Introduction
Peripheral T-cell non-Hodgkin lymphomas (PTCLs)
arising from post-thymic T cells are a heterogeneous group of
neoplasms accounting for ~7–10% of non-Hodgkin lymphomas (NHLs) in
Western countries, compared to 20–30% in East Asia (1,2).
PTCLs usually occur in middle-aged to elderly patients and exhibit
characteristics such as diffuse disease in 68%, systemic symptoms
in ~45%, bone marrow involvement (BMI) in 25.8% and extranodal
disease in 37% of the cases (3).
The 5-year survival rate is 20–40%, which is lower compared to that
of patients with corresponding aggressive B-cell lymphomas
(4).
Several prognostic factors and predictive models for
NHLs have been proposed (5–8) and
β2-microglobulin (β2-MG) levels, lactate dehydrogenase (LDH)
levels, B symptoms (fever, weight loss or night sweats), Ann Arbor
stages, Eastern Cooperative Oncology Group (ECOG) performance
status, bone marrow involvement (BMI), extranodal involvement (ENI)
and International Prognostic Index (IPI) were extensively
investigated and applied (9).
However, previous studies (9) were
mainly focused on diffuse large B-cell lymphomas, which is the most
common type of aggressive lymphoma. Further studies are required to
assess the significance of clinical prognostic factors for PTCL.
Thus, previously described prognostic factors were analyzed in
order to identify favorable prognostic factor combinations
applicable to PTCL patients.
In the present study, we conducted a retrospective
analysis of clinical characteristics from a large series of
patients diagnosed with PTCL according to the criteria of the World
Health Organization Classification (10) and assessed the prognostic
significance of factors such as serum LDH levels, β2-MG levels, IPI
and ECOG score. In addition, the overall survival (OS) rates of
different treatments were compared in order to assess the clinical
characteristics, treatment guidelines, survival and prognostic
factors for PTCL patients.
Patients and methods
Patients
A total of 276 consecutive patients diagnosed with
PTCL who were hospitalized in The First Affiliated Hospital of the
School of Medicine of Zhejiang University between January, 2005 and
December, 2011 were retrospectively reviewed. The diagnoses were
confirmed by histopathological hematoxylin and eosin (H&E)
staining and determination of the immunophenotype according to the
World Health Organization Classification. The immunophenotype was
analyzed using mouse monoclonal antibodies of variable
specificities in order to detect cellular antigens in the frozen or
paraffin-embedded tissue sections. The sections were stained with
T-cell (CD2, CD3 and CD45) and B-cell markers (CD20 and CD79a) and
disease specificity was confirmed by positive staining with one or
more T-cell-specific markers, without any positive B-cell-specific
marker staining. In addition, CD30 and anaplastic lymphoma kinase-1
(ALK-1) immunostaining were used for the differential diagnosis of
systemic anaplastic large-cell lymphoma and CD56, CD57, TIA-1 and
granzyme B were used as markers for the diagnosis of natural
killer/T-cell lymphoma (NKTCL).
Complete clinical profiles were obtained from the
252 patients who completed the follow-up and the clinical data
collected for each patient included age, gender, LDH and β2-MG
levels, IPI, ECOG score, Ann Arbor stage, number of ENI sites, date
of diagnosis and chemotherapeutic regimen. This study was approved
by the Ethics Committee of the First Affiliated Hospital of the
School of Medicine of Zhejiang University and informed consent was
obtained from the participants.
Clinical staging
The disease was graded according to the Ann Arbor
staging system. The performance status was based on the Eastern
Cooperative Oncology Group (ECOG) scale (0–4). The patient IPI
scores (9) were determined and
used in the survival analysis.
Treatment
Chemotherapeutic regimens including
cyclophosphamide, hydroxydaunorubicin, oncovin and prednisone
(CHOP) or CHOP-like regimens (idarubicin, mitoxantrone, liposomal
doxorubicin substituting for epirubicin) were employed. Intensive
chemotherapies included CHOP with etoposide (ECHOP), CHOP with
cytarabine (Ara-C), ifosfamide, 2-mercaptoethane sulfonate Na
(Mesna), mitoxantrone and etoposide (MINE), etoposide,
methylprednisolone, Ara-C and cisplatin (ESHAP), cisplatin,
gemcitabine and dexamethasone (GDP), Ara-C, cisplatin and
dexamethasone (DHAP) and hyperfractionated cyclophosphamide,
doxorubicin, vincristine, dexamethasone and high-dose cytarabine
and methotrexate (hyper-CVAD). Twenty-two patients were
administered L-asparaginase in combination with the regimens
mentioned above and bortezomib treatment was administered to 8
patients. Forty patients received local radiotherapy and 6 patients
underwent autologous hematopoietic stem cell transplantation
(HSCT).
Statistical analysis
The OS rate was calculated from the date of
diagnosis to death or the last date of follow-up. The survival
curves were analyzed by the Kaplan-Meier method and the log-rank
test was used for the comparison between individual clinical
characteristics and survival. The factors associated with survival
as determined by univariate analysis were incorporated into a Cox
proportional hazards model for multivariate analysis. The analyses
were two-sided and P<0.05 was considered to indicate a
statistically significant difference. Data were processed with the
Statistical Package for the Social Sciences software version 16
(SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
The clinical characteristics of the patients are
summarized in Table I. The median
follow-up was 23 months (range, 1–79 months). The median age was 52
years (range, 11–77 years), with 83.3% of the patients aged <60
years. The male:female ratio was 1.90:1. A total of 156 (61.9%)
patients presented with B symptoms, 95 (37.7%) had BMI and 47
(18.7%) had stage III–IV disease. The histological subtypes
included 69 cases (27.4%) with nasal NKTCL, with an OS rate of
26.1%; 9 cases (3.6%) with enteropathy-type T-cell lymphoma (ETTL),
with an OS rate of 11.1%; 11 cases (4.4%) with subcutaneous
panniculitis-like T-cell lymphoma (SCPTCL), with an OS rate of
27.3%; 18 cases (7.1%) with angioimmunoblastic T-cell lymphoma
(AITL), with an OS rate of 44.4%; 3 cases (1.2%) with ALK-negative
anaplastic large-cell lymphoma, (ALK-ALCL), with an OS rate of
66.7%; and 142 cases (56.3%) with peripheral T-cell lymphoma, not
otherwise specified (PTCL-NOS), with an OS rate of 24.6% (Fig. 1).
 | Table IClinical characteristics of the
patients. |
Table I
Clinical characteristics of the
patients.
| Characteristics | Value (%) | OS (%) | P-value |
|---|
| Demographics |
| Age, median
(years) | 52 | | |
| Range | 11–77 | | |
| ≤60 | 210 (83.3) | 23.5 | 0.765 |
| >60 | 42 (16.7) | 26.2 | |
| Gender |
| Male | 165 (65.6) | 21.8 | 0.142 |
| Female | 87 (34.5) | 27.6 | |
| Clinical
characteristics |
| ECOG score |
| 0–1 | 191 (75.8) | 30.9 | <0.001 |
| 2–4 | 61 (24.2) | 11.5 | |
| Ann Arbor stage |
| I–II | 39 (15.5) | 35.9 | 0.007 |
| III–IV | 213 (84.5) | 24.4 | |
| Extranodal
involvement |
| <2 | 202 (80.2) | 27.7 | 0.596 |
| ≥2 | 50 (19.8) | 18.0 | |
| B symptomsa |
| Present | 156 (61.9) | 17.3 | <0.001 |
| Absent | 96 (38.1) | 39.6 | |
| Bone marrow
involvement |
| Present | 95 (37.7) | 14.7 | <0.001 |
| Absent | 157 (62.3) | 32.5 | |
| Serum LDH
levels |
| > Upper limit of
normal | 133 (52.8) | 18.0 | <0.001 |
| ≤ Upper limit of
normal | 119 (47.2) | 34.5 | |
| Serum β2-MG
levels |
| > Upper limit of
normal | 157 (62.3) | 20.5 | <0.001 |
| ≤ Upper limit of
normal | 95 (37.7) | 34.7 | |
| Prognostic index |
| IPI risk |
|
Low/low-intermediate (0–1/2) | 205 (81.3) | 28.3 | 0.001 |
|
Intermediate-high/high (3/4–5) | 47 (18.7) | 19.1 | |
Therapeutic results and survival
analysis
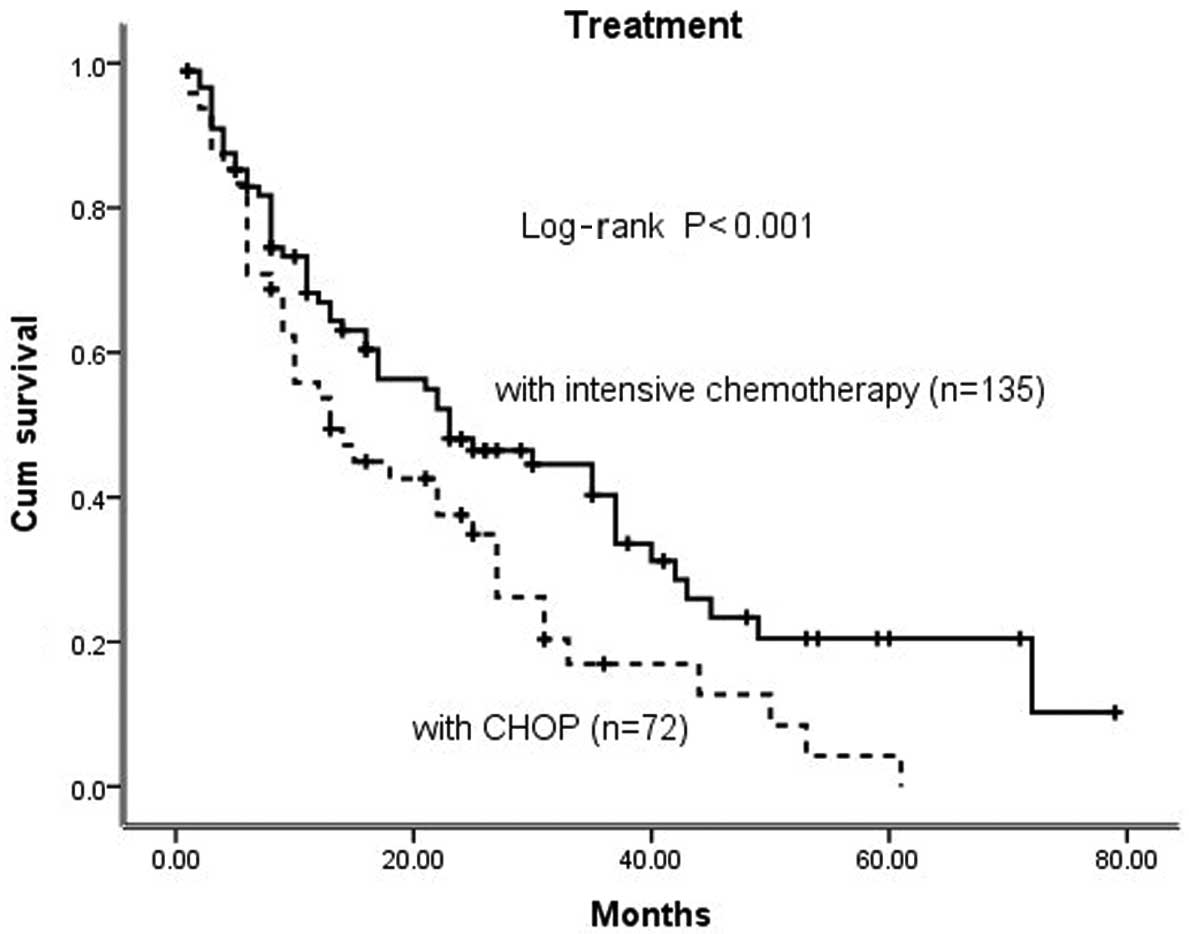
Seventy-two patients (28.6%) received a combination
of chemotherapy with the CHOP regimen, 135 patients (53.5%)
received intensive chemotherapy and 45 patients (17.9%) refused any
type of definitive therapy due to financial or personal reasons.
The estimated OS rate in the intensive chemotherapy group (38.9%)
was significantly higher compared to that in the CHOP group (16.7%)
(Fig. 2).
Prognostic factors
Analyses of prognostic factors are presented in
Table II. A univariate analysis
revealed that ECOG scores (P<0.001), B symptoms (P<0.001),
Ann Arbor stages III/IV (P=0.007), BMI (P<0.001), LDH levels
(P<0.001), β2-MG levels (P<0.001) and IPI (P<0.001) were
poor prognostic factors for PTCL. A multivariate analysis revealed
that ECOG scores (HR=3.49; 95% CI: 2.20–5.54; P<0.001), ENI
(HR=0.51; 95% CI: 0.31–0.85; P=0.001), positive B symptoms
(HR=1.67; 95% CI: 1.12–2.50; P=0.012), BMI (HR=0.39; 95% CI:
0.26–0.61; P<0.001) and LDH levels (HR=2.07; 95% CI: 1.40–3.06;
P<0.001) were significant prognostic factors for OS.
 | Table IIRisk factors for overall survival
(OS). |
Table II
Risk factors for overall survival
(OS).
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age >60 years | 1.07 | 0.67–1.72 | 0.771 | | | |
| Male gender | 0.76 | 0.52–1.11 | 0.153 | | | |
| ECOG score ≥2 | 2.77 | 1.99–3.86 | <0.001 | 3.49 | 2.20–5.54 | <0.001 |
| Stages III/IV | 1.76 | 1.15–2.70 | 0.010 | | | |
| Extranodal
involvement >1 | 0.93 | 0.65–1.31 | 0.666 | 0.51 | 0.31–0.85 | 0.001 |
| Positive B
symptomsa | 2.12 | 1.55–2.91 | <0.001 | 1.67 | 1.12–2.50 | 0.012 |
| Bone marrow
involvement | 0.53 | 0.40–0.71 | <0.001 | 0.39 | 0.26–0.61 | <0.001 |
| LDH level
>ULN | 2.00 | 1.49–2.68 | <0.001 | 2.07 | 1.40–3.06 | <0.001 |
| β2-MG level
>ULN | 2.19 | 1.60–3.00 | <0.001 | | | |
| IPI >2 | 2.08 | 1.48–2.92 | <0.001 | | | |
Association between LDH levels and
prognosis
Patients were divided into normal-level (n=119) and
elevated LDH (n=133)subgroups (LDH titer ≤225 and >225 U/l,
respectively). The OS in the normal-level LDH group (34.5%) was
significantly higher compared to that of the elevated LDH group
(18.0%, P<0.001) (Table I and
Fig. 3A).
Association between serum β2-MG levels
and prognosis
Patients were classified into the normal-level
(n=95) and elevated β2-MG (n=157) subgroups, using 2,200 μg/l β2-MG
as the cut-off point. The OS rate in the normal-level β2-MG group
(34.7%) was significantly higher compared to that of the elevated
β2-M group (20.5%, P<0.001) (Table
I and Fig. 3B).
Association between B symptoms and
prognosis
Patients were reclassified into two groups, those
with (n=96) and those without B symptoms (n=156). The OS rate of
patients with B symptoms (17.3%) was significantly lower compared
to that of patients without B symptoms (39.6%, P<0.001)
(Table I and Fig. 3C).
Association between BMI and
prognosis
Patients were reclassified into two groups, those
with (n=95) and those without BMI (n=157). The OS rate of patients
with BMI (14.7%) was significantly lower compared to that of
patients without BMI (32.5%, P<0.001) (Table I and Fig. 3D).
Association between IPI and
prognosis
Patients were divided into two subgroups, a
low/low-intermediate risk (IPI=0–1/2) and a intermediate-high/high
risk group (IPI=3/4–5), comprising 184 and 68 patients,
respectively. The OS rate in the low/low-intermediate risk group
(28.3%) was significantly higher compared to that of the
intermediate-high/high risk group (19.1%, P<0.001) (Table I and Fig. 4A).
Association between ECOG score and
prognosis
Patients were divided into two subgroups according
to their ECOG scores, a 0–1 (n=191) and a 2–4 score group (n=61).
The OS rate in the former group (30.9%) was significantly higher
compared to that of the latter group (11.5%, P<0.001) (Table I and Fig. 4B).
Association between ENI and
prognosis
Patients were divided into 2 subgroups, an ENI<2
(n=202) and an ENI≥2 group (n=50). The OS rate in the former group
(27.7%) was not significantly higher compared to that of the latter
group (18.0%, P=0.596) (Table I
and Fig. 4C).
Association between Ann Arbor stage and
prognosis
Patients were divided into 2 subgroups, including an
early-stage (I–II) and a late-stage group (III–IV), comprising 39
and 213 patients, respectively. The OS rate in the former group
(35.9%) was significantly higher compared to that of the latter
group (24.4%, P=0.007) (Table I
and Fig. 4D).
Discussion
PTCLs are a heterogeneous group of neoplasms,
usually diagnosed at an advanced stage and characterized by
widespread dissemination, aggressive behavior and poor outcome
(7). Several studies (9,11,12)
were conducted in order to assess the contribution of various
clinical factors to the disease prognosis. In this study, we aimed
to assess the effect of age, gender, LDH and β2-MG levels, B
symptoms, ENI, Ann Arbor stage, IPI and ECOG score on the prognosis
of 252 PTCL patients in our hospital. It was observed that age
(>60 years) was not an independent prognostic factor, which was
not in accordance with the results of previous studies (8,12). A
possible explanation for this inconsistency is that the median age
of the enrolled patients was 52 years, which was younger compared
to the cut-off age of 60 years and the age difference between the
two groups was not particularly notable. In addition, the physical
status of patients aged >60 years was relatively good, due to
the improved overall health status of the Chinese population. Our
results were consistent with those reported by previous studies
(9,11,12)
as regards LDH and β2-MG levels and B symptoms. These three factors
were identified as good prognostic predictors and were
statistically relevant with the OS rates. Therefore, it is
recommended that clinicians routinely measure these factors in PTCL
patients to better evaluate their prognosis and select more potent
regimens for those with poor prognosis.
Among several clinical prognostic factors, the IPI
seems to be suitable for peripheral T-cell lymphomas as well as
diffuse large-cell lymphomas (12–14).
However, in the multivariate analysis, only 3 of 5 parameters
retained their prognostic significance in our study. The clinical
staging and cut-off age of 60 years were no longer significant,
possibly due to the disease being characterized by over four-fifths
of the patients being diagnosed with advanced stage disease at
presentation. The number of ENI sites was also not significant, as
the location of the ENIs was more important. The presence of B
symptoms and BMI have been found to correlate with prognosis and OS
(14). BMI has been associated
with a poor response to treatment and a shorter survival of
patients with diffuse large B-cell lymphoma (15,16).
In PTCL patients BMI occurs in 20–40% of the cases and appears to
be associated with a worse prognosis (17). In the present study, we observed
BMI in a similar proportion of cases and the resulting negative
effect on survival was confirmed, whereas this effect occurred
independently of other IPI prognostic factors. A previous study
conducted by Gallamini et al(11) proposed a new prognostic model for
PTCL-NOS, referred to as PIT, based on 4 simple clinical variables:
age, ECOG score, LDH levels and BMI; however, further studies are
required to assess the prognostic value of this model for PTCL.
Although the elevated β2-MG level is not included in the IPI, there
is strong evidence supporting its independent prognostic relevance
in aggressive non-Hodgkin lymphomas (18). In the present study this factor was
confirmed as being directly associated with poor prognosis.
Although PTCL patients exhibit low survival rates, our data
demonstrated that intense chemotherapy may improve their OS rates.
Therefore, it is reasonable to hypothesize that intense
chemotherapy may prove beneficial for PTCL patients following
failure of the CHOP regimen.
In conclusion, the majority of PTCL cases present
with poor prognostic characteristics at diagnosis, respond poorly
to treatment and exhibit low survival rates. In particular,
elevated LDH and β2-MG levels, B symptoms, Ann Arbor stages III/IV,
BMI, high IPIs and high ECOG scores predict an unfavorable
prognosis for PTCL patients and an intensive chemotherapy may be a
comparatively more efficient PTCL treatment option.
Acknowledgements
This work was supported in part by the Research Plan
of Medical Science from the Health Administration of Zhejiang,
China (2013KYB102) and the Research Plan from the Science
Technology Department of Zhejiang Province, China (2013C33125).
References
|
1
|
Tomita N, Motomura S, Hyo R, et al:
Comparison of peripheral T-cell lymphomas and diffuse large B-cell
lymphoma. Cancer. 109:1146–1151. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kojima H, Hasegawa Y, Suzukawa K, et al:
Clinicopathological features and prognostic factors of Japanese
patients with ‘peripheral T-cell lymphoma, unspecified’ diagnosed
according to the WHO classification. Leuk Res. 28:1287–1292.
2004.
|
|
3
|
Ascani S, Zinzani PL, Gherlinzoni F, et
al: Peripheral T-cell lymphomas. Clinico-pathologic study of 168
cases diagnosed according to the R.E.A.L. Classification Ann Oncol.
8:583–592. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Armitage JO, Vose JM, Linder J, et al:
Clinical significance of immunophenotype in diffuse aggressive
non-Hodgkin’s lymphoma. J Clin Oncol. 7:1783–1790. 1989.
|
|
5
|
Vose J, Armitage J and Weisenburger D;
International T-Cell Lymphoma Project. International peripheral
T-cell and natural killer/T-cell lymphoma study: pathology findings
and clinical outcomes. J Clin Oncol. 26:4124–4130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mourad N, Mounier N, Brière J, et al;
Groupe d’Etude des Lymphomes de l’Adulte. Clinical, biologic, and
pathologic features in 157 patients with angioimmunoblastic T-cell
lymphoma treated within the Groupe d’Etude des Lymphomes de
l’Adulte (GELA) trials. Blood. 111:4463–4470. 2008.PubMed/NCBI
|
|
7
|
Niitsu N, Okamoto M, Nakamine H, Aoki S,
Motomura S and Hirano M: Clinico-pathologic features and outcome of
Japanese patients with peripheral T-cell lymphomas. Hematol Oncol.
26:152–158. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Siegert W, Nerl C, Agthe A, et al:
Angioimmunoblastic lymphadenopathy (AILD)-type T-cell lymphoma:
prognostic impact of clinical observations and laboratory findings
at presentation. The Kiel Lymphoma Study Group Ann Oncol.
6:659–664. 1995.PubMed/NCBI
|
|
9
|
No authors listed. A predictive model for
aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s
Lymphoma Prognostic Factors Project. N Engl J Med. 329:987–994.
1993.
|
|
10
|
Swerdlow SH, Campo E, Harris NL, Jaffe ES,
Pileri SA, Stein H, Thiele J and Vardiman JW: WHO Classification of
Tumours of Haematopoietic and Lymphoid Tissues. 2. 4th edition.
International Agency for Research on Cancer; Lyon: 2008
|
|
11
|
Gallamini A, Stelitano C, Calvi R, et al:
Peripheral T-cell lymphoma unspecified (PTCL-U): a new prognostic
model from a retrospective multicentric clinical study. Blood.
103:2474–2479. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ansell SM, Habermann TM, Kurtin PJ, et al:
Predictive capacity of the International Prognostic Factor Index in
patients with peripheral T-cell lymphoma. J Clin Oncol.
15:2296–2301. 1997.PubMed/NCBI
|
|
13
|
Gisselbrecht C, Gaulard P, Lepage E, et
al: Prognostic significance of T-cell phenotype in aggressive
non-Hodgkin’s lymphomas. Groupe d’Etudes des Lymphomes de l’Adulte
(GELA). Blood. 92:76–82. 1998.
|
|
14
|
López-Guillermo A, Cid J, Salar A, et al:
Peripheral T-cell lymphomas: Initial features, natural history and
prognostic factors in a series of 174 patients diagnosed according
to the R.E.A.L. classification. Ann Oncol. 9:849–855.
1998.PubMed/NCBI
|
|
15
|
Vitolo U, Bertini M, Brusamolino E, et al:
MACOP-B treatment in diffuse large-cell lymphoma: identification of
prognostic groups in an Italian multicenter study. J Clin Oncol.
10:219–227. 1992.PubMed/NCBI
|
|
16
|
Robertson LE, Redman JR, Butler JJ,
Osborne BM, Velasquez WS, McLaughlin P, Swan F, Rodriguez MA,
Hagemeister FB, Fuller LM, et al: Discordant bone marrow
involvement in diffuse large-cell lymphoma: a distinct
clinical-pathologic entity associated with a continuous risk of
relapse. J Clin Oncol. 9:236–242. 1991.PubMed/NCBI
|
|
17
|
Hanson CA, Brunning RD, Gajl-Peczalska KJ,
et al: Bone marrow manifestation of peripheral T-cell lymphoma: a
study of 30 cases. Am J Clin Pathol. 86:449–460. 1986.PubMed/NCBI
|
|
18
|
Rodriguez J, Cabanillas F, McLaughlin P,
et al: A proposal for a simple staging system for intermediate
grade lymphoma and immunoblastic lymphoma based on the ‘tumor
score’. Ann Oncol. 3:711–717. 1992.PubMed/NCBI
|