Introduction
Biliary tract cancer (BTC), including cancer of the
gallbladder, bile ducts and ampulla, is a relatively uncommon type
of cancer, accounting for ∼4% of the malignant neoplasms of the
gastrointestinal tract. This type of cancer is more common in Asia
(1). Based on the data of the
National Cancer Registry in 2009, BTC is the eighth most common
cancer in Korea, with an annual incidence of 4,782 per 100,000
cancer cases (2). Surgical
resection offers patients with resectable BTC the only option for
cure and long-term survival. However, the reported overall 5-year
survival rate following surgical resection was 33.1% for bile duct,
52.8% for ampullary and 41.6% for gallbladder cancer (3). The prognosis for BTC remains poor,
even after extensive surgical resection, due to the high recurrence
rate. Therefore, effective adjuvant therapy is required to prolong
the survival of BTC patients.
However, the role of adjuvant treatment remains
controversial, since the results mentioned above are based on
small-scale studies, rather than large-scale, controlled clinical
trials. The majority of the retrospective trials, which included
limited sample sizes, heterogeneous patient populations and
non-standardized therapies, suggested a marginal benefit of
chemotherapy in reducing recurrence and an uncertain effect on
survival (4,5). The only two prospective randomized
controlled trials (RCT) currently available concluded that adjuvant
chemotherapy did not improve survival (6,7).
Since it was originally synthesized in 1957,
5-fluorouracil (5-FU) has been widely applied in clinical practice.
5-FU is the most extensively investigated drug for BTC, as a single
agent or in combination with other treatment modalities (4–7).
Several mechanisms of resistance to 5-FU were reported in
cholangiocarcinoma cell lines. It was previously demonstrated that
increases in UNG1 and BIRC5 expression and decreases in TP73
expression may be associated with 5-FU resistance (8). Previous studies also demonstrated
that the development of resistance of cancers to 5-FU may involve
mechanisms including alterations in the expression of several 5-FU
metabolic enzyme genes, including thymidylate synthase (TS),
dihydropyrimidine dehydrogenase (DPD) and thymidine phosphorylase
(TP) in colorectal cancer patients (9,10).
TS is the target enzyme for 5-FU and catalyzes the
methylation of deoxyuridine monophosphate (dUMP) to deoxythymidine
monophosphate (dTMP), which is an important process of DNA
biosynthesis (11). Elevated TS
protein levels may interfere with the mechanisms of action of
fluoropyrimidines (12). A recent
meta-analysis confirmed a poorer overall survival of patients with
enhanced TS activity, compared to those with low TS activity
(13). However, conflicting
results have been reported regarding TS expression in gastric
cancer (14,15).
DPD is the initial and rate-limiting enzyme
responsible for the inactivation of 5-FU (11). DPD activity is highly variable in
cancer tissues, which may affect the antitumor efficacy of 5-FU. A
previous in vitro study demonstrated that DPD expression in
cancer cell lines confers resistance to 5-FU (16). It was also reported that the
intratumoral gene expression levels of DPD are associated with
tumor response to 5-FU (17).
TP is a key enzyme in the metabolic activation of
fluoropyrimidines by conversion of doxifluridine (5′-DFUR), which
is an intermediate metabolite of capecitabine, to 5-FU (11). Thus, administration of 5′-DFUR in
cases of tumors with a high TP expression is expected to yield high
concentrations of 5-FU in tumor tissues and thereby a good
chemotherapeutic response. The clinical efficacy of 5′-DFUR was
demonstrated in colorectal cancer patients with high TP expression
tumors, who exhibited a better survival compared to patients with
low TP tumors (18). However, TP
was also identified as an angiogenic factor, identical to the
platelet-derived endothelial cell growth factor (19). Another previous study reported that
high TP immunostaining correlated with more extensive angiogenesis
and poor clinical outcome in colorectal cancer patients (20).
Due to their involvement in 5-FU metabolism, the
expression and activity levels of TS, DPD and TP are potentially
important as predictive markers for the response to 5-FU and as
prognostic factors in colorectal cancer patients (9,10).
However, there is currently no study available on the significance
of these proteins in BTC. The aim of this study was to determine
whether the expression of TS, TP and DPD predicts clinical outcome
in BTC patients treated with adjuvant 5-FU-based chemotherapy.
Patients and methods
Patients
A total of 99 patients who underwent curative
surgery for extrahepatic bile duct, ampullary or gallbladder cancer
at Dong-A University Medical Center between November, 1999 and
February, 2009 were evaluated. Patients with intrahepatic
cholangiocarcinoma were excluded, since this type of cancer has
been known to exhibit different clinicopathological characteristics
from other types of BTC.
Of the 99 patients, 39 (39.4%) had been diagnosed
with gallbladder cancer, 43 (43.4%) with extrahepatic bile duct
cancer and 17 (17.2%) with ampullary cancer. Patients with
extrahepatic bile duct and ampullary cancer typically underwent
pancreatoduodenectomy, with or without pyloric preservation,
whereas the surgical procedure for gallbladder cancer patients
almost always included cholecystectomy, with or without major
hepatectomy. The patients also underwent regional lymph node
dissection. However, dissection of para-aortic lymph nodes was not
routinely performed.
Following tumor resection, the specimens were
pathologically examined and each tumor was classified as well-,
moderately- or poorly-differentiated adenocarcinoma, according to
the predominant pathologic grading of differentiation. Pancreatic,
duodenal and hepatic invasion and lymph node metastasis were
pathologically determined. The surgical margins were considered
positive when infiltrating adenocarcinoma was present at the
proximal hepatic transection line, distal bile duct transection
line, or dissected periductal soft tissue margins. The final stage
of biliary carcinoma was pathologically determined, according to
the tumor-node-metastases staging system of malignant tumors,
published by the American Joint Committee on Cancer (AJCC), 6th
edition.
Clinical records and pathological reports were
retrospectively reviewed. Clinical outcomes were followed from the
date of surgery to either the date of death or August, 2012. The
study was approved by the Institutional Review Board of Dong-A
University Medical Center. Patient consent was obtained from either
the patient of the patient’s family.
Adjuvant chemotherapy
The eligibility criteria for adjuvant therapy
included: i) histologically confirmed preoperative diagnosis of
carcinoma of the gallbladder, extrahepatic bile duct, or ampulla;
ii) stage I–III disease; iii) confirmed resection of the primary
lesion; iv) age <75 years; v) Eastern Cooperative Oncology Group
performance status of 0–2; vi) no previous surgery or chemotherapy;
vii) no serious concomitant disease; viii) no concurrent or
non-concurrent multicentric tumor or double tumor; and ix) at
treatment initiation, a leukocyte count of ≥4,000/mm3, a
platelet count of ≥100,000/mm3, liver enzymes [aspartate
aminotransferase (AST) and alanine aminotransferase (ALT)] ≤100
units and negative urinary protein. External beam radiation or
intraoperative irradiation was not administered to any of the
patients during the study period.
The chemotherapy regimens were FP (5-FU plus
cisplatin) or oral 5-FU (doxfluridine). The FP regimen was as
follows: cisplatin 60 mg/m2 was administered
intravenously on day 1 and 5-FU 1,000 mg/m2 was
administered intravenously on days 1–5. This regimen was repeated
every three weeks for 6 cycles. Oral chemotherapy was initiated at
4 weeks after surgery; 460 mg/m2/day of doxifluridine
was administered daily for 1 year. Chemotherapy was discontinued
for recurrent disease, unacceptable treatment toxicity or at
patient request.
Patient follow up
The postoperative baseline and follow-up
investigations were standardized. Prior to adjuvant chemotherapy,
the baseline assessments included medical history and physical
examination, complete blood count (CBC), renal and liver function
tests, urinalysis, ECG, chest X-ray, radionuclide bone scan and
abdominal computed tomography (CT). Follow-ups for the patients
occurred at 3-month intervals for 2 years, at 6-month intervals for
the following 3 years and annually thereafter. The follow ups
comprised physical examination, CBC, renal and liver function tests
and abdominal ultrasonography or CT scan.
Immunohistochemistry and assessment of
immunostaining
Immunohistochemical study for the detection of TS,
TP and DPD expression was performed on three core cancer tissues (2
mm in diameter) from each individual, which were arranged in tissue
array blocks. The 4–5-μm sections were mounted on Superfrost
Plus glass slides (Thermo Scientific, Braunschweig, Germany). Using
the Discovery XT automated immunohistochemistry stainer (Ventana
Medical Systems, Tucson, AZ, USA), the slides were stained
according to the following procedure: tissue sections were
deparaffinized using the EZ Prep solution (Ventana Medical
Systems). For antigen retrieval, CC1 standard buffer (pH 8.4),
containing Tris/Borate/EDTA, (Ventana Medical Systems) was used for
30 min. Inhibitor D of endogenous peroxidase (3%
H2O2, Ventana Medical Systems) was applied
for 4 min at a temperature of 37°C. The slides were incubated with
anti-thymidine synthase antibody (clone TS106; Millipore Co., CA,
USA; dilution, 1:25), anti-thymidine phosphorylase antibody
(sc-47702; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA;
dilution, 1:100) and anti-dihydropyrimidine dehydrogenase antibody
(sc-50521, Santa Cruz Biotechnology, Inc.; dilution, 1:50) for 1 h
at 37°C, followed by incubation with an HRP-conjugated
anti-rabbit/mouse secondary antibody for 8 min at 37°C. The
reaction was detected with the Dako REAL™ EnVision™ system (Dako,
Glostrup, Denmark). The slides were incubated in
DAB+H2O2 substrate using the Ventana Chromo
Map kit (Ventana Medical Systems) for 8 min at 37°C, followed by
hematoxylin and bluing agent counterstaining.
For the assessment of immunostaining, the intensity
and distribution percentage of stained cancer cells were initially
evaluated. The intensity scores of immunostaining were divided into
negative and positive staining, which were classified as: 0,
negative staining; 1, weak staining; 2, moderate staining; and 3,
strong staining intensity. The distribution scores of
immunostaining were divided into 3 groups as follows: 1, ≤33% of
the tumor cells; 2, 33–66% of the tumor cells; and 3, 66–100% of
the tumor cells. Thereafter, sum scores were calculated by adding
two parameters and were then segregated into low and high
expression groups.
Statistical analysis
The association of the expression of TS, DPD and TP
with the clinicopathological parameters was assessed using the
χ2 or Fisher’s exact tests. Overall survival (OS) was
defined as the length of time from surgery to death. The
Kaplan-Meier method was used to construct curves for OS. The
log-rank test was used to compare distributions. To identify
independent factors significantly associated with patient
prognosis, the Cox’s proportional hazard analysis was used with a
stepwise procedure. The tests were two-sided and P<0.05 was
considered to indicate a statistically significant difference.
Analyses were performed using SPSS software version 19.0 (SPSS Inc,
Chicago, IL, USA).
Results
Patient characteristics
The demographic details of the patients included in
this study are presented in Table
I. The patients included 51 (51.5%) females and 48 (48.5%)
males, with a mean age of 61 years (range, 37–75 years). As regards
differentiation, 56 patients (56.6%) presented with highly, 28
(28.3%) with moderately and 15 (15.2%) with poorly differentiated
carcinomas. Thirty-six patients (36.4%) had pT3 or pT4 tumors and
24 (24.2%) had lymph node metastases. The postoperative stage was
I, II and III in 56 (50.5%), 41 (41.4%) and 8 patients (8.1%),
respectively. The median follow-up duration was 80.4 months (range,
41.3–149.9 months).
 | Table I.Correlation between patient
characteristics and chemotherapy regimen. |
Table I.
Correlation between patient
characteristics and chemotherapy regimen.
| Characteristics | Patient no. (%)
| P-value | Patient no. (%)
| P-value | Patient no. (%)
| P-value |
|---|
| TS (low) | TS (high) | TP (low) | TP (high) | DPD (low) | DPD (high) |
|---|
| Age, years | | | 0.841 | | | 0.504 | | | 0.164 |
| <65 (n=59) | 45 (76.3) | 14 (23.7) | | 19 (32.2) | 40 (67.8) | | 18 (30.5) | 41 (69.5) | |
| ≥65 (n=40) | 29 (72.5) | 11 (27.5) | | 10 (25.0) | 30 (75.0) | | 7 (17.5) | 33 (82.5) | |
| Gender | | | 0.067 | | | 1.000 | | | 0.818 |
| Male (n=48) | 40 (83.3) | 8 (16.7) | | 14 (29.2) | 34 (70.8) | | 13 (27.1) | 35 (72.9) | |
| Female (n=51) | 34 (66.7) | 17 (33.3) | | 15 (29.4) | 36 (70.6) | | 12 (23.5) | 39 (76.5) | |
| Location | | | 0.044 | | | 0.031 | | | 0.351 |
| Gallbladder
(n=39) | 24 (61.5) | 15 (38.5) | | 14 (35.9) | 25 (64.1) | | 11 (28.2) | 28 (71.8) | |
| EHBD (n=43) | 35 (81.4) | 8 (18.6) | | 7 (16.3) | 36 (83.7) | | 8 (18.6) | 35 (81.4) | |
| AOV (n=17) | 15 (88.2) | 2 (11.8) | | 8 (47.1) | 9 (52.9) | | 6 (35.3) | 11 (64.7) | |
| T stage | | | 0.902 | | | 0.067 | | | 0.060 |
| 1 (n=18) | 13 (72.2) | 5 (27.8) | | 9 (50.0) | 9 (50.0) | | 6 (33.3) | 12 (66.7) | |
| 2 (n=45) | 33 (73.3) | 12 (26.7) | | 14 (31.1) | 31 (68.9) | | 15 (33.3) | 30 (66.7) | |
| 3 (n=29) | 22 (75.9) | 7 (24.1) | | 4 (13.8) | 25 (86.2) | | 2 (6.9) | 27 (93.1) | |
| 4 (n=7) | 6 (85.7) | 1 (14.3) | | 2 (28.6) | 5 (71.4) | | 2 (28.6) | 5 (71.4) | |
| N stage | | | 1.000 | | | 0.133 | | | 0.788 |
| N0 (n=75) | 56 (74.7) | 19 (25.3) | | 25 (33.3) | 50 (66.7) | | 20 (26.7) | 55 (73.3) | |
| N1 (n=24) | 18 (75.0) | 6 (25.0) | | 4 (16.7) | 20 (83.3) | | 5 (20.8) | 19 (79.2) | |
| Stagea | | | 0.804 | | | 0.026 | | | 0.041 |
| I (n=50) | 36 (72.0) | 14 (28.0) | | 20 (40.0) | 30 (60.0) | | 17 (34.0) | 3 (66.0) | |
| II (n=41) | 32 (78.0) | 9 (22.0) | | 6 (14.6) | 35 (85.6) | | 5 (12.2) | 36 (87.8) | |
| III (n=8) | 6 (75.0) | 2 (25.0) | | 3 (37.5) | 5 (62.5) | | 3 (37.5) | 5 (62.5) | |
|
Differentiation | | | 0.281 | | | 0.678 | | | 0.061 |
| High (n=56) | 42 (75.0) | 14 (25.0) | | 15 (26.8) | 41 (73.2) | | 19 (339) | 37 (66.1) | |
| Moderate
(n=28) | 23 (82.1) | 5 (17.9) | | 10 (35.7) | 18 (64.3) | | 3 (10.7) | 25 (89.3) | |
| Poor (n=15) | 9 (60.0) | 6 (40.0) | | 4 (26.7) | 11 (73.3) | | 3 (20.0) | 12 (80.0) | |
| Lymphovascular
invasion | | | 0.224 | | | 1.000 | | | 0.112 |
| − (n=83) | 64 (77.1) | 19 (22.9) | | 24 (28.9) | 59 (71.1) | | 18 (21.7) | 65 (78.3) | |
| + (n=16) | 10 (62.5) | 6 (37.5) | | 5 (31.2) | 11 (68.8) | | 7 (43.8) | 9 (56.2) | |
| Resection
margin | | | 0.733 | | | 0.335 | | | 0.507 |
| − (n=86) | 65 (75.6) | 21 (24.4) | | 27 (31.4) | 59 (68.6) | | 23 (26.7) | 63 (73.3) | |
| + (n=13) | 9 (69.2) | 4 (30.8) | | 2 (15.4) | 11 (84.6) | | 2 (15.4) | 11 (84.6) | |
| Chemotherapy
regimen | | | 0.615 | | | 0.235 | | | 0.615 |
| Oral 5-FU
(n=70) | 51 (72.9) | 19 (27.1) | | 18 (25.7) | 52 (74.3) | | 19 (27.1) | 51 (72.9) | |
| FP (n=29) | 23 (79.3) | 6 (20.7) | | 11 (37.9) | 18 (62.1) | | 6 (20.7) | 23 (79.3) | |
Seventy (70.7%) patients received doxifluridine and
50 (71.4%) completed 1-year medication. Twenty-nine (29.3%)
patients received FP chemotherapy, of whom 24 (82.7%) completed 6
cycles. Older patients and patients with positive lymphovasular
invasion were more commonly administered doxifluridine (P=0.003 and
0.034, respectively).
Expression of TS, TP and DPD
The expression rates of TS, TP and DPD were 25.3,
70.7 and 74.7%, respectively. TS, TP and DPD protein expression was
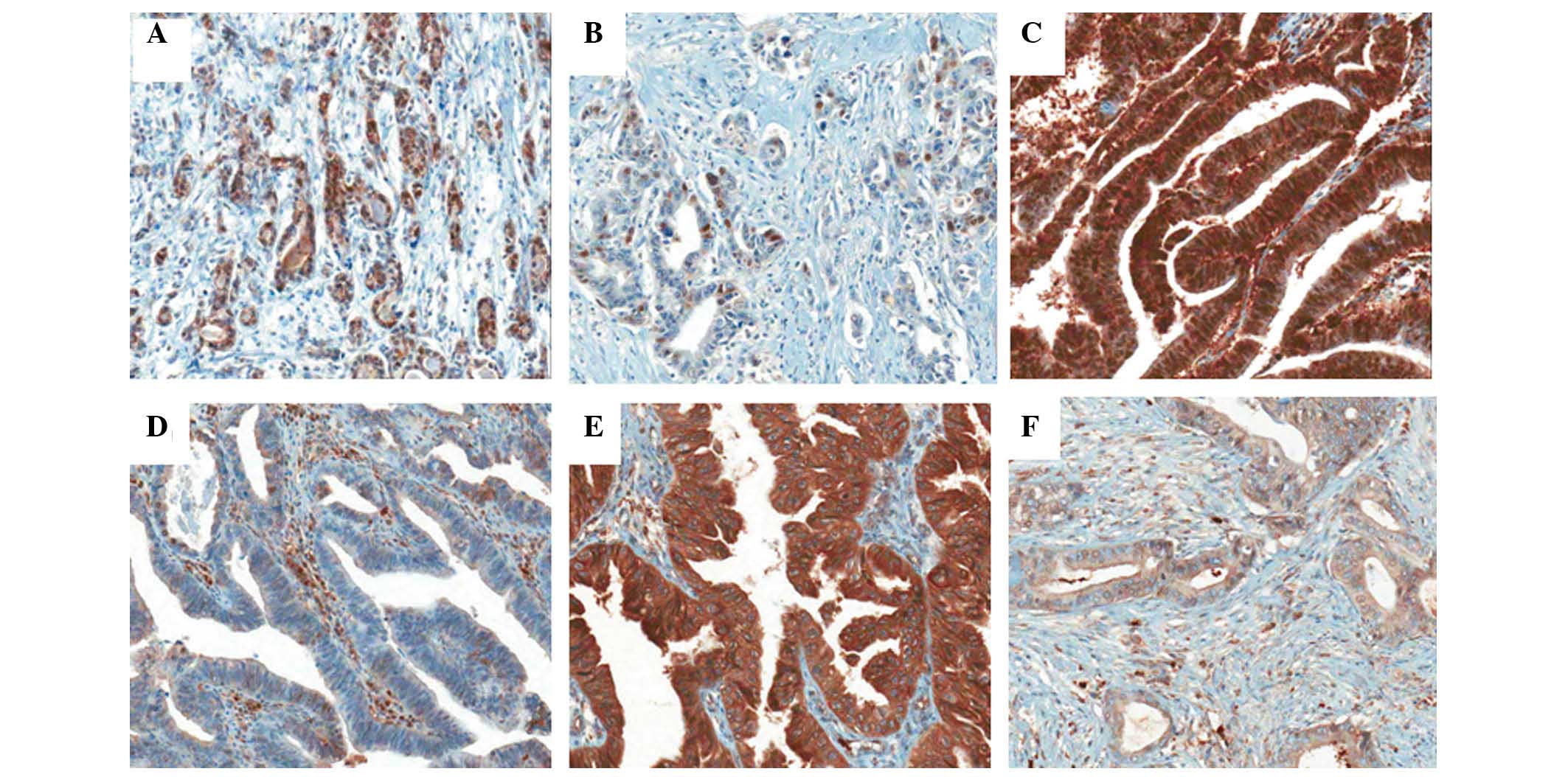
observed in the cytoplasm as well as the nucleus (Fig. 1). The correlation between the
clinicopatholological findings and the expression of these proteins
was analyzed (Table I). TS and TP
expression was significantly correlated with cancer location
(P=0.044 and 0.031, respectively). TS and TP were more commonly
expressed in extrahepatic bile duct cancer. A positive correlation
was observed between the expression of TP and DPD and cancer stage
(P=0.026 and 0.041, respectively). Other parameters, such as age,
gender, differentiation, lymphovascular invasion and resection
margin status, were not associated with the expression of these
proteins.
Association of TS, TP and DPD expression
with clinical outcome
The univariate analysis of clinicopathological
parameters and OS is presented in Table II. Age (P=0.006) and tumor location
(P<0.001) were associated with OS. Patients with extrahepatic
bile duct cancer exhibited shorter survival rates compared to those
with cancer in other sites. T stage (P<0.001), lymph node
metastasis (P<0.001), AJCC stage (P<0.001), differentiation
(P<0.001), resection margin status (P=0.001) and type of
chemotherapy regimen (P=0.015) were also significantly associated
with OS. The patients who were administered FP exhibited improved
survival rates compared to those who were administered
doxifluridine.
 | Table II.Univariate analysis of OS according
to clinicopathological parameters. |
Table II.
Univariate analysis of OS according
to clinicopathological parameters.
| Clinicopathological
parameters | 5-year OS (%) | P-value |
|---|
| Age (years) | | 0.006 |
| <65
(n=59) | 44.3 | |
| ≥65 (n=40) | 29.1 | |
| Gender | | 0.838 |
| Male (n=48) | 36.0 | |
| Female
(n=51) | 40.2 | |
| Location | | <0.001 |
| Gallbladder
(n=39) | 58.3 | |
| Extrahepatic bile
duct (n=43) | 13.8 | |
| Ampulla
(n=17) | 52.9 | |
| T stage | | <0.001 |
| 1 (n=18) | 77.4 | |
| 2 (n=45) | 41.2 | |
| 3 (n=29) | 13.8 | |
| 4 (n=7) | 14.3 | |
| N stage | | <0.001 |
| N0 (n=75) | 47.7 | |
| N1 (n=24) | 8.3 | |
| Stagea | | <0.001 |
| I (n=50) | 61.0 | |
| II (n=41) | 12.4 | |
| III (n=8) | 25.0 | |
|
Differentiation | | <0.001 |
| High (n=56) | 56.2 | |
| Moderate
(n=28) | 21.2 | |
| Poor (n=15) | 0 | |
| Lymphovascular
invasion | | 0.354 |
| − (n=83) | 39.4 | |
| + (n=16) | 25.0 | |
| Resection
margin | | 0.001 |
| − (n=86) | 42.9 | |
| + (n=13) | 7.7 | |
| Chemotherapy
regimen | | 0.015 |
| Oral 5-FU
(n=70) | 31.2 | |
| 5-FU + cisplatin
(n=29) | 54.5 | |
| TS expression | | 0.222 |
| Low (n=74) | 35.1 | |
| High (n=25) | 46.8 | |
| TP expression | | 0.007 |
| Low (n=29) | 65.2 | |
| High (n=70) | 26.9 | |
| DPD expression | | 0.192 |
| Low (n=25) | 44.0 | |
| High (n=74) | 36.0 | |
The prognostic significance of TS, DPD and TP
expression in BTC patients treated with adjuvant chemotherapy was
then investigated (Table II). TS
expression was not found to be significantly correlated with OS
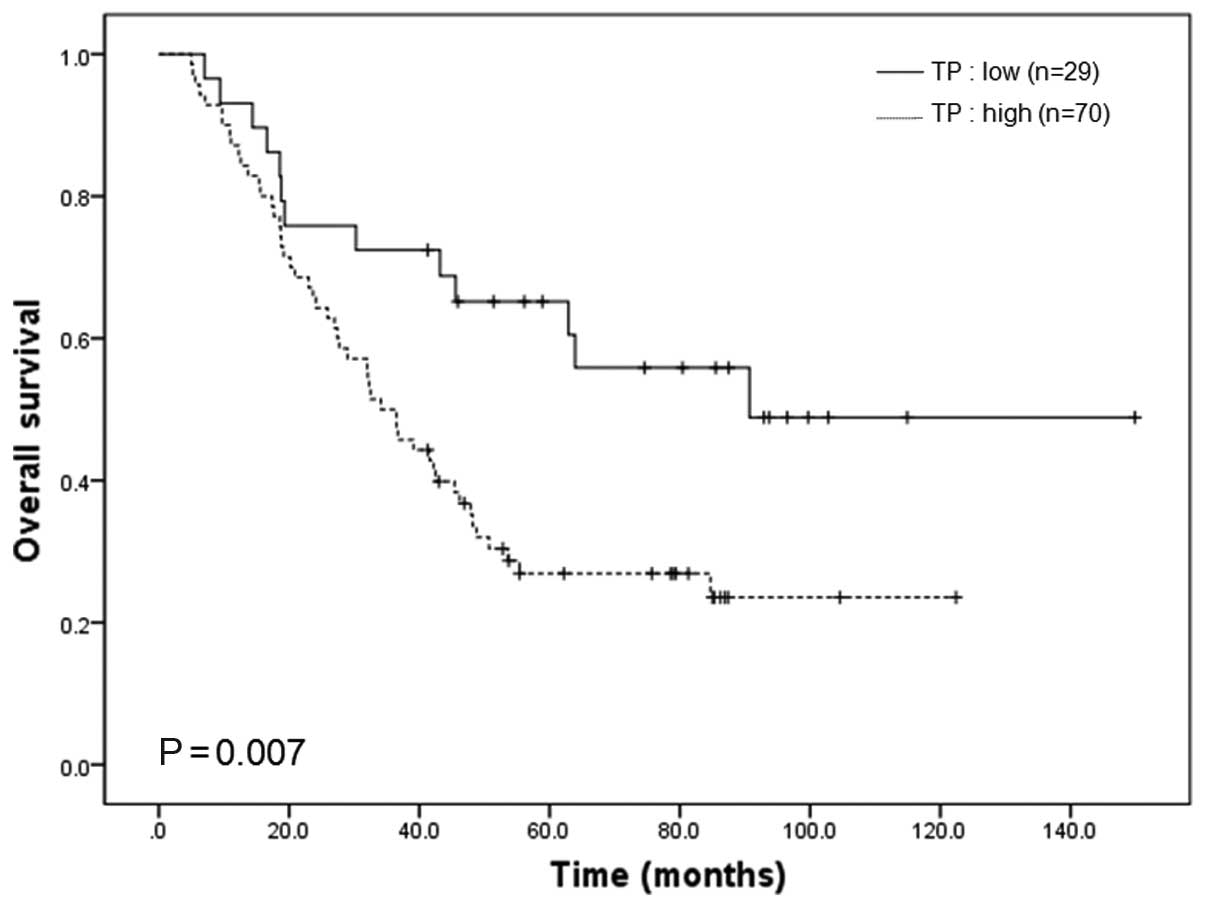
(P=0.222). Patients with high TP-expressing cancers had a
significantly shorter OS compared to those with low TP-expressing
cancers (P=0.007, Fig. 2). DPD
expression was of no prognostic significance in those patients
(P=0.192).
To assess the independent prognostic value of these
markers, a multivariate Cox proportional hazard analysis was used
to control for other prognostic factors (Table III). Accordingly, age [hazard ratio
(HR)=2.157; 95% confidence interval (CI): 1.217–3.822; P=0.008],
stage (HR=2.234; 95% CI: 1.462–3.414; P<0.001), differentiation
(HR=2.566; 95% CI: 1.818–3.620; P<0.001) and resection margin
status (HR=2.748; 95% CI: 1.391–5.432; P=0.004), were independent
prognostic factors for OS after controlling for the other
clinicopathological parameters. Expression of TP also exerted a
significant effect on OS in the multivariate analysis (HR=2.014;
95% CI: 1.035–3.921; P=0.039).
 | Table III.Multivariate analysis of overall
survival. |
Table III.
Multivariate analysis of overall
survival.
| Variable | HR | 95% CI | P-value |
|---|
| Age | 2.157 | 1.217–3.822 | 0.008 |
| Location | 1.332 | 0.909–1.952 | 0.141 |
| Stage | 2.234 | 1.462–3.414 | <0.001 |
|
Differentiation | 2.566 | 1.818–3.620 | <0.001 |
| Resection
margin | 2.748 | 1.391–5.432 | 0.004 |
| Thymidine
phosphorylase | 2.014 | 1.035–3.921 | 0.039 |
| Chemotherapy
regimen | 0.669 | 0.349–1.284 | 0.227 |
Discussion
BTC is a heterogeneous disease and its prognosis
varies according to tumor location (1,3). It
is difficult to elucidate the efficacy of adjuvant chemotherapy for
each type of tumor, due to the limited number of patients (4,5). To
the best of our knowledge, there are currently only two RCTs
available on adjuvant chemotherapy in BTC patients (6,7).
In a previous study by Takada et al (6), patients were randomly assigned to
postoperative chemotherapy (mitomycin C and 5-FU) or surgery alone
groups. The trial included 118 patients with BTC and 112 patients
with gallbladder cancer. The results demonstrated that adjuvant
chemotherapy did not significantly improve the 5-year survival in
either the curative (41% chemotherapy arm vs. 28% surgery alone,
P=0.48) or the non-curative resection patients (8% chemotherapy arm
vs. 16% surgery alone, P=0.30). In the per-protocol analysis, the
5-year survival rate for gallbladder cancer patients was
significantly higher with chemotherapy compared to the control arm
(26 vs. 14%; P=0.0367), However, the intent-to-treat analysis
identified no significant difference in the 5-year survival rate of
gallbladder cancer patients between the chemotherapy and
observation groups. In addition, the 5-year survival rate did not
differ between bile duct and ampullary carcinoma patients.
The results of the ESPAC-3 periampullary cancer
randomized trial, which was a multicenter RCT on adjuvant
chemotherapy (5-FU plus folinic acid or gemcitabine) vs.
observation in patients with ampullary cancer, were recently
reported (7). The median survival
time of the patients in the group who received chemotherapy after
curative resection was not significantly different from that of the
patients in the surgery alone group (43.1 vs. 35.2 months,
respectively, P=0.25). However, the multivariate analysis adjusted
for prognostic factors revealed a significant survival benefit
associated with chemotherapy (HR=0.75; 95% CI: 0.57–0.98; P=0.03).
It was concluded that adjuvant chemotherapy moderately improved
survival in periampullary cancer patients. BTC patients were
treated with FP or doxifluridine and a longer OS was achieved in
the FP chemotherapy group (P=0.015). However, in the multivariate
analysis, a regimen of chemotherapy was not an independent risk
factor (HR=0.669, P=0.227), since elderly patients and
lymphovascular invasion-positive patients were more commonly
administered oral doxifluridine.
A previous meta-analysis revealed an insignificant
benefit for adjuvant therapy in unselected BTC patients (4). However, in subgroups of high-risk
patients, such as lymph node-positive disease (HR=0.49, P=0.004)
and R1 disease (HR=0.36, P=0.002), postoperative adjuvant therapy
appeared to be beneficial. Our data also demonstrated that TNM
stage, differentiation and positive resection margin status were
correlated with OS. Establishing predictive markers for
chemosensitivity, other than the clinicopathological findings, may
contribute to effective adjuvant chemotherapy administration in
patients with a high risk of recurrence. Advances in molecular
pharmacology have refined our understanding of the mechanisms of
action of 5-FU, as well as the mechanisms underlying resistance to
chemotherapy (11). A previous
study by Salonga et al (17) reported that TS, DPD and TP are
independent predictive markers of 5-FU response and that the
measurement of the three markers markedly enhanced the ability to
predict tumor response to 5-FU-based chemotherapy.
The primary biochemical mechanism responsible for
the cytotoxicity of 5-FU is the formation of 5-fluorouridine
monophosphate (FdUMP), which binds closely to and inhibits TS in
the presence of 5,10-methylene tetrahydrofolate. TS catalyzes the
reductive methylation of deoxyuridine-5′-monophosphate (dUMP) to
deoxythymidine-5′-monophosphate (dTMP), which is the only pathway
for the de novo synthesis of dTMP. Therefore, inhibition of
TS by FdUMP disrupts the intracellular nucleotide pools necessary
for DNA synthesis (11,21). Gene amplification of TS, with
consequent increases in TS mRNA and protein expression, has been
observed in cell lines that are resistant to 5-FU (22).
Previous clinical studies measured TS expression by
immunohistochemistry and reverse-transcription PCR and demonstrated
an improved clinical outcome with 5-FU-based therapy in patients
with a low TS expression (23,24).
A previous meta-analysis also reported an HR of 1.35 for a high TS
expression in 2,610 patients with localized colorectal cancer
(13). However, other studies
reported discordant results in gastric cancer patients (14,15).
TS expression in stage III–IV gastric cancer patients who received
curative surgery followed by adjuvant chemotherapy was not
associated with clinical outcomes (14).We also previously demonstrated that
TS expression was not correlated with chemotherapeutic response or
OS in metastatic gastric cancer patients (15) and that TS expression did not
exhibit a statistically significant association with OS. Patients
with lower TS expression levels exhibited a trend to correlate with
a shorter OS compared to those with higher TS expression levels OS
(P=0.222). Such conflicting findings may, in part, be due to the
wide variation in immunohistochemical protocols and the use of
different antibodies to detect p53.
The prognostic and predictive value of DPD was
previously investigated. According to a previous study, low
intratumoral DPD gene expression is associated with improved
response to 5-FU (17). These
findings presumably reflect a higher DPD-mediated degradation of
5-FU in these tumors. Findings of a previous study demonstrated
that a low DPD expression was associated with better survival in
stage III colorectal cancer patients treated with 5-FU chemotherapy
(10). However, the majority of
published studies reported no significant association between DPD
expression and prognosis (25). In
this study, DPD expression was not found to be clinically
significant in BTC.
TP converts 5′-DFUR to the active drug 5-FU by
cleaving the 5-deoxyribose moiety, whereas, through the addition of
2′-deoxyribose-1-phosphate, TP may activate 5-FU to
5-fluoro-2′-deoxyuridine, a precursor of FdUMP, which inhibits TS,
responsible for de novo thymidylate synthesis (11). It was previously demonstrated that
high levels of TP affected 5-FU sensitivity and patients with a
high TP expression exhibited higher survival rates (18). However, the elucidation of the role
of TP in modulating 5-FU responsiveness has been challenging, due
to contradictory preclinical and clinical data. According to
previous findings, a high TP expression correlated with low
sensitivity to 5-FU (17).
TP was found to possess angiogenic properties and to
promote tumor growth. TP was found to be strongly angiogenic in a
rat sponge and freeze-injured skin graft model, whereas the
overexpression of TP in MCF-7 breast cancer cells markedly enhanced
tumor growth in vivo(26).
Consistent with the hypothesis that increased tumor vascularization
leads to a greater metastatic propensity, higher TP levels were
found to be associated with more aggressive bladder tumors
(27). According to evidence
reported in the literature, high TP expression levels are
associated with a negative prognosis (25). According to a recent study, a low
TP expression was associated with a trend for prolonged survival in
stage III colorectal cancer treated with 5-FU, indicative of the
response to chemotherapy in those patients (10). Therefore, a high TP expression may
be a marker for a more invasive and aggressive tumor phenotype that
is less responsive to chemotherapy. Our results have also
demonstrated that TP expression was more common in extrahepatic
bile duct cancer patients and there was a significant correlation
between TP expression levels and OS in BTC cancer patients
receiving postoperative FU-based adjuvant chemotherapy.
In conclusion, based on these findings, an
immunohistochemical evaluation of TP expression may be beneficial
in predicting the survival of BTC patients treated with 5-FU-based
adjuvant chemotherapy. Confirmation of these results by a clinical
trial with a larger patient sample may provide a promising
selection tool for the most appropriate chemotherapeutic regimen in
BTC patients.
Acknowledgements
This study was supported by the Dong-A
University Research Fund.
References
|
1.
|
de Groen PC, Gores GJ, LaRusso NF,
Gunderson LL and Nagorney DM: Biliary tract cancers. N Engl J Med.
341:1368–1378. 1999.
|
|
2.
|
Jung KW, Park S, Kong HJ, et al: Cancer
statistics in Korea: incidence, mortality, survival, and prevalence
in 2009. Cancer Res Treat. 44:11–24. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Miyakawa S, Ishihara S, Horiguchi A,
Takada T, Miyazaki M and Nagakawa T: Biliary tract cancer
treatment: 5,584 results from the Biliary Tract Cancer Statistics
Registry from 1998 to 2004 in Japan. J Hepatobiliary Pancreat Surg.
16:1–7. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Horgan AM, Amir E, Walter T and Knox JJ:
Adjuvant therapy in the treatment of biliary tract cancer: a
systematic review and meta-analysis. J Clin Oncol. 30:1934–1940.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Cereda S, Belli C and Reni M: Adjuvant
treatment in biliary tract cancer: to treat or not to treat? World
J Gastroenterol. 18:2591–2596. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Takada T, Amano H, Yasuda H, et al: Is
postoperative adjuvant chemotherapy useful for gallbladder
carcinoma? A phase III multicenter prospective randomized
controlled trial in patients with resected pancreaticobiliary
carcinoma. Cancer. 95:1685–1695. 2002. View Article : Google Scholar
|
|
7.
|
Neoptolemos JP, Moore MJ, Cox TF, et al:
Effect of adjuvant chemotherapy with fluorouracil plus folinic acid
or gemcitabine vs. observation on survival in patients with
resected periampullary adenocarcinoma: the ESPAC-3 periampullary
cancer randomized trial. JAMA. 308:147–156. 2012. View Article : Google Scholar
|
|
8.
|
Namwat N, Amimanan P, Loilome W, et al:
Characterization of 5-fluorouracil-resistant cholangiocarcinoma
cell lines. Chemotherapy. 54:343–351. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Yamada H, Iinuma H and Watanabe T:
Prognostic value of 5-fluorouracil metabolic enzyme genes in Dukes’
stage B and C colorectal cancer patients treated with oral
5-fluorouracil-based adjuvant chemotherapy. Oncol Rep. 19:729–735.
2008.PubMed/NCBI
|
|
10.
|
Soong R, Shah N, Salto-Tellez M, et al:
Prognostic significance of thymidylate synthase, dihydropyrimidine
dehydrogenase and thymidine phosphorylase protein expression in
colorectal cancer patients treated with or without
5-fluorouracil-based chemotherapy. Ann Oncol. 19:915–919. 2008.
View Article : Google Scholar
|
|
11.
|
Longley DB, Harkin DP and Johnston PG:
5-Fluorouracil: mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Marsh S and McLeod HL: Cancer
pharmacogenetics. Br J Cancer. 90:8–11. 2004. View Article : Google Scholar
|
|
13.
|
Popat S, Matakidou A and Houlston RS:
Thymidylate synthase expression and prognosis in colorectal cancer:
a systematic review and meta-analysis. J Clin Oncol. 22:529–536.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Kim JS, Kim MA, Kim TM, et al: Biomarker
analysis in stage III–IV (M0) gastric cancer patients who received
curative surgery followed by adjuvant 5-fluorouracil and cisplatin
chemotherapy: epidermal growth factor receptor (EGFR) associated
with favourable survival. Br J Cancer. 100:732–738. 2009.
|
|
15.
|
Kwon HC, Roh MS, Oh SY, et al: Prognostic
value of expression of ERCC1, thymidylate synthase, and glutathione
S-transferase P1 for 5-fluorouracil/oxaliplatin chemotherapy in
advanced gastric cancer. Ann Oncol. 18:504–509. 2007. View Article : Google Scholar
|
|
16.
|
Takebe N, Xu LC, MacKenzie KL, Bertino JR
and Moore MA: Methotrexate selection of long-term culture
initiating cells following transduction of CD34+ cells
with a retrovirus containing a mutated human dihydrofolate
reductase gene. Cancer Gene Ther. 9:308–320. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Salonga D, Danenberg KD, Johnson M, et al:
Colorectal tumors responding to 5-fluorouracil have low gene
expression levels of dihydropyrimidine dehydrogenase, thymidylate
synthase, and thymidine phosphorylase. Clin Cancer Res.
6:1322–1327. 2000.
|
|
18.
|
Hasegawa S, Seike K, Koda K, et al:
Thymidine phosphorylase expression and efficacy of adjuvant
doxifluridine in advanced colorectal cancer patients. Oncol Rep.
13:621–626. 2005.PubMed/NCBI
|
|
19.
|
Brown NS and Bicknell R: Thymidine
phosphorylase, 2-deoxy-D-ribose and angiogenesis. Biochem J. 334(Pt
1): 1–8. 1998.PubMed/NCBI
|
|
20.
|
Takebayashi Y, Akiyama S, Akiba S, et al:
Clinicopathologic and prognostic significance of an angiogenic
factor, thymidine phosphorylase, in human colorectal carcinoma. J
Natl Cancer Inst. 88:1110–1117. 1996. View Article : Google Scholar
|
|
21.
|
Diasio RB and Harris BE: Clinical
pharmacology of 5-fluorouracil. Clin Pharmacokinet. 16:215–237.
1989. View Article : Google Scholar
|
|
22.
|
Johnston PG, Drake JC, Trepel J and
Allegra CJ: Immunological quantitation of thymidylate synthase
using the monoclonal antibody TS 106 in 5-fluorouracil-sensitive
and -resistant human cancer cell lines. Cancer Res. 52:4306–4312.
1992.
|
|
23.
|
Edler D, Kressner U, Ragnhammar P, et al:
Immunohistochemically detected thymidylate synthase in colorectal
cancer: an independent prognostic factor of survival. Clin Cancer
Res. 6:488–492. 2000.PubMed/NCBI
|
|
24.
|
Lenz HJ, Hayashi K, Salonga D, et al: p53
point mutations and thymidylate synthase messenger RNA levels in
disseminated colorectal cancer: an analysis of response and
survival. Clin Cancer Res. 4:1243–1250. 1998.PubMed/NCBI
|
|
25.
|
Soong R and Diasio RB: Advances and
challenges in fluoropyrimidine pharmacogenomics and
pharmacogenetics. Pharmacogenomics. 6:835–847. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Moghaddam A, Zhang HT, Fan TP, et al:
Thymidine phosphorylase is angiogenic and promotes tumor growth.
Proc Natl Acad Sci USA. 92:998–1002. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
O’Brien TS, Fox SB, Dickinson AJ, et al:
Expression of the angiogenic factor thymidine
phosphorylase/platelet-derived endothelial cell growth factor in
primary bladder cancers. Cancer Res. 56:4799–4804. 1996.PubMed/NCBI
|