Introduction
In developed countries, the life expectancy of the
general population is on the increase, leading to an increased
incidence of malignant diseases among elderly individuals. Among
malignant diseases, the incidence and mortality of lung cancer has
increased worldwide over the last decades (1,2). Due
to the recent advances in the medical management of lung cancer,
the development of new drugs such as epidermal growth factor
receptor-tyrosine kinase inhibitors (EGFR-TKIs), the higher
standards of medical care and the more widely available health
services, survival of elderly patients may have been altered. Due
to the increase in the incidence of lung cancer among elderly
individuals, the efficacy and safety of EGFR-TKIs for the treatment
of elderly patients with non-small-cell lung cancer (NSCLC) have
been investigated in previous clinical trials (3–7),
although those in clinical practice have not yet been evaluated.
Therefore, additional studies are required, specifically focusing
on EGFR-TKI efficacy and safety in a population-based evaluation in
unselected patients.
Erlotinib, similar to gefitinib, is a reliable
EGFR-TKI and has been prescribed for numerous NSCLC patients
(8). In a previous phase III study
(BR.21) that compared erlotinib with placebo in the second- or
third-line treatment of NSCLC patients who were not responding to
standard chemotherapy, erlotinib was confirmed to significantly
prolong overall survival (OS), progression-free survival and the
time to deterioration of lung cancer-related symptoms (cough,
dyspnea and pain) as a quality of life measure (9). Successful results were also reported
by a combined analysis of two phase II clinical studies (JO16565
and JO18396) conducted in Japan. The objective response and disease
control rates were 28% [95% confidence interval (CI): 20.0–37.9%]
and 49% (95% CI: 39.2–59.0%), respectively, whereas the time to
progression was 10.7 weeks (95% CI: 8.1–18.3 weeks) and the OS was
13.8 months (95% CI: 11.4–18.1 months) (10). Erlotinib was demonstrated to be
effective in EGFR mutation-positive patients (11,12),
similar to gefitinib, although it was also suggested to be
effective in EGFR mutation-negative patients (11,13).
A population-based observational study was recently
conducted in the Ibaraki prefecture to investigate the usefulness
of erlotinib in lung cancer treatment by collecting and analyzing
data from all the patients receiving erlotinib, irrespective of
their individual characteristics (14). In this subset analysis, we
evaluated the association of age with the treatment results of
erlotinib in patients with NSCLC, by comparing the outcomes between
the elderly (≥75 years) and younger patients (<75 years) who
were enrolled in this population-based observational study.
Materials and methods
Patients
Fourteen institutions (17 departments) located in
the Ibaraki prefecture (area, 6,095 km2; population, ~3
million) participated in the present retrospective study, which
included patients who were treated with erlotinib at these
institutions between December, 2007 and December, 2010. In total,
307 patients were included in the study. Of these, 74 were aged ≥75
years (elderly group) and 233 were aged <75 years (younger
group). All the patients demonstrated histological or cytological
evidence of NSCLC. Histopathological diagnoses were defined
according to the World Health Organization (WHO) classification
system and the patients were staged according to the Union for
International Cancer Control (UICC) tumor-node-metastasis (TNM)
staging system.
The patient characteristics, efficacy and safety
were evaluated using patient data extracted from the database of
each institution. Tumor responses were classified as complete
response (CR), partial response (PR), stable disease (SD),
progressive disease or not evaluable, according to the response
evaluation criteria in solid tumors (RECIST), version 1.1.
The present observational study conformed to the
Ethical Guidelines for Clinical Studies issued by the Ministry of
Health, Labor and Welfare of Japan.
In this subset analysis, patients were divided into
two groups: those aged ≥75 years (elderly group) and those aged
<75 years (younger group). Patient characteristics, efficacy and
toxicity of erlotinib, time to treatment failure (TTF) and OS were
compared between the two groups.
Statistical analysis
Patient survival time was calculated from the date
of erlotinib therapy initiation to the date of death or latest
follow-up contact of the patient. The survival rate was analyzed by
the Kaplan-Meier method and comparisons were performed using the
log-rank test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
During the study period, a total of 307 patients
were treated with erlotinib and they were all evaluated for
efficacy and safety in this analysis. Of the 307 patients, 74 were
aged ≥75 years (elderly group) and 233 were aged <75 years
(younger group). There were no clinically significant differences
in baseline characteristics between the two age groups, apart from
PS and treatment line of erlotinib (Table I).
 | Table ICharacteristics of the elderly and
younger groups of patients. |
Table I
Characteristics of the elderly and
younger groups of patients.
| Characteristics | Patients ≥75 years
(elderly group) | Patients <75 years
(younger group) |
|---|
| Total no. of
patients | 74 | 233 |
| Gender
(male/female) | 50/24 | 152/81 |
| Performance
status |
| 0–1/2/3–4 | 49/21/4 | 158/42/33 |
| Pathology |
| AD/SQ/other | 53/9/12 | 180/33/20 |
| Smoking |
|
Never/smoker/unknown | 30/42/2 | 76/152/5 |
| Treatment line |
| 1st/2nd/3rd/4th or
later | 9/21/18/26 | 11/50/58/114 |
| EGFR mutation
status |
|
Positive/negative/unknown | 9/23/42 | 46/62/125 |
Efficacy
Tumor response was determined among patients
according to age and treatment (Table
II). Of the 74 elderly patients, 1 (1.4%) exhibited CR, 5
(6.8%) exhibited PR and 37 (50%) had SD. Of the 233 younger
patients, 1.3, 10.7 and 30.5% had CR, PR and SD, respectively. The
disease control rate (CR+PR+SD) was 58.2 and 42.5% in the elderly
and younger groups, respectively, with a statistically significant
difference (P=0.0228).
 | Table IITumor response in patients aged ≥75
and <75 years. |
Table II
Tumor response in patients aged ≥75
and <75 years.
| Response (%) | Patients ≥75 years
(elderly group) | Patients <75 years
(younger group) |
|---|
| Complete
response | 1 (1.4) | 3 (1.3) |
| Partial response | 5 (6.8) | 25 (10.7) |
| Stable disease | 37 (50.0) | 71 (30.5) |
| Progressive
disease | 26 (35.1) | 103 (44.2) |
| Not evaluable | 5 (6.8) | 31 (13.3) |
| Response rate | 6 (8.2) | 28 (12.0) |
| Disease control
rate | 43 (58.2) | 99 (42.5) |
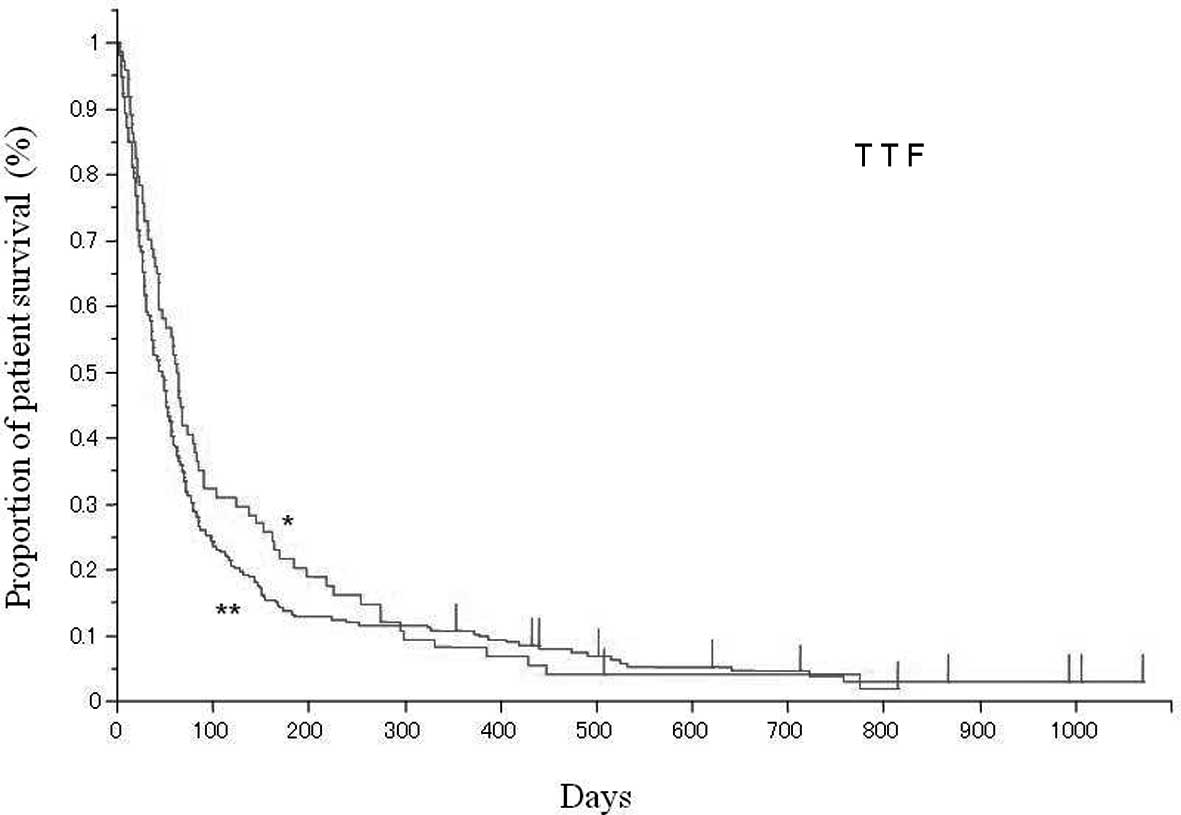
TTF in the two age groups is shown in Fig. 1. The median TTF was 62 days (95%
CI: 44–80 days) and 46 days (95% CI: 35–53 days) in the elderly and
younger group, respectively. There was no statistical difference
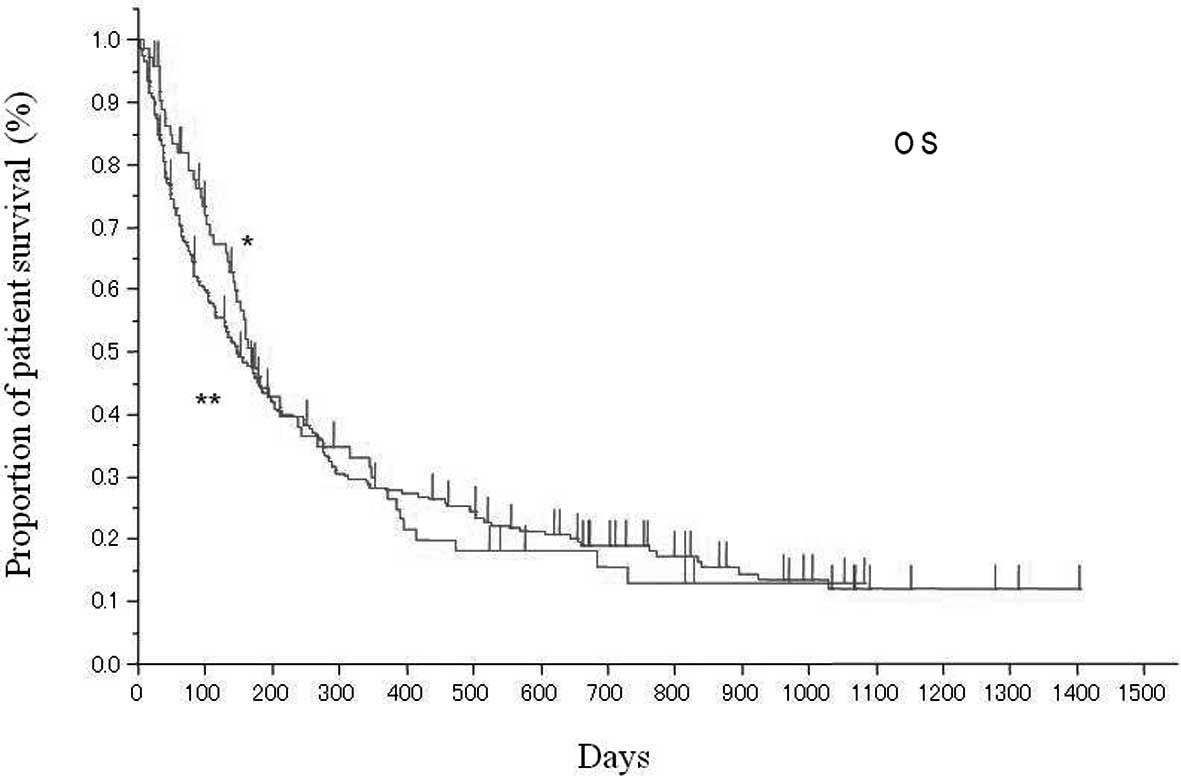
between the two groups (P=0.2475). The OS in the two age groups is
shown in Fig. 2. The median OS was
170 days (95% CI: 142–239 days) and 148 days (95% CI: 114–185 days)
in the elderly and the younger group, respectively. There was no
statistical difference between the two groups (P=0.7642).
In the elderly group of patients, TTF was not
different between EGFR mutation-positive and -negative patients (73
vs. 44 days, respectively; P=0.4585). No difference was observed in
OS between the two types (268 vs. 160 days, respectively;
P=0.5386).
Toxicity
The incidence of adverse events >grade 3 in the
two patient groups is presented in Table III. The elderly group exhibited a
slightly higher incidence in hepatotoxicity, diarrhea and pulmonary
toxicity, although the difference was not statistically
significant. The younger group of patients exhibited a slightly
higher incidence in skin rash, which was of no statistical
significance.
 | Table IIIToxicity >grade 3 in the elderly
and younger groups of patients. |
Table III
Toxicity >grade 3 in the elderly
and younger groups of patients.
| Toxicity (%) | Patients ≥75 years
(elderly group) | Patients <75 years
(younger group) |
|---|
| Skin rash | 2 (2.7) | 21 (9.0) |
| Hepatotoxicity | 3 (4.1) | 2 (0.9) |
| Diarrhea | 1 (1.4) | 1 (0.4) |
| Pulmonary
toxicity | 4 (5.4) | 7 (3.0) |
Efficacy and toxicity in patients aged
≥80 years
The differences between 20 patients aged ≥80 years
and 287 aged <80 years were also analyzed. The disease control
rate (CR+PR+SD) was 60.0% in the former and 45.3% in the latter
group. There was no statistically significant difference between
the two groups (P=0.2484). There was also no statistically
significant difference in TTF and OS between the two groups (TTF,
67 vs. 49 days, respectively, P=0.6538; OS, 153 vs. 160 days,
respectively, P=0.4956). There was no difference in the incidence
of >grade 3 skin rash, hepatotoxicity, diarrhea or pulmonary
toxicity between the two groups.
Discussion
Prior to EGFR-TKI application in the treatment of
elderly NSCLC patients, single-agent therapy with vinorelbine or
docetaxel was the most frequently used systemic treatment option
(15). Over the last few years,
treatment of elderly NSCLC patients with EGFR-TKIs has attracted
attention and has been investigated by previous studies (3–7). A
phase II clinical trial conducted by Jackman et al(6) on chemotherapy-naive NSCLC patients
aged ≥70 years, evaluated 80 patients and reported that the disease
control rate was 51%, whereas time to progression and median
survival time were 3.5 and 10.9 months, respectively (6). Inoue et al(5) prescribed gefitinib, another EGFR-TKI,
for 30 patients with poor PS or those aged 75–79 years and reported
that the disease control rate was 90%, whereas median
progression-free survival and median survival time were 6.5 and
17.8 months, respectively. Their study group recently verified
safety and efficacy of first-line gefitinib in 31 patients aged ≥75
years harboring EGFR mutations (7). Platania et al(4) reported their results from 43 patients
aged ≥70 years. In their retrospective study, the disease control
rate was 49% and the median progression-free survival and median
survival time were 3 and 8.4 months, respectively (4). Recently, a phase II randomized trial
conducted by Chen et al(3)
investigated the use of erlotinib in 57 chemonaive patients aged
≥70 years and reported a response rate of 22.7%, with a median
progression-free survival and median survival time of 4.57 and
11.67 months, respectively (3). Of
note, that study also reported that EGFR mutation-positive patients
exhibited better survival compared to those with EGFR wild-type
disease (3).
Our POSITIVE study was a relatively large
population-based observational study on 307 NSCLC patients and the
results supported the use of erlotinib as a treatment for NSCLC
(14). Elderly patients aged ≥75
years constituted 24.1% of the POSITIVE study population. The
subgroup analysis of the POSITIVE population demonstrated that
older and younger patients benefited equally from erlotinib
treatment, without significant differences in TTF and OS. In the
present subset analysis, the median TTF and OS in the elderly group
of patients was 62 and 170 days, respectively. In the majority of
the above-mentioned studies, the enrolled patients were aged ≥70
years and erlotinib was administered as first-line therapy
(3–6). By contrast, the elderly patients in
our study were unselected ordinary patients aged ≥75 years and
87.8% were administered erlotinib as second- or later-line therapy.
This may be the reason for the OS among our elderly patients being
worse compared to that reported by those previous studies. In
addition, differences in the treatment following erlotinib therapy
may affect OS. In this study, no unexpected adverse events
attributable to advanced age were observed. Rash, diarrhea,
hepatotoxicity and pulmonary toxicity were the most common
toxicities and they were mostly manageable. Pulmonary toxicity was
of no particular concern in the elderly patients, although grade 3
or higher pulmonary toxicity was observed in 4 patients (5.4%) in
the elderly and 7 (3.0%) in the younger group. Of note, the disease
control rate (CR+PR+SD) was higher in the elderly compared to the
younger group of patients. Since over half of the patients included
in this study had an unknown EGFR mutation status, the results may
be attributable to the characteristics of the EGFR mutation in this
study population. In this retrospective subgroup analysis of
POSITIVE data, TTF and OS did not differ between the two age groups
and the disease control rate was approximately equal in the two
groups. The treatment efficacy was almost the same as that
previously reported (3–7), although our study involved unselected
ordinary patients. There was no notable difference in the frequency
or severity of erlotinib-related toxicity between the two groups.
Therefore, erlotinib treatment may be effective against NSCLC and
tolerable in clinical practice, regardless of age. In addition, the
efficacy and safety of erlotinib in our patients aged ≥80 and in
those aged ≥75 years were approximately the same.
This study had several limitations. This was a
retrospective subset analysis of a population-based observational
study. Therefore, it was not designed to assess statistically
significant differences in treatment effects between the elderly
and younger subgroups. The limited sample size and imbalance of the
size between the two study populations may influence the results.
Approximately half of the patients from each age group had an
unknown EGFR mutation status. The treatment lines were different
between the two age groups, which may also affect the results.
However, the data presented in this study may contribute important
information and key study data on the elderly and younger subgroups
receiving erlotinib therapy. These analyses provide informative
results that may assist clinicians in selecting the appropriate
targeted therapy and may also provide guidance in the design of
trials involving targeted therapies for the elderly NSCLC
patients.
The efficacy of erlotinib was maintained in patients
aged ≥75 years. This result, combined with an acceptable toxicity
profile in both the elderly and the younger patient groups,
supports the use of erlotinib as a treatment for advanced NSCLC in
all age groups.
References
|
1
|
Bilello KS, Murin S and Matthay RA:
Epidemiology, etiology, and prevention of lung cancer. Clin Chest
Med. 23:1–25. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gabrielson E: Worldwide trends in lung
cancer pathology. Respirology. 11:533–538. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen YM, Tsai CM, Fan WC, et al: Phase II
randomized trial of erlotinib or vinorelbine in chemonaive,
advanced, non-small cell lung cancer patients aged 70 years or
older. J Thorac Oncol. 7:412–418. 2012.PubMed/NCBI
|
|
4
|
Platania M, Agustoni F, Formisano B, et
al: Clinical retrospective analysis of erlotinib in the treatment
of elderly patients with advanced non-small cell lung cancer.
Target Oncol. 6:181–186. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Inoue A, Kobayashi K, Usui K, et al; North
East Japan Gefitinib Study Group. First-line gefitinib for patients
with advanced non-small-cell lung cancer harboring epidermal growth
factor receptor mutations without indication for chemotherapy. J
Clin Oncol. 27:1394–1400. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jackman DM, Yeap BY, Lindeman NI, et al:
Phase II clinical trial of chemotherapy-naive patients > or=70
years of age treated with erlotinib for advanced non-small-cell
lung cancer. J Clin Oncol. 25:760–766. 2007.
|
|
7
|
Maemondo M, Minegishi Y, Inoue A, et al:
First-line gefitinib in patients aged 75 or older with advanced
non-small cell lung cancer harboring epidermal growth factor
receptor mutations: NEJ 003 study. J Thorac Oncol. 7:1417–1422.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reck M, Mok T, Wolf J, et al: Reviewing
the safety of erlotinib in non-small cell lung cancer. Expert Opin
Drug Saf. 10:147–157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bezjak A, Tu D, Seymour L, et al; National
Cancer Institute of Canada Clinical Trials Group Study BR.21.
Symptom improvement in lung cancer patients treated with erlotinib:
quality of life analysis of the National Cancer Institute of Canada
Clinical Trials Group Study BR.21. J Clin Oncol. 24:3831–3837.
2006. View Article : Google Scholar
|
|
10
|
Kubota K, Nishiwaki Y, Tamura T, et al:
Efficacy and safety of erlotinib as monotherapy for Japanese
patients with advanced non-small-cell lung cancer: a phase II
study. J Thoracic Oncol. 3:1439–1445. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pirker R, Su W, Rooneem R, et al: Clinical
outcome with erlotinib in relation to biomarker status: analysis
from the open-label TRUST study in advanced non-small-cell lung
cancer (NSCLC). Ann Oncol. 19(Suppl 8): 265P2008.
|
|
12
|
Massuti B, Moran T, Porta R, et al:
Multicenter prospective trial of customized erlotinib for advanced
non-small-cell lung cancer (NSCLC) patients (p) with epidermal
growth factor receptor (EGFR) mutations: Final results of the
Spanish Lung Cancer Group (SLCG) trial. J Clin Oncol. 27(Suppl):
abstract 80232009.
|
|
13
|
Cappuzzo F, Ciuleanu T, Stelmakh L, et al:
SATURN: A double-blind, randomized, phase III study of maintenance
erlotinib vs. placebo following nonprogression with first-line
platinum-based chemotherapy in patients with advanced NSCLC. J Clin
Oncol. 27(Suppl): abstract 80012009.
|
|
14
|
Kaburagi T, Satoh H, Hayashihara K, et al:
Observational study on efficacy and safety of erlotinib in patients
with non-small-cell lung cancer in Ibaraki, Japan. Oncol Lett.
5:435–439. 2013.PubMed/NCBI
|
|
15
|
Ardizzoni A and Tiseo M: Second-line
chemotherapy in the treatment of advanced non-small cell lung
cancer (NSCLC). J Chemother. 16(Suppl 4): 104–107. 2004. View Article : Google Scholar : PubMed/NCBI
|