Introduction
Metanephric adenoma (MA) is a rare renal neoplasm,
accounting for 0.2% of adult renal epithelial neoplasms (1). It is usually benign and was first
described by Brisigotti et al (2). The majority of MAs occur in patients
50–60 years of age with a female:male ratio of 2:1 (3). However, a case of a MA in a
7-year-old female has also been reported (4). According to the 2004 WHO
Classification of Renal Cell Tumors, MA was commonly described as a
unilateral lesion, although a multifocal case of childhood MA has
been reported (5). The clinical
and radiological characteristics of MA are non-specific and a
histopathological examination is required in order to establish a
definitive diagnosis. Partial nephrectomy should be considered as a
therapeutic option and regular follow-up is required to detect late
recurrences. In this study we present two patients with MA and a
supplementary review of previously published cases and related
literature.
Case reports
A 35-year-old female was admitted to Peking
University Shenzhen Hospital (Shenzen, China) with an incidental
finding of a right flank mass 1 week earlier. The hemoglobin level
was 146 g/l, the red blood cell count was 5.31×1012/l
and other routine hematological examinations and biochemical tests
were within normal limits. Computed tomography (CT) revealed a
solid tumor in the upper pole of the right kidney, measuring 5.5 ×
5.4 cm. The tumor was well-demarcated, 44 HU and marginally
enhanced following contrast enhancement and was characterized by
septation and cystic areas in its interior. Considering the size of
the mass and the possibility of malignancy, a radical nephrectomy
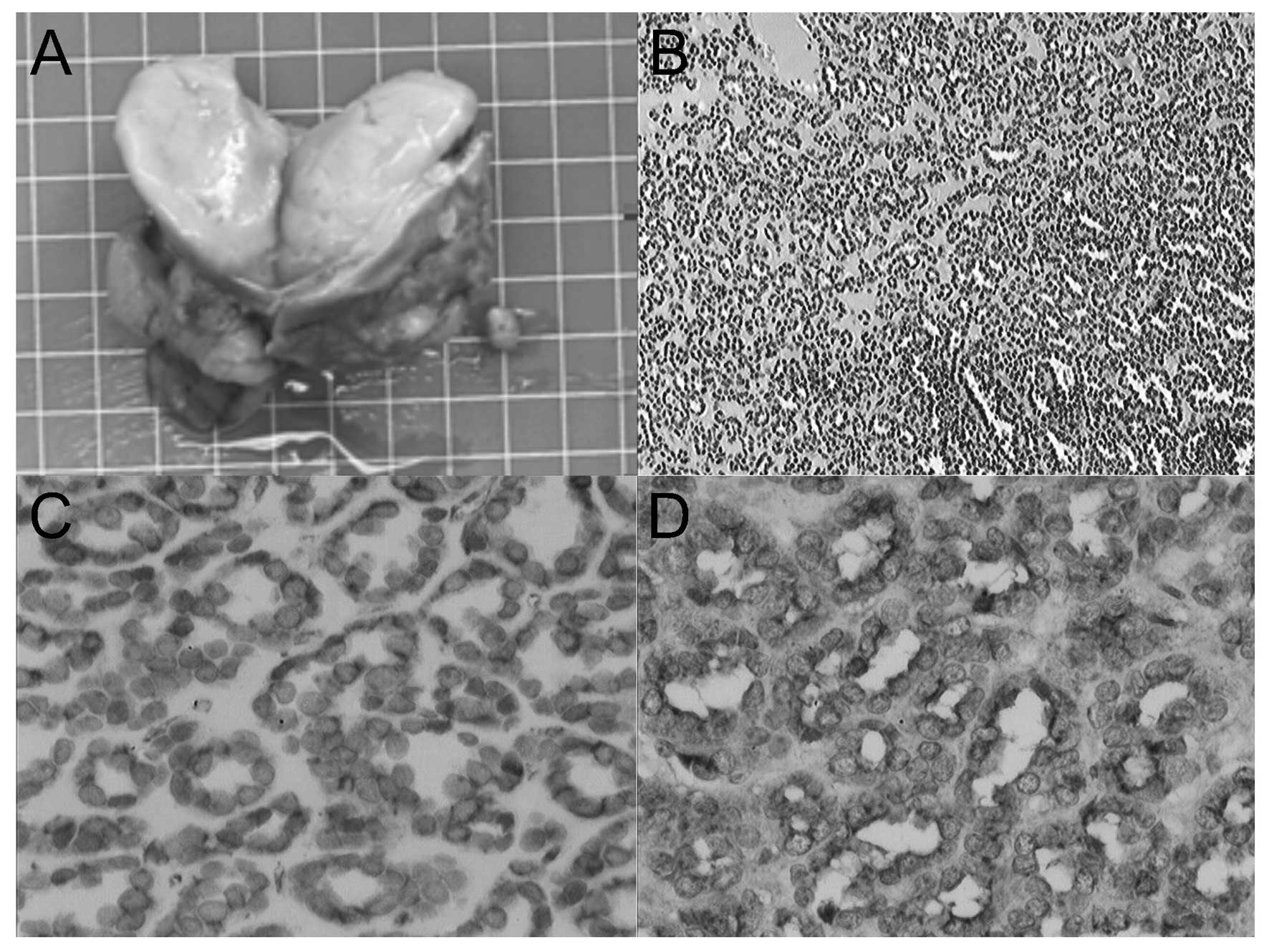
was performed. Microscopically, the excised tumor exhibited
characteristically monotonous, small, acinar and tubular structures
lined by small, uniform epithelial cells with scanty cytoplasm and
hyperchromatic round nuclei. Based on the histolological and
cytogenetic characteristics, a diagnosis of MA was suggested. The
patient was free from recurrence after 2 years of follow-up.
The second patient was a 46-year-old female with no
previous health problems, who presented with flank pain persisting
for 2 months and was admitted to Peking University Shenzhen
Hospital. The hemoglobin level was 109 g/l and the red blood cell
count was 5.34 × 1012/l. The ultrasound examination
revealed a hypoechoic mass with a solid aspect and irregular limits
in the upper pole of the right kidney. CT confirmed the presence of
a 4-cm expansive tumoral formation with intermediate attenuation
and minimal venous contrast enhancement at that anatomical site.
The patient was subjected to a successful partial nephrectomy by
open surgery. Microscopically, the tumor was composed of tightly
packed, uniform, small epithelial cells with small regular nuclei,
a high nucleus-to-cytoplasm ratio and the absence of mitotic
figures. Immunohistochemically, the tumor exhibited diffuse
positive staining for CD57 and vimentin and was only focally and
weakly positive for Wilm’s tumor 1 (WT1). Immunostaining for
epithelial membrane antigen (EMA), CD10 and CD34 was negative in
the tumor. The findings were compatible with a diagnosis of MA
(Fig. 1). The patient remains
disease-free 18 months following the surgery.
Discussion
Approximately 50% of MAs are not clinically
symptomatic, although flank pain, haematuria, fever, hypertension
and polycythaemia have been reported. Among renal lesions, MA is
associated with the highest incidence (10%) of polycythemia
(6), which may be attributed to
the paraneoplastic syndrome. Yoshioka et al (7) detected high concentrations of
erythropoietin, granulocyte macrophage colony-stimulating factor,
granulocyte colony-stimulating factor, interleukin (IL)-5 and IL-8
in the cell culture medium of MA. However, futher evidence is
required in order to establish an association between polycythemia
and these biomolecules. In addition, hypercalcemia and chyluria
were reported in rare cases (8).
The imaging characteristics of the lesions have not
been definitively described. On sonography, MA more commonly
presents as a well-circumscribed, round or oval, hypo- or
hyperechoic solid mass, occasionally with a hypoechoic rim, and may
appear as a fluid-containing mass. The power Doppler evaluation
demonstrated that this type of lesion is hypovascular. In detail,
MA appears as an iso- or hyperdense mass in relation to adjacent
renal parenchyma on pre-contrast CT, with minimal enhancement on
contrast CT. A case reported by Jinzaki et al (9) exhibited peak enhancement of the
neoplasm in the late nephrographic phase. On magnetic resonance
imaging, MA appears as an isointense or hypointense mass on
T1-weighted images and as a heterogeneous hyperintense mass on
T2-weighted images.
Pathological examination of the surgical specimen
revealed a firm, light brown lesion with a reticulated central area
and clear delimitation from the adjacent parenchyma. Unlike renal
adenoma, which is by definition <5 mm in diameter, the diameter
of MA ranges from 6–200 mm. The renal tumors presented in this
study were 40 and 55 mm in diameter. Microscopically, the tumor is
composed of small, uniform epithelial cells with scant cytoplasm
and hyperchromatic round nuclei, that form tubular or
glomerular-like structures. Abundant psammoma bodies are commonly
present, while mitotic figures are absent. Large tumors may appear
as heterogeneous, hypovascular masses with frequent foci of
hemorrhage, necrosis and calcifications.
Despite the absence of consistent
immunohistochemical staining patterns, there is frequently focal
positivity for CD7 and CD57, diffuse positivity for vimentin, WT1
and AE1/AE3 and negativity for EMA, S-100, carcinoembryonic
antigen, chromogranin A, synaptophysin, actin and α-methylacyl-CoA
racemase (10). This marker
profile may be used in the differential diagnosis of papillary
renal cell carcinoma (PRCC), since PRCC is positive for EMA and CK7
(11).
MA has been linked to PRCC due to certain overlaps
of histological characteristics and common molecular alterations,
such as the gains of chromosomes 7 and 17 and loss of gender
chromosomes. However, MA exhibits no duplication of chromosomes 7
and 17q21.32, which is a consistent molecular characteristic of
PRCC. A previous case report described a balanced translocation
[t(9;15)(p24;q24)] and a balanced paracentric inversion of
chromosome 12 [inv(12) (q13q15)]
(12).
Fine-needle aspiration may be used as a less
invasive method for the diagnosis of MA, although it is less
accurate than nephrectomy. Additionally, cytological diagnosis
using fine-needle aspiration may be challenging (10).
The differential diagnosis of renal MA includes PRCC
and epithelial Wilm’s tumor (WT). PRCC accounts for 7–15% of renal
cell cancer cases. Histologically, the tumor cells are arranged in
an entirely papillary pattern with fibrovascular cores. The tumor
cells have abundant oncocytic cytoplasm and exhibit stratification
or pseudostratification. The nuclei are large and hyperchromatic,
with distinct nucleoli. Mitotic figures are evident. Scattered
psammoma bodies, Prussian blue-positive hemosiderin granules and
pale foamy cells are observed. Trisomies of the chromosomes 7 and
17 and loss of gender chromosomes may also be detected by genetic
studies. WTs are three-phase embryonic renal tumors consisting of
varying amounts of blastemic, epithelial and mesenchymal
structures, with a low incidence rate in adults. MA may be
difficult to distinguish from epithelial (tubular predominant) type
Wilms’ tumor, although the latter grows quickly and exhibits
abundant mitotic figures with distinct cellular atypia.
MA is an invariably benign renal tumor with a
favorable prognosis. However, the majority of reported cases of MA
were subjected to nephrectomy due to the difficult preoperative
differentiation from malignant renal tumors. The biological
behavior of MA is benign and is always accompanied by a successful
outcome with nephrectomy or mass resection. However, a case of MA
with metastases in the periaortic, hilar and aortic bifurcation
lymph nodes was reported in a 7-year-old female (13) and a case of MA containing foci of
papillary carcinoma and a regional metastatic lymph node was
reported in an 11-year-old female (14). Furthermore, bone metastases in
adults have been described (15).
In conclusion, MA is a rare, benign neoplasm of
epithelial cells, which is complicated to preoperatively
distinguish from other malignant neoplasms. In this study we
present the cases of two patients with MA. The two patients
underwent surgery and the pathological findings indicated that MA
was the correct diagnosis. The two patients were free of recurrence
following longterm follow-up. Given the rarity of this tumor and
the lack of highly predictive clinical or radiographic criteria, we
suggest that a histopathological examination is necessary in order
to establish a definitive diagnosis.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (Grant No. 81101922),
the Medical Scientific Research Foundation of Guangdong Province of
China (Grant Nos. A2012584 and A2013606) and the Science and
Technology Development Fund Project of Shenzhen (Grant No.
JCYJ20130402114702124).
References
|
1.
|
Amin MB, Amin MB, Tamboli P, Javidan J,
Stricker H, de-Peralta Venturina M, Deshpande A and Menon M:
Prognostic impact of histologic subtyping of adult renal epithelial
neoplasms: an experience of 405 cases. Am J Surg Pathol.
26:281–291. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Brisigotti M, Cozzutto C, Fabbretti G,
Sergi C and Callea F: Metanephric adenoma. Histol Histopatho1.
7:689–692. 1992.
|
|
3.
|
Schmelz HU, Stoschek M, Schwerer M, Danz
B, Hauck EW, Weidner W and Sparwasser C: Metanephric adenoma of the
kidney: case report and review of the literature. Int Urol Nephrol.
37:213–217. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Liniger B, Wolf RW, Fleischmann A and
Kluwe W: Local resection of metanephric adenoma with kidney
preservation. J Pediatr Surg. 44:E21–E23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Kohashi K, Oda Y, Nakamori M, Yamamoto H,
Tamiya S, Toubo T, Kinoshita Y, Tajiri T, Taguchi T and Tsuneyoshi
M: Multifocal metanephric adenoma in childhood. Pathol Int.
59:49–52. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Bastide C, Rambeaud JJ, Bach AM and Russo
P: Metanephric adenoma of the kidney: clinical and radiological
study of nine cases. BJU Int. 103:1544–1548. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Yoshioka K, Miyakawa A, Ohno Y, Namiki K,
Horiguchi Y, Murai M, Mukai M and Tachibana M: Production of
erythropoietin and multiple cytokines by metanephric adenoma
resu1ts in erythrocytosis. Pathol Int. 57:529–536. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
McNeil JC, Corbett ST, Kuruvilla S and
Jones EA: Metanephric adenoma in a five-year-old boy presenting
with chyluria: case report and review of literature. Urology.
72:545–547. 2008.PubMed/NCBI
|
|
9.
|
Jinzaki M, Tanimoto A, Mukai M, Ikeda E,
Kobayashi S, Yuasa Y, Narimatsu Y and Murai M: Double-phase helical
CT of small renal parenchymal neoplasms: correlation with
pathologic findings and tumor angiogenesis. J Comput Assist Tomogr.
24:835–842. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Patel NP, Geisinger KR, Zagoria RJ and
Bergman S: Fine needle aspiration biopsy of metanephric adenoma: a
case report. Acta Cyto1. 53:327–331. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Olgac S, Hutchinson B, Tickoo SK and
Reuter VE: Alpha-methylacyl CoA racemase as a marker in the
differential diagnosis of metanephric adenoma. Mod Pathol.
19:218–224. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Rakheja D, Lian F, Tomlinson GE, Ewalt DH,
Schultz RA and Margraf LR: Renal metanephric adenoma with
previously unreported cytogenetic abnormalities: case report and
review of the literature. Pediatr Dev Pathol. 8:218–223. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Renshaw AA, Freyer DR and Hanlners YA:
Metastatic meta-nephric adenoma in a child. Am J Surg Pathol.
24:570–574. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Drut R, Drut RM and Ortolani C: Metastatic
metanephric adenoma with foci of papilary carcinoma in a child: a
combined histologic, immunohistochemical, and FISH study. Int J
Surg Pathol. 9:241–247. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Pins MR, Jones EC, Martul EV, Kamat BR,
Umlas J and Renshaw AA: Metanephric adenoma-like tumors of the
kidney: report of 3 malignancies with emphasis on discriminating
features. Arch Pathol Lab Med. 123:415–20. 1999.PubMed/NCBI
|