Introduction
Renal cell carcinoma (RCC) is a relatively common
tumor, accounting for ∼3% of the adult malignancies (1). In Japan, a previous nationwide study
investigating RCC revealed 7,405 patients (5,063 males, 2,342
females) with a positive diagnosis in 2002 (2).
In general, only ≤5% of RCC patients develop
paraneoplastic erythropoietin (EPO) overproduction-induced
polycythemia (2). However, several
studies are available on EPO-producing RCC. In the present study,
the first case of a patient exhibiting a therapeutic effect on
EPO-producing advanced RCC subsequent to targeted pre-surgical
sunitinib therapy is reported, with a review of the literature.
Case report
A 62-year-old male individual identified a
malformation of the left scrotum. On physical examination, a left
unilateral varicocele was diagnosed, and redness of the face was
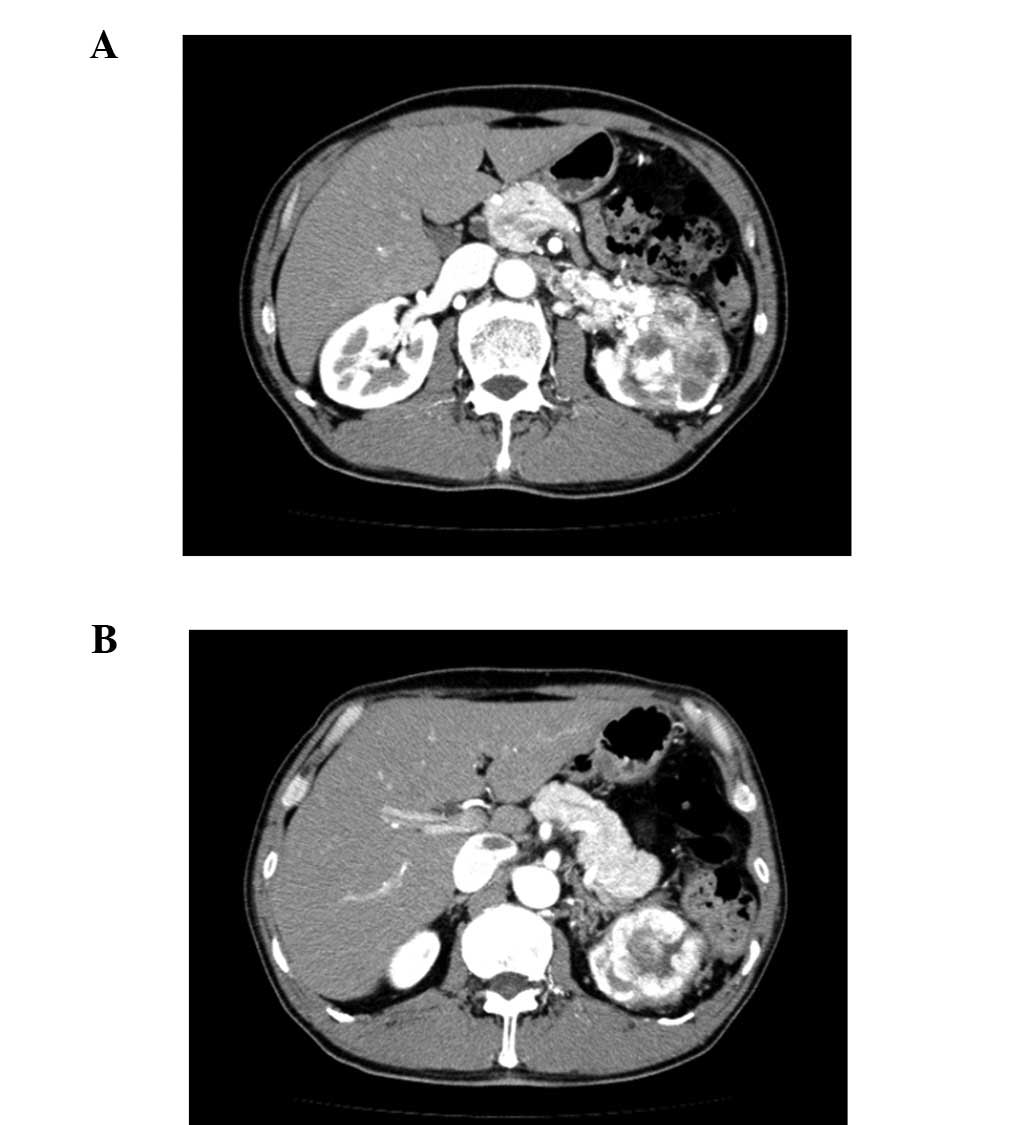
observed. Abdominal ultrasonography and computer tomography (CT)
scans detected a tumor of 73 mm in diameter at the upper pole of
the left kidney and tumor embolism in the renal vein and the
inferior vena cava (Fig. 1).
Furthermore, lymph node metastasis was detected in the left hilus
renalis. A thoracic CT scan demonstrated several nodules in the
lung, while the laboratory analysis showed red blood cell (RBC)
count, 682x106/mm3; hemoglobin (Hb), 21.3 g/dl and hematocrit (Ht)
61.6%. The patient’s serum EPO level was 35.8 mU/ml (normal range,
8–36 mU/ml). Written consent was obtained from the patient prior to
inclusion in this case study.
The percutaneous biopsy of the left renal tumor was
performed in an outpatient setting under ultrasonographic guidance
for the histological diagnosis. Subsequent to microscopic
examination, the histology of the renal tumor showed clear cell RCC
(Fig. 2), which was classified as
clinical stage T3bN1M1, according to TNM classification. The
patient was classifiated to be at intermediate risk, according to
the Memorial Sloan-Kettering Cancer Center (MSKCC) criteria. The
patient first received phlebotomy 3 times/week for polycythemia and
then sunitinib (50 mg/day) was administered for 4 weeks at 6-week
intervals as pre-surgical therapy. However, as grade 3 liver
dysfunction emerged after 12 days of the first treatment cycle,
sunitinib administration was transiently terminated and reinitiated
at 37.5 mg/day upon improvement. Although grade 1 dysgeusia was
observed as a further adverse event during the treatment, sunitinib
administration was well-tolerated with no grade 4 treatment-related
adverse events. Moreover, white blood cell (WBC) and platelet
counts were normal. During sunitinib treatment, hemoglobin and
hematocrit counts remained within the normal ranges (11–13 g/dl and
33–46%, respectively).
After 3 cycles of treatment, 20.4% regression of the
tumor was observed on CT based on the response evaluation criteria
in solid tumors (RECIST), while the intravenous tumor embolism had
decreased in size. A radical nephrectomy and lymph node resection
were performed through an abdominal approach 2 weeks subsequent to
the cessation of sunitinib. At the time of the nephrectomy,
adherence surrounding the left hilus renalis and the distention of
the left renal vein, as well as the gonadal vessels, were observed.
Although a left renal vein tumor embolism was detected, no embolism
was detected in the inferior vena cava. The surgery lasted for 5 h
32 min, with an estimated blood loss of 2,040 ml. During the
perioperative period, part of the wound was dissociated, without
additional complications.
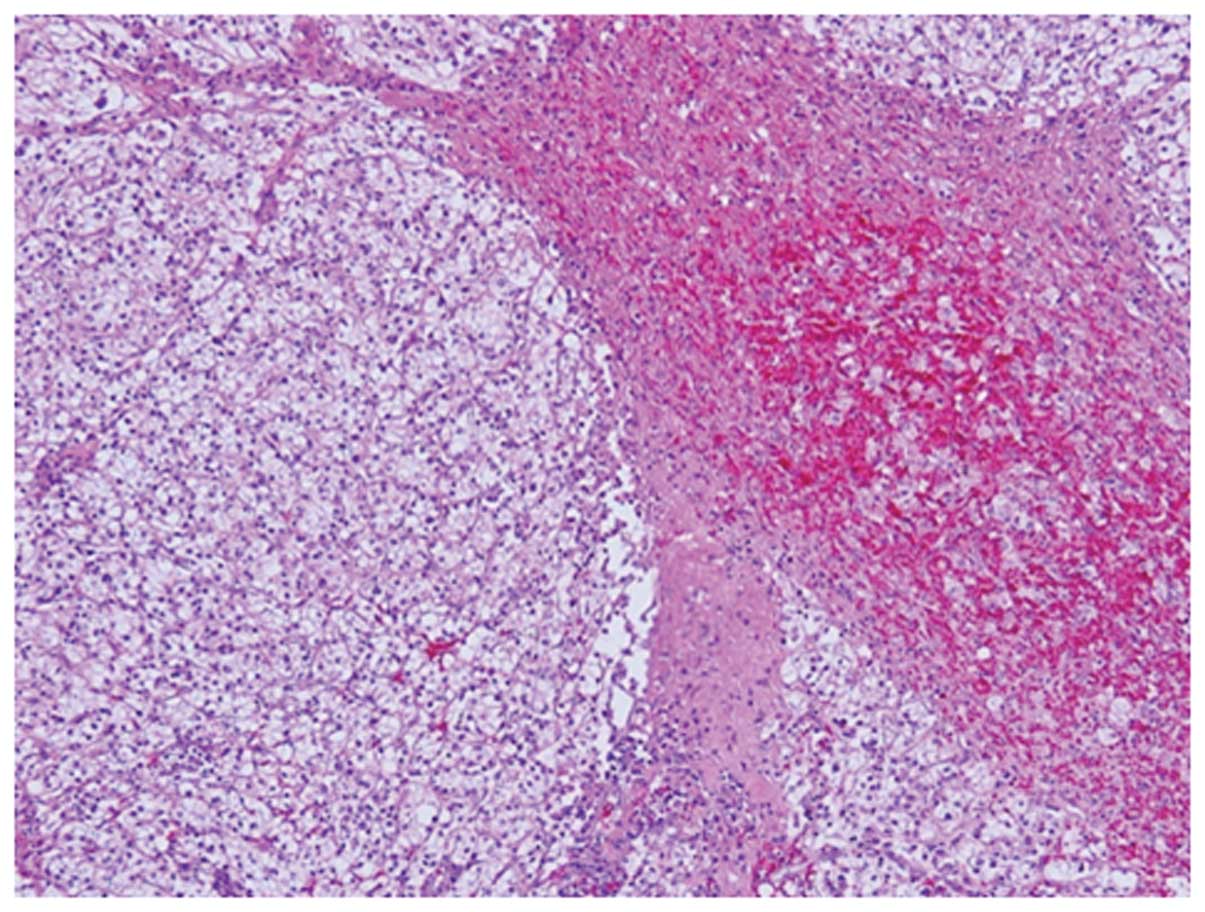
The histological examination of the tumor showed a
clear cell RCC, which had proliferated in an alveolar pattern
(Fig. 3). Although most of the
tumor contained viable cells, hemorrhage and necrosis were
detected. The capsular invasion of the tumor was not observed,
rather viable RCC cells were detected in the left renal vein and
the gonadal vessels. Furthermore, the lymph node of the hilus
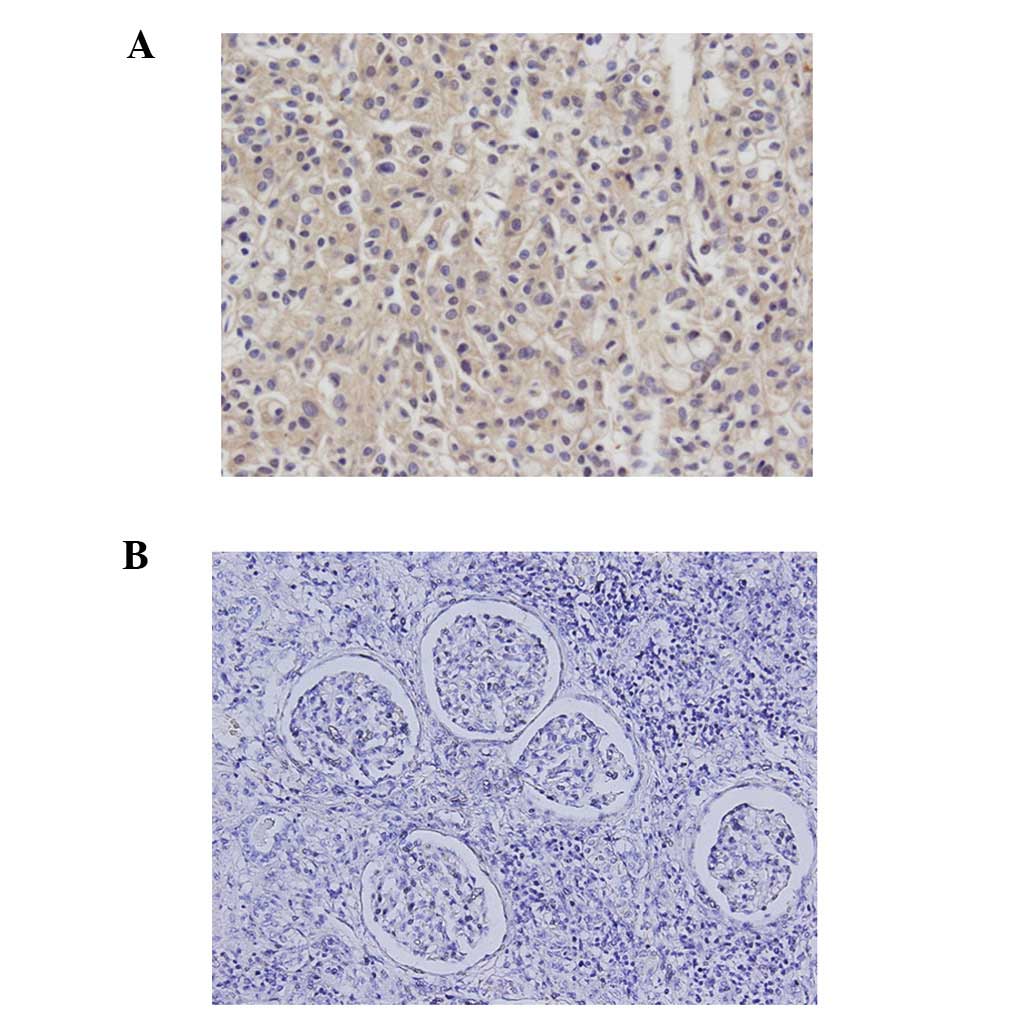
renalis contained viable RCC cells. Immunohistochemistry
demonstrated EPO in cancer cells, but not in normal kidney tissues
(Fig. 4). From the abovementioned
result, this was diagnosed as a case of EPO-producing RCC with
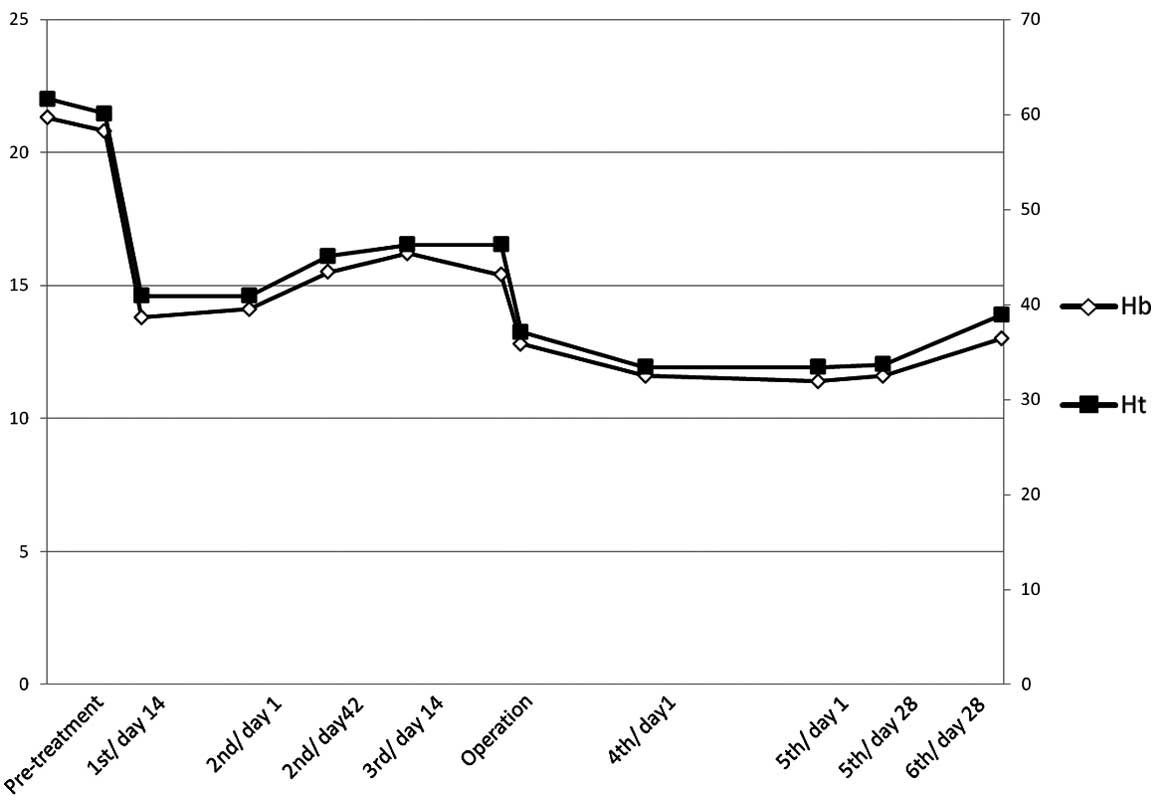
polycythemia. Subsequent to nephrectomy, sunitinib was
re-administered at 37.5 mg per day. The metastatic lesion of the
lung was stable at 6 months after surgery, while the hemoglobin and
hematocrit were maintained within normal ranges (Fig. 5).
Discussion
EPO, a glycoprotein produced mainly by the kidney,
controls erythropoiesis (3).
Increased EPO production results in secondary erythrocytosis, which
has been reported in a variety of tumors including RCC (4), hepatocellular carcinoma (5) and cerebellar hemangioblastoma
(6). Additionally, EPO-producing
renal cyst, metanephric adenoma, renal hemangioma and
polycythemia-induced hydronephrosis have been thoroughly described
(7). In general, only ≤5% of
patients with RCC develop paraneoplastic EPO overproduction-induced
polycythemia, while approximatrly 35% develop significant anemia
(4,7,8). The
glycoprotein hormone EPO is an essential viability and growth
factor for the erythrocytic progenitors. The oxygen-dependent
regulation of the EPO gene expression is postulated to be
controlled by a hypoxia-inducible transcription factor-1α (HIF-1α).
HIF-1α interacts with the Von Hippel-Lindau (VHL) protein and is
degraded by ubiquitin-mediated proteolysis in the presence of
oxygen. Accumulation occurs under hypoxia due to the inhibition of
its proteolytic degradation through the ubiquitin proteasome
pathway, while promoting EPO gene transcription. Furthermore, VHL
gene product (pVHL) loss has also been detected in 45–80% of clear
cell RCC cases (9–11). RCC continuously activates the HIF
pathway, while promoting EPO gene transcription (12).
Recent studies have reported that the receptor
tyrosine kinase inhibitors of the vascular endothelial growth
factor (VEGF) receptor family, such as sunitinib and sorafenib,
induce EPO upregulation (13–15).
However, in the present study, the administration of sunitinib was
successful in inhibiting tumor progression, as well as controlling
polycythemia. The present case was most likely controlled by
damaging cancer cells and decreasing EPO production to inhibit
angiogenesis by not signaling the HIF-VEGF or HIF-EPO pathways.
Hokayama et al(18)
suggested various criteria for erythropoietin-producing tumors: i)
coexistence of the tumor and polycythemia, ii) polycythemia
consumption following tumor resection, iii) increasing activation
of serum or urinary EPO, iv) EPO activation from tumor extracts, v)
no additional evidence of the cause of erythrocytosis or -poiesis,
including mRNA in the immunohistochemistry as additional
evidence.
In the present study, the immunohistochemical
staining of tumor tissue also revealed EPO production in tumor
cells subsequent to pre-surgical sunitinib therapy. The mechanism
of EPO elevation in RCC has been shown to be due to the fact that
the tumor is self-producing, as well as to EPO overproduction in
normal renal tissue resulting in local hypoxia arising from the
tumor. However, in the present study, immunohistochemical staining
of normal renal tissue was detected. To the best of our knowledge,
17 patients (14 males and 3 females; median age, 57 years; range,
44–83), including the present case, have been reported thus far
with EPO-producing RCC (16–19)
(Tables I and II). Most of these patients are male, with
the most common histological cell type being RCC (14 cases). Due to
the limited number of reports available the prognosis has been
insufficient, although in their studies, Sufrin et
al(20) and Ljungherg et
al(21) demonstrated that 63
(36/57) and 33% (55/167) of RCC cases showed elevated serum EPO,
respectively, whereas a few cases showed polycythemia (20,21).
Several studies have delineated that ∼8% renal tumors show
polycythemia, while ∼1.2% of the cases show serum EPO elevation.
Michael et al(22) reported
that the immunohistological expression of EPO in RCC is adversely
associated with poor prognosis (22). Furthermore, Westenfelder and
Baranowski (4), as well as
Westenfelder et al(23)
showed that EPO is likely to stimulate malignant tumor cells
(4,23).
 | Table IPatient characteristics (n=17). |
Table I
Patient characteristics (n=17).
| Characteristics | Value |
|---|
| Median age, years
(range) | 57 (44–83) |
| Gender, n | |
| Males | 14 |
| Females | 3 |
| Median RBC,
104/μl (range) | 709 (570–791) |
| Median Hb, g/dl
(range) | 19.2 (17.2–23.2) |
| Median Ht, %
(range) | 59.25 (52.1–70) |
| Nephrectomy, n | 17 |
| Other therapy, n | 9 |
| IFNα | 5 |
| UFT | 2 |
| Embolization,
n | 1 |
| Sunitinib, n | 1 |
| Histological type,
n | |
| Clear cell
type | 14 |
| Unknown | 3 |
| Stage, n | |
| I | 2 |
| II | 2 |
| III | 4 |
| IV | 7 |
| Unknown | 2 |
 | Table IIStudy characteristics. |
Table II
Study characteristics.
| Patient number | Study (Refs.) | Year | Age
(years)/gender | RBC
(104/μl) | Hb (g/dl) | Ht (%) | Treatment | Stage |
|---|
| 1 | Fujii et al
(18) | 1976 | 66/M | 650 | 20.5 | 63.4 | RN | NS |
| 2 | Hokayama et al
(18) | 1976 | 64/M | NS | 21.3 | NS | RN | IV |
| 3 | Shiga et al
(18) | 1981 | 44/M | 682 | 21.5 | 65.0 | RN | IV |
| 4 | Ishihara et al
(18) | 1988 | 56/F | 682 | 18 | 54.3 | RN | NS |
| 5 | Ishibashi et
al (18) | 1990 | 58/M | 631 | 17.2 | 52.1 | RN, IFNα, UFT | IV |
| 6 | Kanamaru et al
(18) | 1992 | 51/M | 762 | 18.4 | 59.2 | RN | III |
| 7 | Furukawa et al
(18) | 1992 | 83/M | 737 | 17.9 | 60.5 | RN, UFT | IV |
| 8 | Yomogi et al
(24) | 1994 | 56/M | 712 | 23.2 | 70.0 | RN | III |
| 9 | Teishima et al
(25) | 1996 | 52/M | 660 | 19.4 | 59.2 | RN, IFNα | IV |
| 10 | Yoshida et al
(16) | 1997 | 59/F | 709 | 17.9 | 55.5 | RN | II |
| 11 | Sato et al
(26) | 1998 | 56/F | 714 | 19 | 59.3 | RN | III |
| 12 | Noguchi et al
(17) | 1999 | 57/M | 789 | 20.4 | 65.0 | RN | I |
| 13 | Morita et al
(27) | 2001 | 52/M | 791 | 19.4 | 61.3 | RN, IFNα | IV |
| 14 | Okuyama et
al (18) | 2002 | 46/M | 775 | 18.9 | 58.5 | RN, IFNα | III |
| 15 | Rad et al
(19) | 2008 | 76/M | NS | NS | 55.0 | RN, IFNα | I |
| 16 | Hirose et al
(28) | 2011 | 80/M | 570 | 18.7 | 55.2 | Embolization,
RN | II |
| 17 | Present case | 2012 | 62/M | 682 | 21.3 | 61.6 | Sunitinib, RN | IV |
At present, there are few studies available using
targeted therapy for EPO-producing advanced RCC. This is the first
report to demonstrate a successful treatment of EPO-producing
advanced RCC subsequent to targeted presurgical sunitinib therapy.
Although the mechanism and the effect of the multi-targeted
tyrosine kinase inhibitor on EPO-producing RCC remain to be
determined, the present case suggests that sunitinib is likely to
inhibit tumor progression, as well as control polycythemia.
Consequently, additional cases are necessary to confirm these
results.
Abbreviations:
|
CT
|
computer tomography
|
|
EPO
|
erythropoietin
|
|
MRI
|
magnetic resonance imaging
|
|
RCC
|
renal cell carcinoma
|
References
|
1.
|
Chow WH, Devesa SS, Warren JL and Fraumeni
JF Jr: Rising incidence of renal cell carcinoma in the United
States. JAMA. 281:1628–1638. 1999.
|
|
2.
|
Marumo K, Kanayama H, Miyao N, Nakazawa H,
Ozono S, Horie S, Nagamori S, Igarashi T, Hasegawa M, Kimura G,
Nakao M, Nakamoto T and Naito S: Prevalence of renal cell
carcinoma: a nation-wide survey in Japan, 2002. Int J Urol.
14:479–482. 2007.
|
|
3.
|
Da Silva JL, Lacombe C, Bruneval P,
Casadevall N, Leporrier M, Camilleri JP, Bariety J, Tambourin P and
Varet B: Tumor cells are the site of erythropoietin synthesis in
human renal cancers associated with polycythemia. Blood.
75:577–582. 1990.
|
|
4.
|
Westenfelder C and Baranowski RL:
Erythropoietin stimulates proliferation of human renal carcinoma
cells. Kidney Int. 58:647–657. 2000.
|
|
5.
|
Muta H, Funakoshi A, Baba T, Uike N,
Wakasugi H, Kozuru M and Jimi A: Gene expression of erythropoietin
in hepatocellular carcinoma. Intern Med. 33:427–431. 1994.
|
|
6.
|
Trimble M, Caro J, Talalla A and Brain M:
Secondary erythrocytosis due to a cerebellular hemangioblastoma:
demonstration of erythropoietin mRNA in the tumor. Blood.
78:599–601. 1991.
|
|
7.
|
Nseyo UO, Williams PD and Murphy GP:
Clinical significance of erythropoietin levels in renal carcinoma.
Urology. 28:301–306. 1986.
|
|
8.
|
Motzer RJ, Bander NH and Nanus DM:
Renal-cell carcinoma. N Engl J Med. 335:865–875. 1996.
|
|
9.
|
Linehan WM, Pinto PA, Srinivasan R, Merino
M, Choyke P, Choyke L, Coleman J, Toro J, Glenn G, Vocke C, Zbar B,
Schmidt LS, Bottaro D and Neckers L: Identification of the genes
for kidney cancer: opportunity for disease-specific targeted
therapeutics. Clin Cancer Res. 13:671–679. 2007.
|
|
10.
|
Prowse AH, Webster AR, Richards FM,
Richard S, Olschwang S, Resche F, Affara NA and Maher ER: Somatic
inactivation of the VHL gene in von Hippel-Lindau disease tumours.
Am J Hum Genet. 60:765–771. 1997.
|
|
11.
|
Na X, Wu G, Ryan CK, Schoen SR,
di’Santagnese PA and Messing EM: Overproduction of vascular
endothelial growth factor related to von Hippel-Lindau tumour
suppressor gene mutations and hypoxia-inducible factor 1α
expression in renal cell carcinoma. J Urol. 170:588–592. 2003.
|
|
12.
|
Gong K, Zhang N, Zhang K and Na Y: The
relationship of erythropoietin overexpression with von
Hippel-Lindau tumour suppressor gene mutations between
hypoxia-inducible factor-1α and -2α in sporadic clear cell renal
carcinoma. Int J Mol Med. 26:907–912. 2010.
|
|
13.
|
Alexandrescu DT, McClure R, Farzanmehr H
and Dasanu CA: Secondary erythrocytosis produced by the tyrosine
kinase inhibitors sunitinib and sorafenib. J Clin Oncol.
26:4047–4078. 2008.
|
|
14.
|
Vroling L, van der Veldt AA, de Haas RR,
Haanen JB, Schuurhuis GJ, Kuik DJ, van Cruijsen H, Verheul HM, van
den Eertwegh AJ, Hoekman K, Boven E, van Hinsbergh VW and
Broxterman HJ: Increased numbers of small circulating endothelial
cells in renal cell cancer patients treated with sunitinib.
Angiogenesis. 12:69–79. 2009.
|
|
15.
|
Ebos JM, Lee CR, Christensen JG, Mutsaers
AJ and Kerbel RS: Multiple circulating proangiogenic factors
induced by sunitinib malate tumor-independent and correlate with
antitumor efficacy. Proc Natl Acad Sci USA. 104:17069–17074.
2007.
|
|
16.
|
Yoshida T, Kin S and Kounami T: A case of
erythropoietin-producing renal cell carcinoma proved by
immunohistochemistry. Hinyokika Kiyo. 43:339–342. 1997.(In
Japanese).
|
|
17.
|
Noguchi Y, Goto T, Yufu Y, Uike N,
Hasegawa Y, Fukuda T, Jimi A and Funakoshi A: Gene expression of
erythropoietin in renal cell carcinoma. Intern Med. 38:991–994.
1999.
|
|
18.
|
Okuyama M, Nishihara M, Hashimoto H,
Kaneko S and Yachiku S: A case of erythropoietin–producing renal
cell carcinoma with polycythemia. Hinyokika Kiyo. 48:531–534.
2002.(In Japanese).
|
|
19.
|
Rad FH, Ulusakarya A, Gad S, Sibony M,
Juin F, Richard S, Machover D and Uzan G: Novel somatic mutations
of the VHL gene in an erythropoietin-producing renal carcinoma
associated with secondary polycythemia and elevated circulating
endothelial progenitor cells. Am J Hematol. 83:155–158. 2008.
|
|
20.
|
Sufrin G, Mirand EA, Moore RH, Chu TM and
Murphy GP: Hormones in renal cancer. J Urol. 117:433–438. 1997.
|
|
21.
|
Ljungherg B, Rasmuson T and Grankvist K:
Erythropoietin in renal cell carcinoma: evaluation of its
usefulness as a tumor marker. Eur Urol. 21:160–163. 1992.
|
|
22.
|
Michael A, Politi E, Havranek E,
Corbishley C, Karapanagiotou L, Anderson C, Relph K, Syrigos KN and
Pandha H: Prognostic significance of erythropoietin expression in
human renal cell carcinoma. BJU Int. 100:291–294. 2007.
|
|
23.
|
Westenfelder C, Biddle DL and Baranowski
RL: Human, rat, and mouse kidney cells express functional
erythropoietin receptors. Kidney Int. 55:808–820. 1999.
|
|
24.
|
Yomogi H, Odajima K, Nakamura H, et al: A
case of erythropoietin-producing renal cell carcinoma. Rinsho
Hinyokika. 48:139–141. 1994.
|
|
25.
|
Teishima T, Sumi H, Ikemoto H, Himeno Y
and Ishino T: A case of erythropoietin-producing renal cell
carcinoma. Jpn J Urol Surg. 9:1181–1183. 1996.
|
|
26.
|
Sato T, Hukui J, Okamoto S, et al: A case
of renal cell carcinoma with polycythemia. Jpn J Urol Surg.
11:85–87. 1998.
|
|
27.
|
Morita S, Watanabe K, Sakamoto H, et al: A
case of erythropoietin-producing renal cell carcinoma. Rinsho
Hinyokika. 55:651–653. 2001.
|
|
28.
|
Hirose Y, Ando R, Nakane A, Akita H and
Okamura T: A case of erythropoietin-producing renal cell carcinoma.
Jpn J Urol Surg. 24:187–190. 2011.
|