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamauchi K, Sakurai H, Kimura T,
Wiriyasermkul P, Nagamori S, Kanai Y and Kohno N: System L amino
acid transporter inhibitor enhances anti-tumor activity of
cisplatin in a head and neck squamous cell carcinoma cell line.
Cancer Lett. 276:95–101. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sakata T, Ferdous G, Tsuruta T, et al:
L-type amino-acid transporter 1 as a novel biomarker for high-grade
malignancy in prostate cancer. Pathol Int. 59:7–18. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim DK, Ahn SG, Park JC, et al: Expression
of L-type amino acid transporter 1 (LAT1) and 4F2 heavy chain
(4F2hc) in oral squamous cell carcinoma and its precursor lesion.
Anticancer Res. 24:1671–1675. 2004.PubMed/NCBI
|
|
9
|
Kobayashi H, Ishii Y and Takayama T:
Expression of L-type amino acid transporter 1 (LAT1) in esophageal
carcinoma. J Surg Oncol. 90:233–238. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nawashiro H, Otani N, Shinomiya N, et al:
L-type amino acid transporter 1 as a potential molecular target in
human astrocytic tumors. Int J Cancer. 119:484–492. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakanishi K, Ogata S, Matsuo H, et al:
Expression of LAT1 predicts risk of progression of transitional
cell carcinoma of the upper urinary tract. Virchows Arch.
451:681–690. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kaira K, Oriuchi N, Imai H, et al:
Prognostic significance of L-type amino acid transporter 1
expression in resectable stage I–III non-small cell lung cancer. Br
J Cancer. 98:742–748. 2008.
|
|
13
|
Takeuchi K, Ogata S, Nakanishi K, et al:
LAT1 expression in non-small cell lung carcinomas: analyses by
semiquantitative reverse transcription-PCR (237 cases) and
immunohistochemistry (295 cases). Lung Cancer. 68:58–65. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Furuya M, Horiguchi J, Nakajima H, Kanai Y
and Oyama T: Correlation of L-type amino acid transporter 1 and
CD98 expression with triple negative breast cancer prognosis.
Cancer Sci. 103:382–389. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kaira K, Sunose Y, Segawa A, et al:
Prognostic significance of L-type amino acid transporter 1
expression in surgically resected pancreatic cancer. Br J Cancer.
107:632–638. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM Classification of Malignant Tumors (UICC International Union
Against Cancer). 7th edition. Wiley-Blackwell; Oxford: 2009
|
|
17
|
Epstein JI, Allsbrook WC Jr, Amin MB and
Egevad LL; ISUP Grading Committee: The 2005 International Society
of Urological Pathology (ISUP) consensus conference on Gleason
grading of prostatic carcinoma. Am J Surg Pathol. 29:1228–1242.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Buck AC, Schirrmeister HH, Guhlmann CA, et
al: Ki-67 immunostaining in pancreatic cancer and chronic active
pancreatitis: does in vivo FDG uptake correlate with proliferative
activity? J Nucl Med. 42:721–725. 2001.PubMed/NCBI
|
|
19
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumor angiogenesis and metastasis - correlation in invasive
breast carcinoma. N Engl J Med. 324:1–8. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kaira K, Oriuchi N, Imai H, et al:
Prognostic significance of L-type amino acid transporter 1 (LAT1)
and 4F2 heavy chain (CD98) expression in stage I pulmonary
adenocarcinoma. Lung Cancer. 66:120–126. 2009. View Article : Google Scholar
|
|
21
|
D’Amico AV, Whittington R, Malkowicz SB,
et al: Biochemical outcome after radical prostatectomy, external
beam radiation therapy, or interstitial radiation therapy for
clinically localized prostate cancer. JAMA. 280:969–974. 1998.
|
|
22
|
Stark JR, Perner S, Stampfer MJ, et al:
Gleason score and lethal prostate cancer: does 3+4=4+3? J Clin
Oncol. 27:3459–3464. 2009.PubMed/NCBI
|
|
23
|
Wright JL, Salinas CA, Lin DW, Kolb S,
Koopmeiners J, Feng Z and Stanford JL: Prostate cancer specific
mortality and Gleason 7 disease differences in prostate cancer
outcomes between cases with Gleason 4+3 and Gleason 3+4 tumors in a
population-based cohort. J Urol. 182:2702–2707. 2009.
|
|
24
|
Laitinen S, Martikainen PM, Tolonen T,
Isola J, Tammela T and Visakorpi T: EZH2, Ki-67 and MCM7 are
prognostic markers in prostatectomy-treated patients. Int J Cancer.
122:595–602. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mastroberardino L, Spindler B, Pfeiffer R,
et al: Amino-acid transport by heterodimers of 4F2hc/CD98 and
members of a permease family. Nature. 395:288–291. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim DK, Kanai Y, Matsuo H, et al: The
human T-type amino acid transporter-1: characterization, gene
organization, and chromosomal location. Genomics. 79:95–103. 2002.
View Article : Google Scholar : PubMed/NCBI
|