Introduction
Colorectal cancer is one of the leading causes of
cancer-related mortality, being the third most commonly diagnosed
cancer among men and the second among women (1). The incidence rates of colorectal
cancer are rapidly increasing in several areas that were
historically at minimal risk, including several countries within
Eastern Asia (2). Early diagnosis
and intervention may be crucial in improving therapeutic
effectiveness and prolonging survival time. In the initial stage of
tumorigenesis, tumor markers are widely used for diagnosis,
staging, and monitoring of colorectal cancer in clinical laboratory
tests (3). These markers are
usually proteins released from dying tumor cells or produced by
neoplastic cells. Certain specific proteins are expressed only in
tumor cells and are useful for the detection and diagnosis of
specific malignant tumors. Non-specific proteins or markers
associated with malignant tumor cells are oncofetal or carcinogenic
antigens, such as carcinoembryonic antigen (CEA), α-fetoprotein,
carbohydrate antigen 125, carbohydrate antigen 19-9 (CA19-9),
tissue plasminogen activator and tissue polypeptide-specific
antigen (4).
Koprowski et al (5) described CA19-9 as a monoclonal
antibody in 1979. Since then, CA19-9 has been increasingly used to
detect serum antigens associated with specific malignancies. It was
previously demonstrated that CA19-9 is produced by adenocarcinomas
of the pancreas, stomach, gallbladder, colon, ovary and lung and is
released into the circulation. Elevated serum CA19-9 levels have
been associated with a range of gastrointestinal malignant tumors,
including colorectal carcinoma. CA19-9 may also be useful in
determining the nature of pancreatic masses (6). Tumor markers are most useful for
monitoring the response to therapy and detecting early relapses.
Each tumor marker has a variable range of application for
screening, determining diagnosis and prognosis, assessing the
response to therapy and monitoring cancer recurrence. CEA is most
frequently used to detect gastrointestinal malignant tumors and the
variation of CEA values reflects individual response to clinical
therapy (7). By contrast, in the
screening of gastrointestinal malignancies, the American Society of
Clinical Oncology guidelines suggested that serum testing for
CA19-9 is an integral part of the diagnosis and management of
colorectal carcinomas (8).
Numerous studies addressed the potential utility of CA19-9
assessment in adenocarcinomas of the colon and rectum (9). The reported incidence of elevated
serum CA19-9 levels in colorectal cancer ranges from 20–40%
(10). The incidence of elevated
CA19-9 levels is stage-related, with the highest sensitivity
observed in patients with metastases. However, the sensitivity of
CA19-9 has always been lower compared to that of the CEA for all
the stages of this disease. The false-positive rate is 15–30% in
patients with non-neoplastic diseases of the pancreas, liver and
biliary tract. Consequently, CA19-9 may not be used for screening
asymptomatic populations (11,12).
Serum screening tests require sufficient specificity and high
sensitivity to detect early-stage carcinoma. Individuals who
undergo serum tests display varying results. Benign conditions,
such as cirrhosis, cholestasis, cholangitis and pancreatitis, may
also result in an elevation of the CA19-9 levels. False-positive
results prompt the research for more specific and sensitive tumor
markers.
Colorectal cancer is a major contributor to
cancer-related mortality and morbidity (1). The diagnosis and therapy of colon and
rectal cancer as a single entity has attracted considerable
attention. Although these two types of cancer have similar
etiology, incidence rates, surgical and radiotherapeutic management
implications, accumulating evidence reveals notable differences.
The differences between the colon and rectum are largely anatomical
and biological and may affect prognosis. Cancers of the colon and
rectum may develop differently due to their distinctive
embryological origin (midgut/hindgut and hindgut, respectively) and
differential exposure to bowel content. Furthermore, colon and
rectal cancers have differences regarding anatomy and blood
circulation. Venous blood from the colon flows to the liver via the
portal vein, whereas rectal venous blood partially bypasses the
liver. Blood circulation often affects tumor relapse. Rectal
cancers exhibit higher rates of localized regional relapse and lung
metastases, whereas colon cancers have a higher tropism for liver
spread (13). The serum
concentration of tumor markers may be affected by metabolism in the
liver. This may explain the differential expression of CA19-9
between these two malignancies. There is also a difference in
clinical presentation, prognosis and, possibly, in genetic and
environmental epidemiology (14).
The differential behavior of single molecules in colon and rectal
tumors may help elucidate the molecular basis of these two types of
cancer and their prognostic and therapeutic implications (15). Despite clinical evidence of the
differences between colon and rectal cancer, the number of studies
that have addressed the molecular differences between the two
diseases is limited. Through the analysis of several molecular
markers, Kapitejin et al (16) demonstrated a significantly
different β-catenin and p53 expression between colon and rectal
cancers and concluded that these two malignancies may follow
different mechanisms of oncogenesis (17). Furthermore, the analysis of KRAS
mutations revealed that they are more specifically detectable in
colon compared to rectal cancer (18). As regards epidemiological,
morphological and molecular characteristics, the mechanisms of
colorectal carcinogenesis may differ according to tumor location.
It was suggested that a mechanism exists that promotes the
progression of mucosal lesions to invasive cancers in the colon and
rectum (19). Therefore, we
decided to investigate whether there are differences in the serum
levels of CA19-9 between patients with colon and those with rectal
cancer.
In our study, a differential analysis of serum
CA19-9 levels according to gender, age, Dukes’ stage and distant
metastasis for human colon and rectal cancer was conducted. As a
significant predictor for colorectal cancer invasion and
metastasis, serum CA19-9 levels in colon cancer displayed a notable
upregulated behavior in advanced stages of the tumor with distant
metastasis. By contrast, the upregulated serum CA19-9 levels in the
early stages of rectal cancer without distant metastasis further
supported our hypothesis that the expression of CA19-9 displayed a
site-specific differential behavior. The integrative analysis
suggested a significant difference between colon and rectal cancer
and also indicated an important role for CA19-9 in early diagnosis
and individualized therapy of human colorectal cancer.
Materials and methods
Patient specimens
Preoperative serum samples were obtained from 227
patients (135 men and 92 women) with histologically verified
colorectal cancer. The patients were classified into the younger
group (<60 years old, 90 cases) and the elder group (≥60 years
old, 137 cases). As the focus of our study, 116 colon and 111
rectal cancers were staged according to the modified Dukes’
classification (stage A, 54; stage B, 51; stage C, 57; and stage D,
65 cases). As mentioned above, 116 patients had colon cancer (30
patients had stage A, 28 stage B, 28 stage C and 30 stage D) and
111 patients had rectal cancer (24 patients had stage A, 23 stage
B, 29 stage C and 35 stage D). According to our statistics,
specimens with Dukes’ stage A, B and C colorectal cancer did not
exhibit significant differences. However, patients with Dukes’
stage D disease were quite different. Therefore, patients with
Dukes’stages A–C were considered as having early-stage disease,
whereas those with Dukes’ stage D were considered as having
advanced-stage disease. Patients in the distant metastasis group
had either lymph node or distant metastases. The CA19-9 values were
obtained from the serum of the patients who underwent surgical
resection at the First and Second Affiliated Hospitals of Dalian
Medical University beteween 2010 and 2012.
Serum collection and CA19-9 assay
The preoperative serum samples were obtained prior
to the administration of radiation treatment or chemotherapy. Blood
samples were collected, separated by centrifugation and the serum
samples were stored at −20°C until the assays were performed. The
CA19-9 kit was provided by Diagnostic Products Corporation (DPC,
Tianjin, China). The serum CA19-9 levels were determined by the DPC
Gamma C12 immunoradiometric gamma counter (DPC). Data on patient
specimens were furnished by the First and Second Affiliated
Hospitals of Dalian Medical University between 2010 and 2012.
Statistical analysis
A differential analysis of the 227 samples according
to serum CA19-9 levels, gender, age, Dukes’ stage and metastasis
was separately conducted for human colon and rectal cancer. The
serum levels of CA19-9 did not follow a normal distribution and the
significance between the groups was calculated by non-parametric
statistical methods (Mann-Whitney and Kruskall-Wallis tests). The
centralized tendency of each group was described by geometric mean
due to the right skewness of the frequency distribution. The
statistical analysis was performed with SPSS software for Windows,
version 11.5 (SPSS Inc., Chicago, IL, USA). Our results were
accurate to four digits. P<0.05 was considered to indicate a
statistically significant difference.
Results
Upregulated serum CA19-9 levels in
colorectal cancer with advanced stage and distant metastasis
A total of 227 colorectal cancer patients, including
116 colon and 111 rectal cancer cases, were clinically diagnosed by
imaging and histopathology. For the mean values (mean ± SEM) of the
CA19-9 levels, there was no statistical difference between the sera
collected from colon cancer (45.85±11.05 U/ml) and those collected
from rectal cancer patients (44.88±9.150 U/ml) (P= 0.9467).
Therefore, we analyzed them first as a single entity (Table I and Fig. 1). We observed that the serum CA19-9
levels between the gender groups (135 men and 92 women) and the age
groups (90 patients included in the younger and 137 patients in the
elder group) exhibited no statistically significant difference
(P>0.05). However, the mean values of serum CA19-9 levels
exhibited a significant correlation with Dukes’ stage (P=0.005) and
distant metastasis (P= 0.016). The mean values of the serum CA19-9
levels in patients with advanced-stage disease (74.30±11.29 U/ml)
and distant metastasis (71.33±18.49 U/ml) were more upregulated
compared to those in patients with early-stage disease (33.77±6.284
U/ml) and without distant metastasis (33.86±6.322 U/ml). The
expression of CA19-9 exhibited a tendency for increase, suggesting
that the mean values of serum CA19-9 levels reflected tumor
progression and were a significant predictor for colorectal
carcinoma invasion and metastasis (Table I and Fig. 1). This finding may be associated
with the function of CA19-9 in the differentiation and migration of
tumor cells.
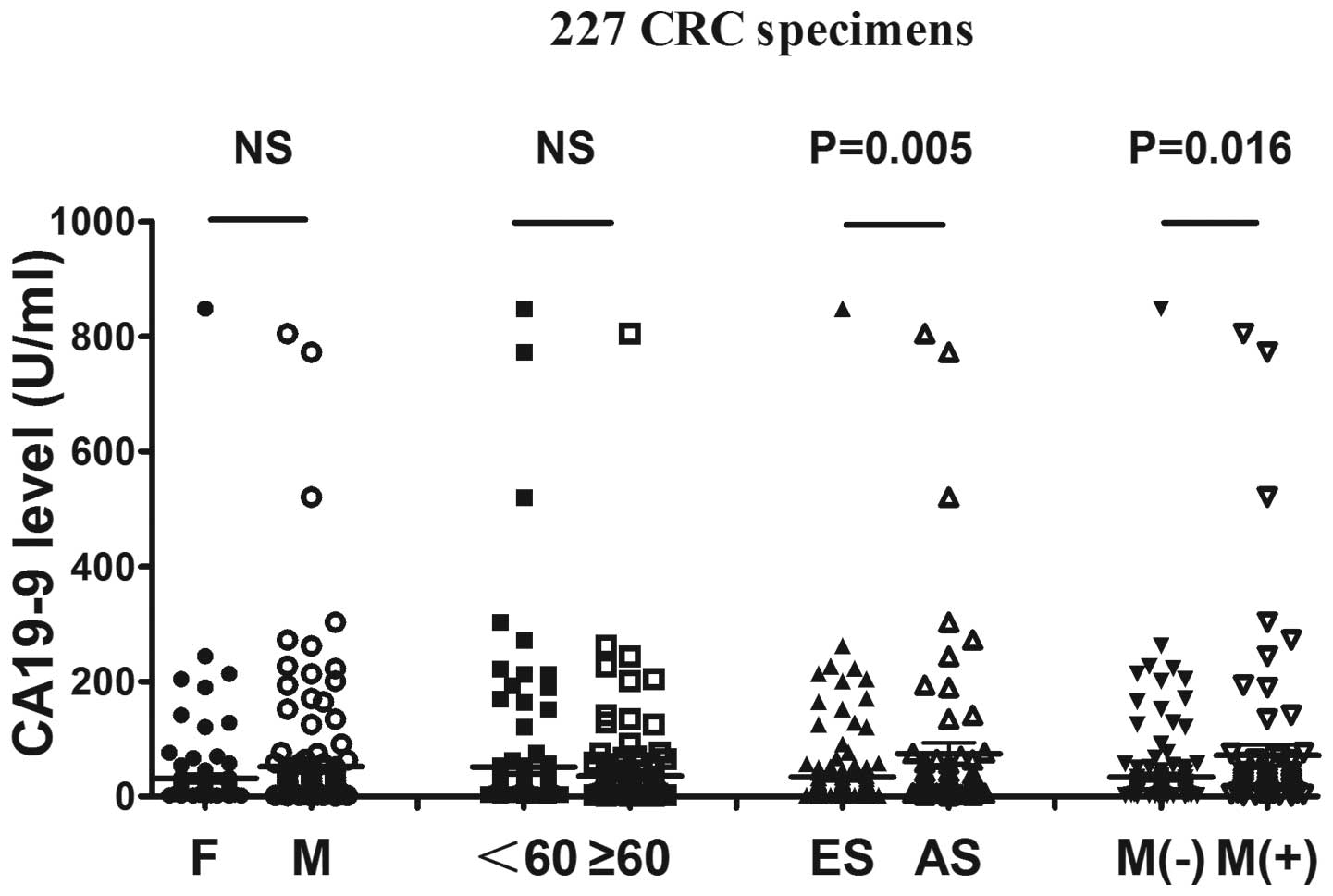 | Figure 1.Different mean values of carbohydrate
antigen 19-9 (CA19-9) among the 227 CRC specimens according to
different groups. The 227 CRC specimens were divided into 4 groups
according to gender, age (<60 and ≥60 years), Dukes’ stage and
metastasis. Although patients with different gender and age,
exhibited no statistically significant differences, the differences
between patients with early- and advanced-stage disease (P= 0.005)
and between patients with and those without metastasis (P=0.016)
were statistically significant. The results indicated that high
CA19-9 values were more significant for patients with Dukes’ stage
D disease or with metastasis. Furthermore, the expression of CA19-9
was increased during tumorigenesis. CRC, colorectal cancer; F,
female; M, male; <60, patients younger than 60 years; ≥60,
patients older than 60 years; ES, early stage (Dukes’ A, B and C);
AS, advanced stage (Dukes’ D); M(−), patients without metastasis;
M(+), patients with metastasis; NS, difference without statistical
significance. |
 | Table I.Comparison of serum CA19-9 levels in
colorectal cancer among different groups. |
Table I.
Comparison of serum CA19-9 levels in
colorectal cancer among different groups.
| Pathological
characteristics | No. | Median CA19-9
(U/ml) | P-value |
|---|
| Gender | | | 0.615 |
| Male | 135 | 15.30 | |
| Female | 92 | 13.48 | |
| Age (years) | | | 0.665 |
| <60 | 90 | 14.65 | |
| >60 | 137 | 14.20 | |
| Dukes’ stage | | | 0.005a |
| Early | 162 | 12.75 | |
| Advanced | 65 | 18.64 | |
| Distant
metastasis | | | 0.016a |
| − | 161 | 12.70 | |
| + | 66 | 18.06 | |
Upregulated serum CA19-9 levels in colon
cancer, but not in rectal cancer, with advanced stage and distant
metastasis
A number of studies indicated the differences
between colon and rectal cancer (14,16,21,24);
therefore, we conducted further separate analyses of the serum
CA19-9 levels in colon and rectal cancer (Tables II and III, Fig.
2). In colon cancer specimens, the results demonstrated that
the serum CA19-9 levels in colon cancer were significantly
correlated with Dukes’ tumor stage (P=0.002) and distant metastasis
(P=0.003). The upregulated gradient of CA19-9 levels was quite
notable. The mean CA19-9 values in patients with advanced-stage
disease (113.7±25.32 U/ml) and distant metastasis (111.1±25.03
U/ml) were significantly higher compared to those in patients with
early-stage disease (19.86±2.468 U/ml) and without distant
metastasis (19.77±2.481 U/ml). Similar to the holistic analyses of
colorectal cancer, the differences in colon cancer specimens
between gender and age groups were not statistically significant.
We confirmed that CA19-9 expression in colon cancer patients was
significantly upregulated during the process of tumorigenesis and
metastasis (Table II and Fig. 2A). However, the mean values of
serum CA19-9 levels in rectal cancer displayed no statistically
significant difference among any of the 4 groups (Table III and Fig. 2B). This finding indicated that
CA19-9 expression was not only associated with invasion and
metastasis, but also varied with tumor location. The mean CA19-9
values in patients with advanced-stage disease (74.30±19.27 U/ml)
and distant metastasis (73.48±19.00 U/ml) were significantly higher
compared to those in patients with early-stage disease (33.75±6.284
U/ml) and without distant metastasis (33.86±6.322 U/ml).
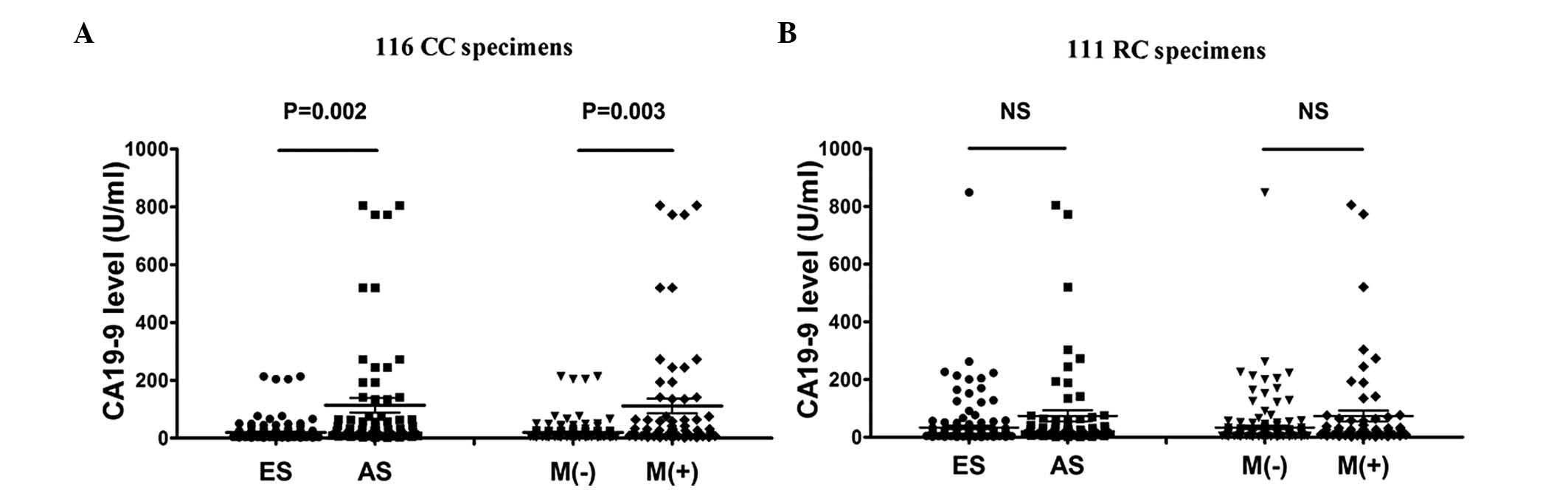 | Figure 2.Different mean values of carbohydrate
antigen 19-9 (CA19-9) among the 116 CC and the 111 RC specimens
separately. The 227 CRC specimens included 116 CC and 111 RC
patients. The 116 CC and 111 RC specimens were separately divided
into 4 groups according to gender, age (<60 and ≥60 years),
Dukes’ stage and metastasis. (A) The differences between early- and
advanced-stage disease (P=0.002) and the differences between
patients with and those without metastasis (P=0.003) in the 116 CC
specimens were statistically significant. There were no significant
differences regarding the remaining two variables. (B) Among the
111 RC patients divided into the 4 different groups, there were no
statistically significant differences in the mean CA19-9 values.
CC, colon cancer; RC, rectal cancer; CRC, colorectal cancer;
<60, patients younger than 60 years; ≥60, patients aged 60 years
or older; ES, early stage (Dukes’ A, B and C); AS, advanced stage
(Dukes’ D); M(−), patients without metastasis; M(+), patients with
metastasis; NS, difference without statistical significance. |
 | Table II.Comparison of serum CA19-9 levels in
colon cancer among different groups. |
Table II.
Comparison of serum CA19-9 levels in
colon cancer among different groups.
| Pathological
characteristics | No. | Median CA19-9
(U/ml) | P-value |
|---|
| Gender | | | 0.960 |
| Male | 68 | 12.55 | |
| Female | 48 | 12.35 | |
| Age (years) | | | 0.522 |
| <60 | 42 | 10.03 | |
| >60 | 74 | 13.28 | |
| Dukes’ stage | | | 0.002a |
| Early | 86 | 11.38 | |
| Advanced | 30 | 28.99 | |
| Distant
metastasis | | | 0.003a |
| − | 84 | 11.38 | |
| + | 32 | 26.38 | |
 | Table III.Comparison of serum CA19-9 levels in
rectal cancer among different groups. |
Table III.
Comparison of serum CA19-9 levels in
rectal cancer among different groups.
| Pathological
characteristics | No. | Median CA19-9
(U/ml) | P-value |
|---|
| Gender | | | 0.583 |
| Male | 67 | 17.11 | |
| Female | 44 | 14.77 | |
| Age (years) | | | 0.270 |
| <60 | 48 | 16.98 | |
| >60 | 63 | 15.30 | |
| Dukes’ stage | | | 0.570 |
| Early | 76 | 15.21 | |
| Advanced | 35 | 17.35 | |
| Distant
metastasis | | | 0.452 |
| − | 77 | 15.11 | |
| + | 34 | 17.41 | |
Differential expression of serum CA19-9
levels between colon and rectal cancer in early-stage disease
without distant metastasis
Previous studies indicated that colon and rectal
cancer were similar but different types of cancer (14,16,24,26).
Therefore, we continued our study by analyzing colon and rectal
cancer as matched pairs (Table IV
and Fig. 3). According to our
statistical data, the mean values of serum CA19-9 levels in
early-stage disease (P=0.015) and without distant metastasis
(P=0.021) were significantly more upregulated in rectal compared to
colon cancer, with a statistically significant difference (Table IV and Fig. 3B). There was no distinction between
colon and rectal cancer in the cohort with gender and age groups
(Table IV and Fig. 3A). However, the serum CA19-9 levels
in rectal cancer patients with early-stage disease (76 patients)
was significantly more upregulated compared to those in colon
cancer patients with the same Dukes’ stage (86 patients) (P=0.015).
In addition, the CA19-9 levels in rectal cancer patients without
distant metastasis (including 84 colon cancer and 77 rectal cancer
patients) was also statistically more upregulated compared to those
in colon cancer patients in the same matched pairs (P=0.021)
(Table IV and Fig. 3B). These results demonstrated that
the expression of CA19-9 displayed a site-specific differential
behavior. Moreover, they suggested that serum CA19-9 levels may be
more sensitive in the early diagnosis of rectal cancer. The
differential expression of CA19-9 during the early stages of
tumorigenesis also indicates the distinction between colon and
rectal carcinomas.
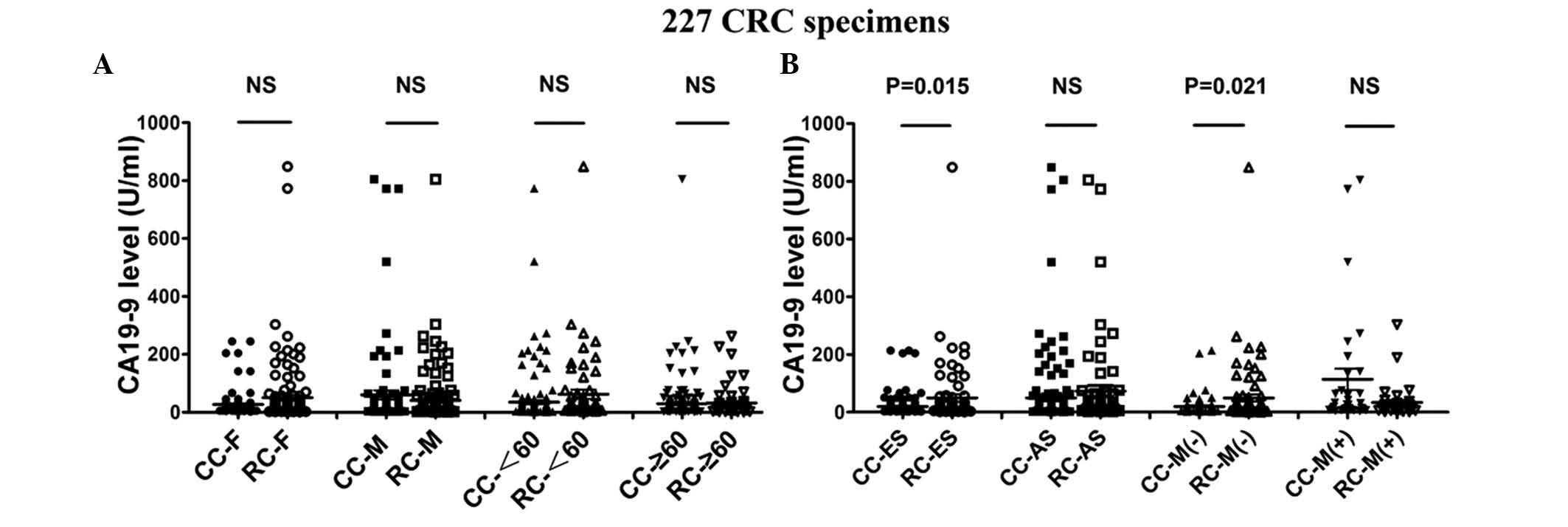 | Figure 3.Different mean values of carbohydrate
antigen 19-9 (CA19-9) between CC and RC patients according to
gender, age, Dukes’ stage and metastasis. A total of 227 CRC
patients, including 116 CC and 111 RC patients, were divided into 4
groups according to gender, age (<60 and ≥60 years), Dukes’
stage and metastasis. Furthermore, CA19-9 values were compared
between CC and RC as different aspects of tumor location. Among the
patients of different gender and age, female RC and male CC
patients exhibited a tendency for high CA19-9 levels. Of note, the
two age groups exhibited high mean CA19-9 values in RC patients.
However, there were no significant differences in the mean CA19-9
values between gender and age difference groups (A) Among the
patients with advanced Dukes’ stage and metastasis, although CC
patients exhibited higher CA19-9 levels compared to RC patients
during the same period, the differences were not statistically
significant. Among these 4 groups, we demonstrated that RC patients
with early-stage disease (P=0.0015) and without metastasis (P=
0.0021) exhibited statistically significant higher mean CA19-9
values compared to CC patients. CC, colon cancer; RC, rectal
cancer; CRC, colorectal cancer; F, female; M, male; <60,
patients younger than 60 years; ≥60, patients aged 60 years or
older; ES, early stage (Dukes’ A, B and C); AS, advanced stage
(Dukes’ D); M(−), patients without metastasis; M(+), patients with
metastasis; NS, difference without statistical significance. |
 | Table IV.Comparison of serum carbohydrate
antigen 19-9 (CA19-9) levels between colon and rectal cancer. |
Table IV.
Comparison of serum carbohydrate
antigen 19-9 (CA19-9) levels between colon and rectal cancer.
| Pathological
characteristics | Cases
| Median CA19-9
(U/ml)
| P-value |
|---|
| Colon cancer | Rectal cancer | Colon cancer | Rectal cancer |
|---|
| Gender | | | | | |
| Male | 68 | 67 | 12.55 | 17.11 | 0.165 |
| Female | 48 | 44 | 12.35 | 14.77 | 0.298 |
| Age (years) | | | | | |
| <60 | 42 | 48 | 10.03 | 16.98 | 0.075 |
| >60 | 74 | 63 | 13.28 | 15.30 | 0.433 |
| Dukes’ stage | | | | | |
| Early | 86 | 76 | 11.38 | 15.21 | 0.015a |
| Advanced | 30 | 35 | 28.99 | 17.35 | 0.231 |
| Distant
metastasis | | | | | |
| − | 84 | 77 | 11.38 | 15.11 | 0.021a |
| + | 32 | 34 | 26.38 | 17.41 | 0.346 |
Discussion
Similar to other tumor-associated antigens, it
appears that elevated serum CA19-9 levels are associated with
gastrointestinal malignancies, particularly advanced colorectal
cancer. Of more relevance to the potential use of CA19-9 as a
screening test is the comparison of CA19-9 serum levels between
individuals with colon and rectal cancer. To identify possible
biological differences between colon and rectal tumors, we
conducted a differential analysis of 227 specimens, analyzing serum
CA19-9 levels according to gender, age, Dukes’ stage and distant
metastasis in human colon and rectal cancer. We demonstrated that
the serum CA19-9 levels in colorectal cancer of advanced stage and
with distant metastasis were significantly upregulated, suggesting
that the expression of CA19-9 reflects tumor invasion and
metastasis. Correspondingly, we confirmed that serum CA19-9 levels
displayed a notable upregulation in colon cancer specimens of
advanced stage and with distant metastasis. However, we failed to
demonstrate this upregulation of CA19-9 in rectal cancer specimens,
suggesting that colon and rectal cancer are similar but different
types of cancer. In our continued comparison, by analyzing colon
and rectal cancer as matched pairs, the serum CA19-9 levels in
early-stage disease and without distant metastasis exhibited
statistically more significant upregulation in rectal compared to
colon cancer. It was evident that there was no distinction between
colon and rectal cancer in the cohort of gender and age groups.
These results further supported our hypothesis that the expression
of CA19-9 displayed a site-specific behavior.
The issue of whether colon and rectal cancer should
be considered as a single entity or two distinct entities is still
debated upon. There are differences between colon and rectal cancer
with respect to patient gender and age, as well as tumor
progression and adjuvant treatments (17). Despite a similar etiology and
cancer incidence rates, the anatomical and clinical distinction
should not be overlooked. A previous study attempted to identify
and characterize the genetic changes involved in the colorectal
malignant transformation process (18). Through the analysis of several
molecular markers, Kapitejin et al demonstrated a
significantly different β-catenin and p53 expression between colon
and rectal cancers and concluded that these two types of cancer may
follow separate mechanisms of oncogenesis (16). Furthermore, several critical genes
and pathways have been shown to be involved in the initiation and
progression of colorectal cancer. Large-scale sequencing analyses
identified numerous recurrently mutated genes and a recurrent
chromosomal translocation (20–22).
These include the cyclin A2, COX2, RAS-MAPK, PI3K, TGF-β, p53 and
DNA mismatch-repair pathways (22). Moreover, colon cancers exhibit a
higher number of mutations, including KRAS and BRAF mutations
(15). The CIN pathway is far more
common in rectal compared to colon cancers (24,25).
In addition, several homeobox genes were found to be associated
with tumor location (26). A
different number of mutations induce varying mechanisms of
oncogenesis in colon and rectal cancer. The site-specific
differential behavior of CA19-9 in colon and rectal carcinoma
demonstrated a different tumor identification and progression.
It was previously demonstrated that the serum CA19-9
levels were the most significant prognostic indicator of patients
with metastatic colorectal cancer (27). Our results, although similar,
differed in detail. The serum CA19-9 levels were significantly
upregulated in association with advanced-stage disease and distant
metastasis in colon cancer, but not in rectal cancer. In our
continued study, serum CA19-9 levels in early-stage disease and
without distant metastasis were statistically significantly more
upregulated in rectal cancer compared to colon cancer, suggesting
that CA19-9 is more sensitive for early diagnosis of rectal cancer.
The differential expression of CA19-9 between colon and rectal
cancer further supports that colon and rectal cancer are similar
but different types of cancer.
In conclusion, our study strongly suggests that the
expression of CA19-9 displays a site-specific differential
behavior. The internal mechanism underlying our results has not
been elucidated, although a consistent difference was observed
between colon and rectal cancer. This observation indicates an
important role for CA19-9 in early diagnosis and tumor metastasis.
In our future study, we aim to recommend the individualization of
treatment for human colon and rectal cancer.
Acknowledgements
This study was supported by a grant
from NSFC (31270867), Chinese State Key Program in Basic Research
(2012CB822103) and Hall of Liaoning Province Science and Technology
Grant (2012225020).
References
|
1.
|
Weitz J, Koch M, Debus J, Höhler T, Galle
PR and Büchler MW: Colorectal cancer. Lancet. 365:153–165. 2005.
View Article : Google Scholar
|
|
2.
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
3.
|
Ludwig JA and Weinstein JN: Biomarkers in
cancer staging, prognosis and treatment selection. Nat Rev Cancer.
5:845–856. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Chan CC, Fan CW, Kuo YB, Chen YH, Chang
PY, Chen KT, Hung RP and Chan EC: Multiple serological biomarkers
for colorectal cancer detection. Int J Cancer. 126:1683–1690.
2010.PubMed/NCBI
|
|
5.
|
Koprowski H, Steplewski Z, Mitchell K,
Herlyn M, Herlyn D and Fuhrer P: Colorectal carcinoma antigens
detected by hybridoma antibodies. Somatic Cell Genet. 5:957–971.
1979. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Goonetilleke KS and Siriwardena AK:
Systematic review of carbohydrate antigen (CA 19-9) as a
biochemical marker in the diagnosis of pancreatic cancer. Eur J
Surg Oncol. 33:266–270. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Yakabe T, Nakafusa Y, Sumi K, Miyoshi A,
Kitajima Y, Sato S, Noshiro H and Miyazaki K: Clinical significance
of CEA and CA19-9 in postoperative follow-up of colorectal cancer.
Ann Surg Oncol. 17:2349–2356. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Bast RC Jr, Ravdin P, Hayes DF, Bates S,
Fritsche H Jr, Jessup JM, Kemeny N, Locker GY, Mennel RG and
Somerfield MR; American Society of Clinical Oncology Tumor Markers
Expert Panel: 2000 update of recommendations for the use of tumor
markers in breast and colorectal cancer: clinical practice
guidelines of the American Society of Clinical Oncology. J Clin
Oncol. 19:1865–1878. 2001.PubMed/NCBI
|
|
9.
|
Duffy MJ, van Dalen A, Haglund C, Hansson
L, Klapdor R, Lamerz R, Nilsson O, Sturgeon C and Topolcan O:
Clinical utility of biochemical markers in colorectal cancer:
European Group on Tumour Markers (EGTM) guidelines. Eur J Cancer.
39:718–727. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Gupta MK, Arciaga R, Bocci L, Tubbs R,
Bukowski R and Deodhar SD: Measurement of a
monoclonal-antibody-defined antigen (CA19-9) in the sera of
patients with malignant and nonmalignant diseases. Comparison with
carcinoembryonic antigen. Cancer. 56:277–283. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Thomas WM, Robertson JF, Price MR and
Hardcastle JD: Failure of CA19-9 to detect asymptomatic colorectal
carcinoma. Br J Cancer. 63:975–976. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Iemura K and Moriya Y: A comparative
analysis of the serum levels of NCC-ST-439, CEA and CA19-9 in
patients with colorectal carcinoma. Eur J Surg Oncol. 19:439–442.
1993.PubMed/NCBI
|
|
13.
|
Tan KK, Lopes Gde L Jr and Sim R: How
uncommon are isolated lung metastases in colorectal cancer? A
review from database of 754 patients over 4 years. J Gastrointest
Surg. 13:642–648. 2009.PubMed/NCBI
|
|
14.
|
Iacopetta B: Are there two sides to
colorectal cancer? Int J Cancer. 101:403–408. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Fearon ER: Molecular genetics of
colorectal cancer. Annu Rev Pathol. 6:479–507. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Kapiteijn E, Liefers GJ, Los LC,
Kranenbarg EK, Hermans J, Tollenaar RA, Moriya Y, van de Velde CJ
and van Krieken JH: Mechanisms of oncogenesis in colon versus
rectal cancer. J Pathol. 195:171–178. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Weissenberger C, Von Plehn G, Otto F,
Barke A, Momm F and Geissler M: Adjuvant radiochemotherapy of stage
II and III rectal adenocarcinoma: role of CEA and CA 19-9.
Anticancer Res. 25:1787–1793. 2005.PubMed/NCBI
|
|
18.
|
Hinoue T, Weisenberger DJ, Lange CP, Shen
H, Byun HM, Van Den Berg D, Malik S, Pan F, Noushmehr H, van Dijk
CM, Tollenaar RA and Laird PW: Genome-scale analysis of aberrant
DNA methylation in colorectal cancer. Genome Res. 22:271–282. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Birkenkamp-Demtroder K, Olesen SH,
Sørensen FB, Laurberg S, Laiho P, Aaltonen LA and Ørntoft TF:
Differential gene expression in colon cancer of the caecum versus
the sigmoid and rectosigmoid. Gut. 54:374–384. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Cui H, Cruz-Correa M, Giardiello FM,
Hutcheon DF, Kafonek DR, Brandenburg S, Wu Y, He X, Powe NR and
Feinberg AP: Loss of IGF2 imprinting: a potential marker of
colorectal cancer risk. Science. 299:1753–1755. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Jorissen RN, Gibbs P, Christie M, Prakash
S, Lipton L, Desai J, Kerr D, Aaltonen LA, Arango D, Kruhøffer M,
Orntoft TF, Andersen CL, Gruidl M, Kamath VP, Eschrich S, Yeatman
TJ and Sieber OM: Metastasis-associated gene expression changes
predict poor outcomes in patients with Dukes stage B and C
colorectal cancer. Clin Cancer Res. 15:7642–7651. 2009. View Article : Google Scholar
|
|
22.
|
Dunican DS, McWilliam P, Tighe O,
Parle-McDermott A and Croke DT: Gene expression differences between
the microsatellite instability (MIN) and chromosomal instability
(CIN) phenotypes in colorectal cancer revealed by high-density cDNA
array hybridization. Oncogene. 21:3253–3257. 2002. View Article : Google Scholar
|
|
23.
|
Ahlquist DA, Skoletsky JE, Boynton KA,
Harrington JJ, Mahoney DW, Pierceall WE, Thibodeau SN and Shuber
AP: Colorectal cancer screening by detection of altered human DNA
in stool: feasibility of a multitarget assay panel.
Gastroenterology. 119:1219–1227. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Frattini M, Balestra D, Pilotti S,
Bertario L and Pierotti MA: Tumor location and detection of k-ras
mutations in stool from colorectal cancer patients. J Natl Cancer
Inst. 95:72–73. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Li FY and Lai MD: Colorectal cancer, one
entity or three. J Zhejiang Univ Sci B. 10:219–229. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Sanz-Pamplona R, Cordero D, Berenguer A,
Lejbkowicz F, Rennert H, Salazar R, Biondo S, Sanjuan X, Pujana MA,
Rozek L, Giordano TJ, Ben-Izhak O, Cohen HI, Trougouboff P, Bejhar
J, Sova Y, Rennert G, Gruber SB and Moreno V: Gene expression
differences between colon and rectum tumors. Clin Cancer Res.
17:7303–7312. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Wang WS, Lin JK, Chiou TJ, Liu JH, Fan FS,
Yen CC, Lin TC, Jiang JK, Yang SH, Wang HS and Chen PM: CA19-9 as
the most significant prognostic indicator of metastatic colorectal
cancer. Hepatogastroenterology. 49:160–164. 2002.PubMed/NCBI
|

















