Introduction
Multiple myeloma involves the clonal proliferation
of plasma cells based in the bone marrow, with various degrees of
differentiation (1). Neoplastic
cells usually produce large amounts of monoclonal immunoglobulin
light or heavy chains that can be detected in serum or urine
(2). Although multiple myeloma is
the most common primary bone cancer in adults, in ∼95% of cases, it
involves several bones (3).
The etiology of this disease remains to be
determined. However, some occupations, exposure to certain
chemicals, overdose irradiation, viruses and genetic factors are
considered to be etiologic factors (4).
Myeloma is slightly more prevalent in males and
individuals of African-American descent (5). In western countries, the disease is
more prevalent in males, with a median and average age at diagnosis
of 66 and ∼60 years, respectively (6). By contrast, the median and average
age at diagnosis is 57 and 55–65 years, respectively (7). The first manifestations that usually
present at diagnosis include bone pain (58%), fatigue (32%) and
weight loss (24%) (6). The
diagnosis of myeloma is usually confirmed by the demonstration of a
monoclonal protein (M-protein) in the serum or urine and/or lytic
lesions on X-ray together with histological confirmation of a
malignant proliferation of plasma cells (8). Treatment involves mainly irradiation,
chemotherapy, autologous stem cell transplantation. Prognosis is
determined via risk classification by the International Staging
System (ISS) (9).
The present study reports a case of painful
ulcer-like maxillary mass with multiple myeloma, which was
diagnosed based on biopsy of the oral lesion.
Case report
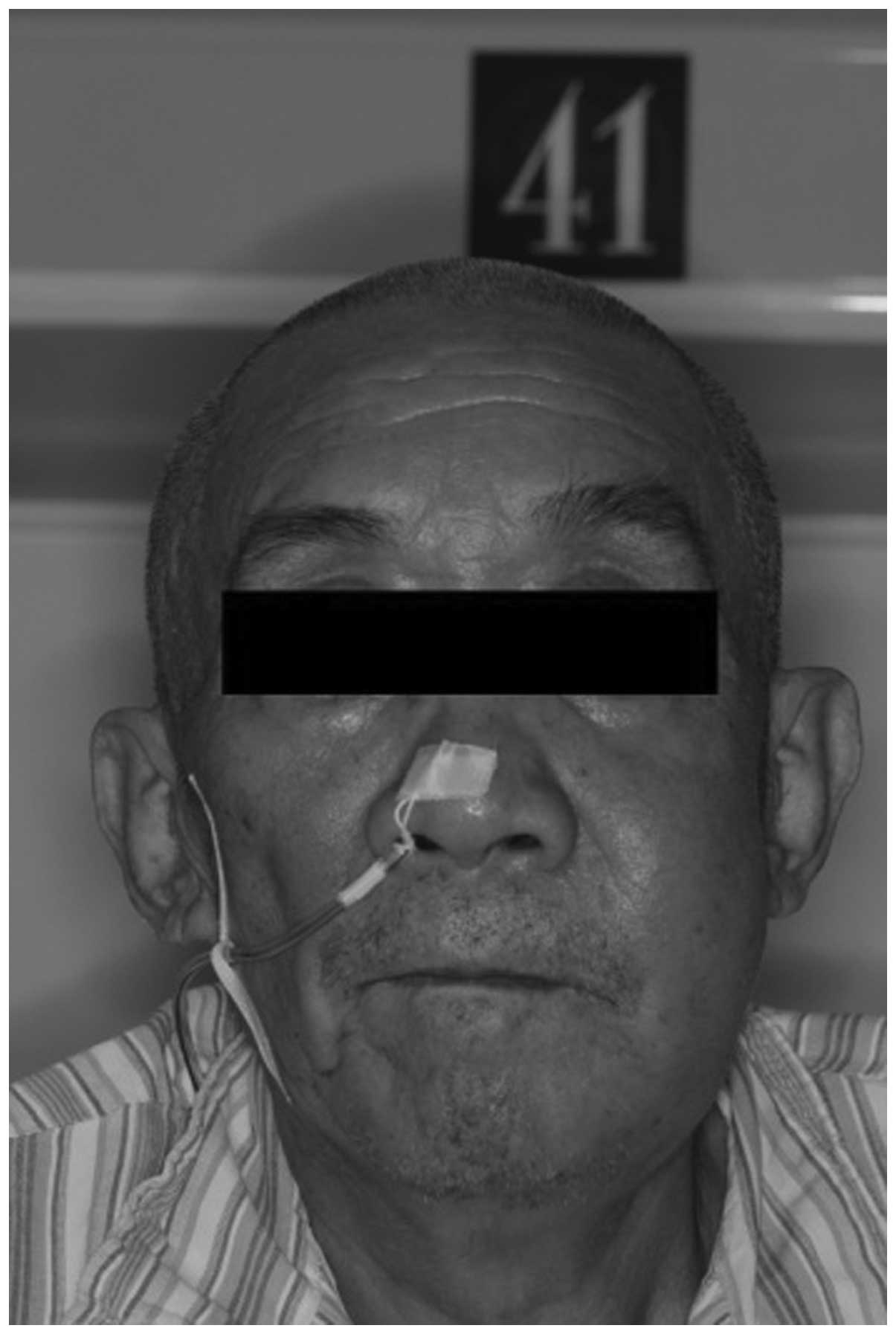
A 69-year-old male patient presented with a chief
complaint of a painful ulcerated lesion in left maxilla, for ∼1
month. Although the patient was treated with antibiotics and
cortisol, the lesion was non-healing and became enlarged and
painful, resulting in restriction of mouth opening and a weight
loss of 4 kg during the month he was observed. Subsquently, the
patient presented to the Shanghai Ninth Peoples’ Hospital
Affiliated to Shanghai Jiaotong University School of Medicine
(Shanghai, China) for further oral and maxillofacial surgery. His
previous medical history revealed an episode of lumbar
intervertebral disc prolapsed over a period of five years. The pain
was exacerbated subsequent to heavy lifting, but had not
progressed. The patient had no family history of cancer. Ethics
approval for this study was granted by the ethics committee of
Shanghai Ninth People’s Hospital affiliated to Shanghai Jiaotong
University, School of Medicine (Shanghai, China). The patient
provided written informed consent.
Peripheral lymphadenopathy was not observed, while
the liver and spleen were unpalpable. Intraoral examination
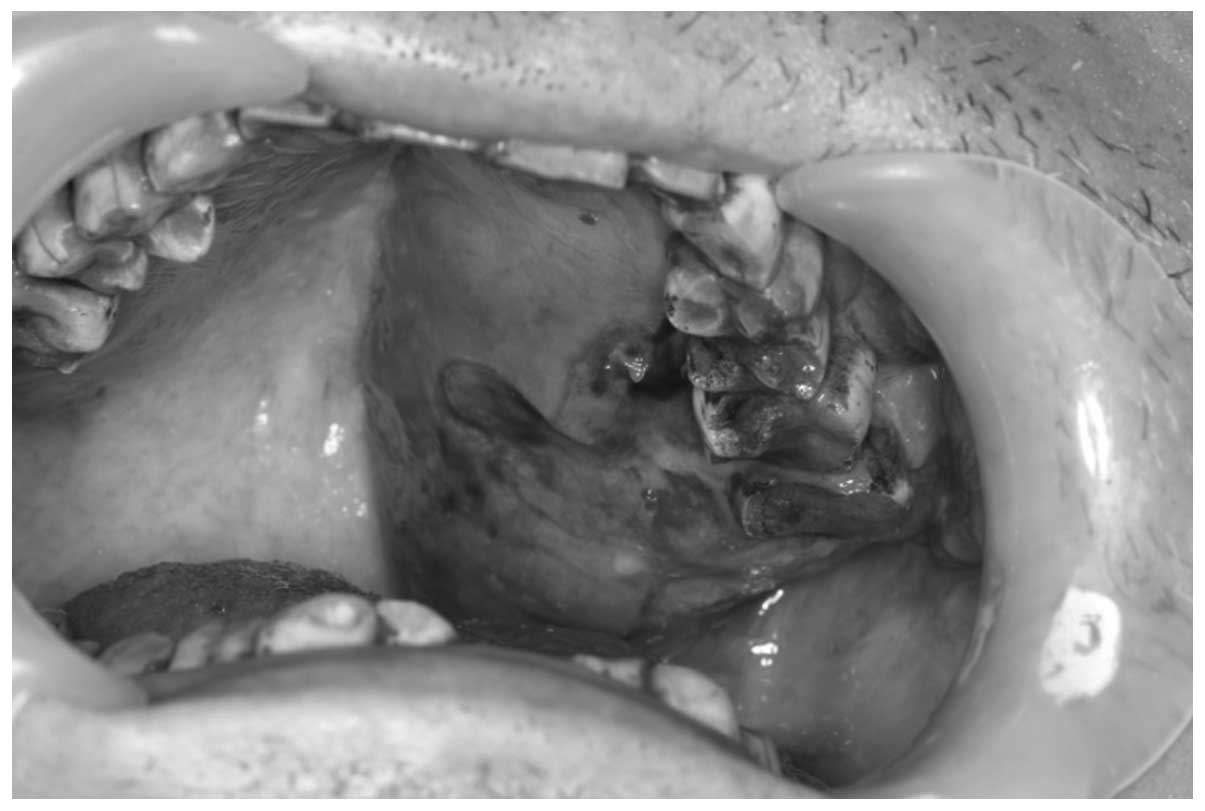
revealed an ulcerated and hemorrhagic mass in the left posterior
palate near the maxilla body. The lesion extended from maxillary
left third molar to maxillary left seventh molar. It had an uneven
bleeding surface and measured ∼5 cm in its maximum diameter
impairing the upper left buccal and palatine gingiva (Figs. 1 and 2).
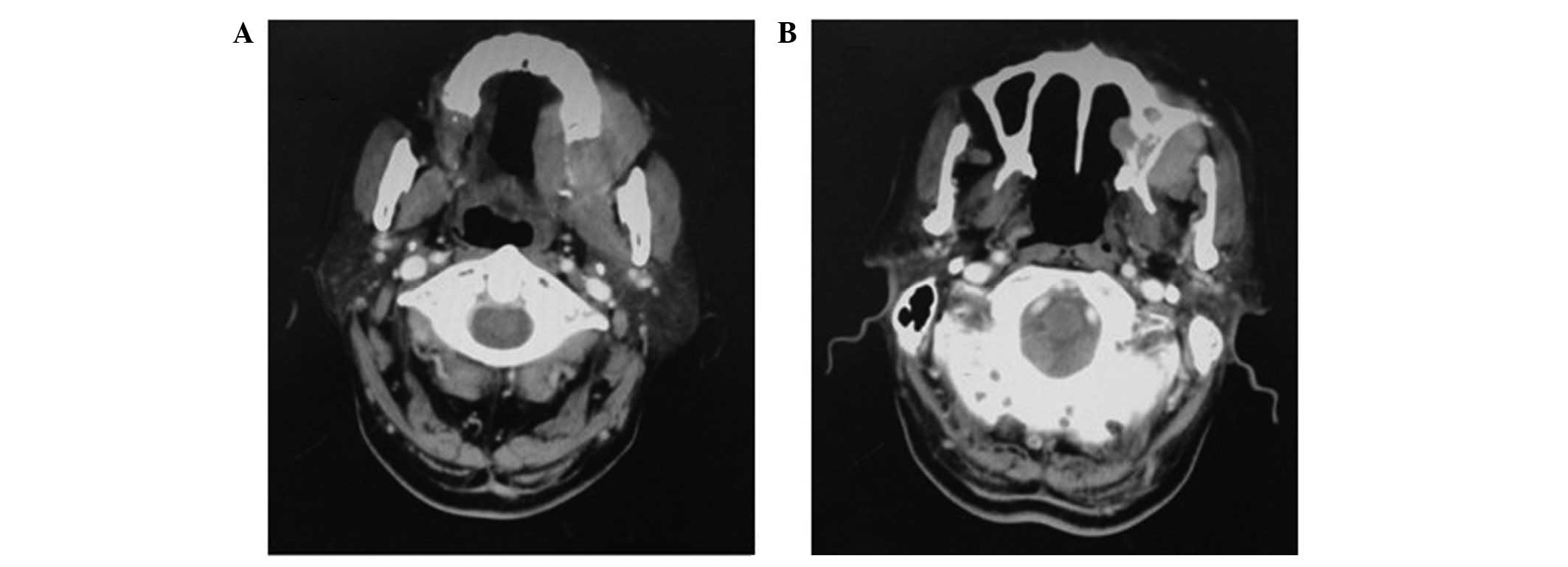
Contrasted oral and maxillary computed tomography
(CT) and magnetic resonance imaging (MRI) showed irregular soft
tissue with destruction of the left upper-alveolar bone,
periodontium and the maxillary sinus, suggesting a malignant mass
(Figs. 3 and 4).
Elevated serum creatinine levels were indicative of
multiple organ infiltrations. Consequently, the patient was
referred for consultation to the Department of Nephrology and
underwent additional examinations.
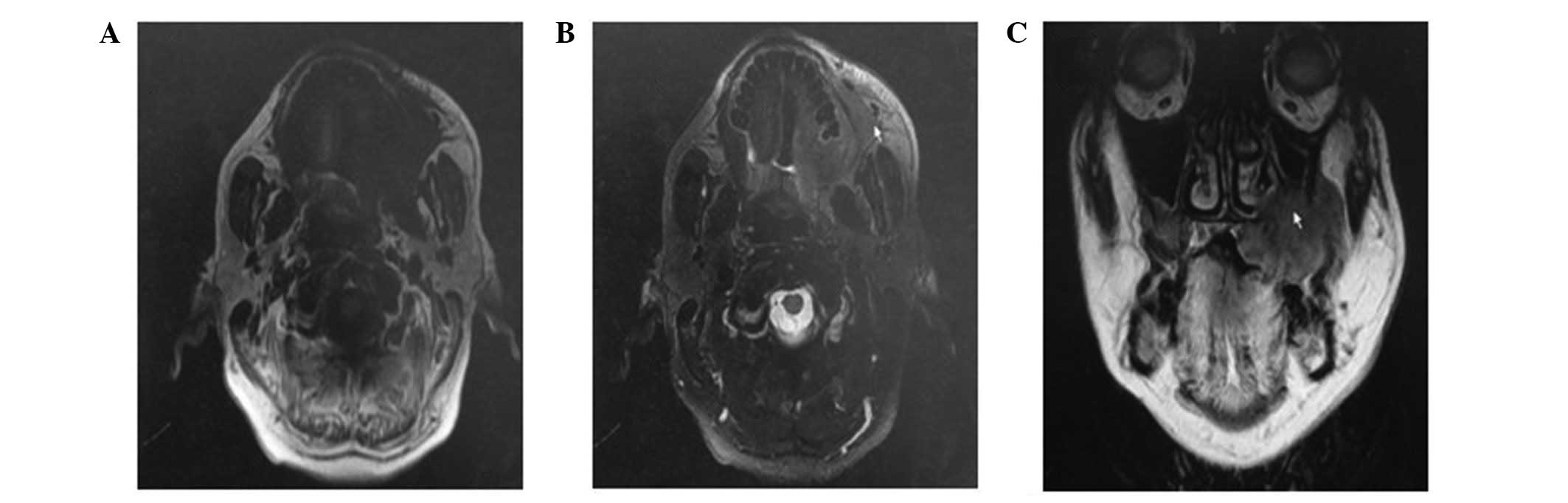
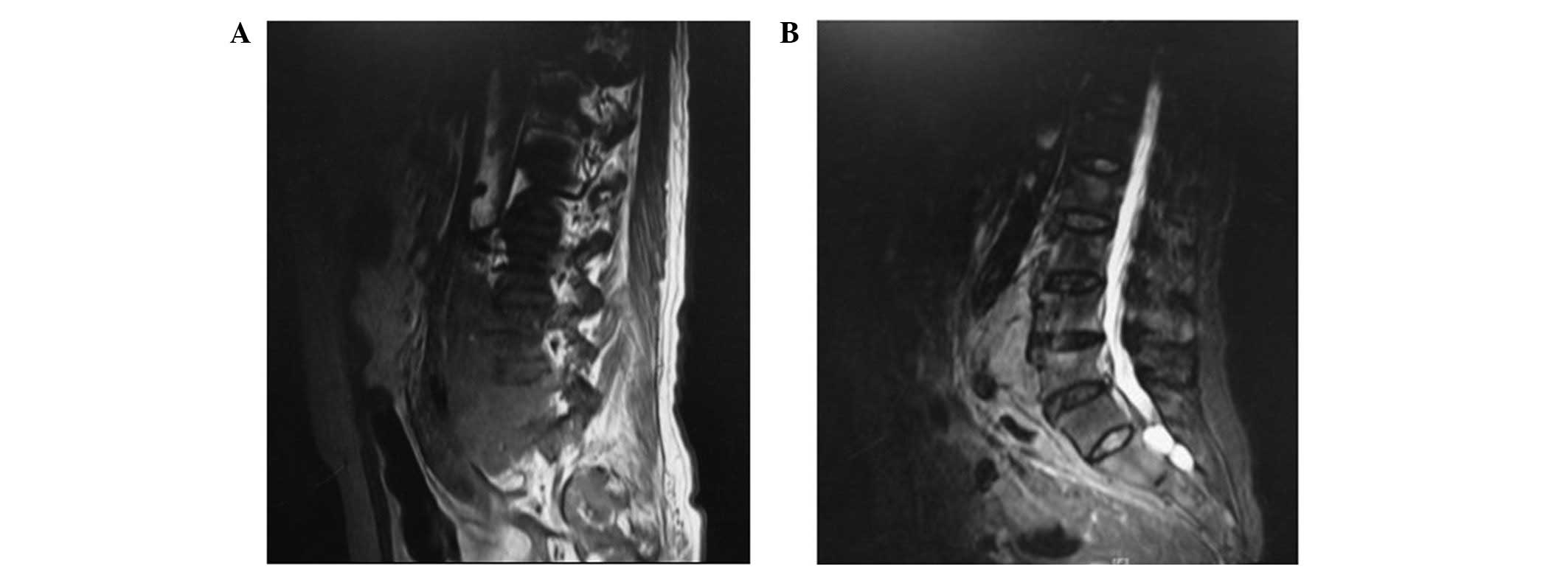
The lumbar spine MRI revealed multiple vertebral
destruction (Fig. 5).
A high serum level of β2-microglobulin,
hypercalcemia and lactate dehydrogenase were observed, whereas
urinary Bence-Jones protein was not identified.
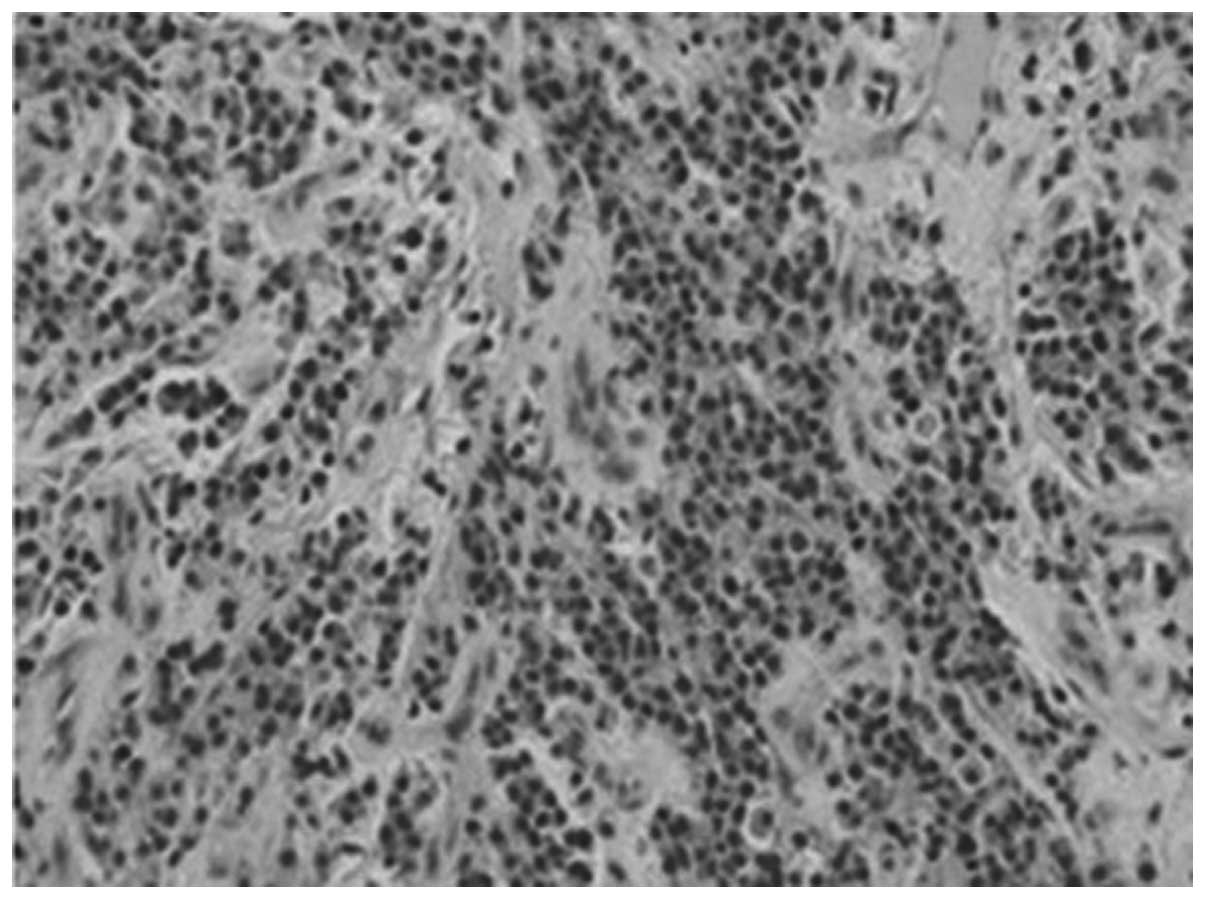
An incisional biopsy was performed under local
anesthesia. The histological features indicated sheets of atypical
plasma cells (Fig. 6). The
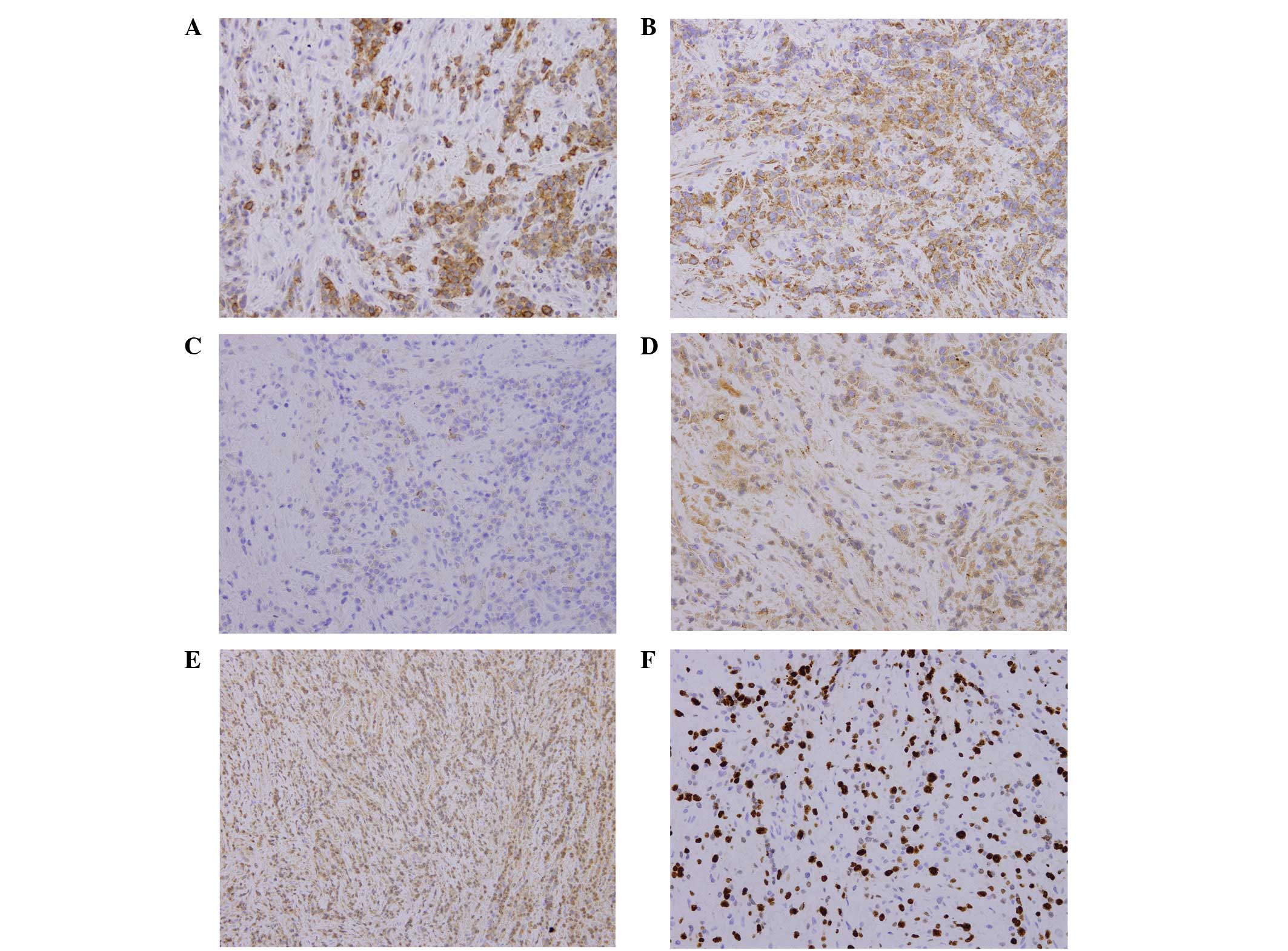
immunohistochemical results were positive for CD138, Vs38c, EMA and
immunoglobulin G (IgG) (Fig. 7),
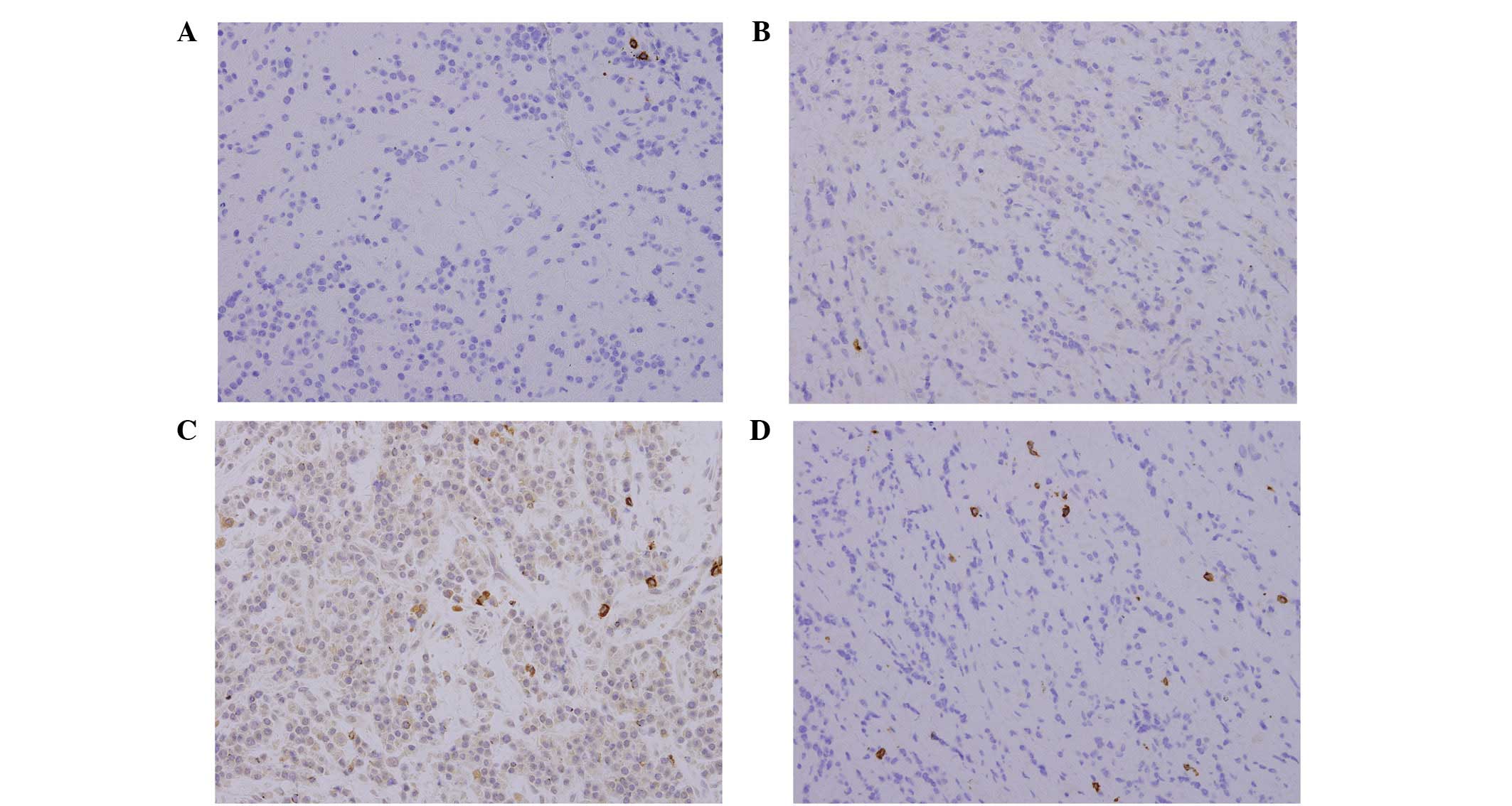
negative for L26, CD79a and CD3, and ∼60–70% positive for Ki-67
(Fig. 8). Monoclonal staining for
λ was positive, whereas κ was negative. A bone marrow aspiration
demonstrated 44% plasmacytosis.
Serum electrophoresis revealed myeloma protein
(M-protein) secreting IgG (72.4 g/l) as well as λ light chains.
According to these results the diagnosis of multiple myeloma was
established as stage IIIB (Durie/Salmon staging system) and
symptomatic myeloma presenting as myeloma-related organ and tissue
impairment (ROTI, adapted from the International Myeloma Working
Group, 2003) (Table I) (10).
 | Table I.Values of complete blood count,
chemistry measurements and serum protein electrophoresis. |
Table I.
Values of complete blood count,
chemistry measurements and serum protein electrophoresis.
| Item | Results | Normal range |
|---|
| Hemoglobin | 123 g/l | 103–151 g/l |
| Platelets |
171×109/l |
101–320×109/l |
| Serum creatinine | 180
μmol/l | 44–97
μmol/l |
| Calcium | 2.82
μmol/l | 2.08–2.65
μmol/l |
| Lactate
dehydrogenase | 220 U/l | 100–190 U/l |
| Serum globulins | 25 g/l | 32–48 g/l |
| β-2
microglobulin | 16 mg/l | 0.7–1.8 mg/l |
| γ globulins | 60.1 g/l | 6–25 g/l |
| Globulin peak in the
urine | 2.75 g/24 h | 0–0.15 g/24 h |
The patient was referred for consultation to the
Deparments of Hematology and Nephrology. Chemotherapy comprising
thalidomide combined with bortezomib-mitoxantronedexamethasone
(PMD) was administered. The patient subsequently received
autologous stem cell transplantation and remained in remission at
the date of the writing of this manuscript.
Discussion
Multiple myeloma accounts for ∼1% of all types of
malignancy and slightly >10% of hematologic malignancies
(11). Bone marrow examination
reveals a large amount of these abnormal plasma cells. Myeloma
cells produce abnormal immunoglobulin (M-protein), light chain
proteins (κ and λ) and other factors, such as cytokines. Excessive
M-protein causes hyperviscosity of the blood. The excessive
production of a monoclonal protein (M-protein) may lead to renal
dysfunction. Lesions of bone are largely caused by the release of
cytokines that promote bone resorption through the upregulation of
osteoclast activity, differentiation and maturation (12,13).
Initial findings of the examinations conducted were:
anemia in 73% of patients, bone pain in 58%, renal insufficiency in
48%, hypercalcemia in 28%, palpable liver in 4% and palpable spleen
in 1% of patients. Lymphadenopathy was observed in 1% of patients
(6). Maxillofacial presentations
in patients with multiple myeloma are not uncommon, however,
multiple myeloma is often overlooked. Epstein et al
(14) examined 783 patients in the
literature and indicated that ∼14% of patients had oral
manifestations. Oral lesions rarely occured as the first indication
of the disease (15–17), whereas jaw lesions are the more
common manifestation of multiple myeloma with an incidence varying
from 8–15% (18). As the symptoms
vary, multiple myeloma may be misdiagnosed or overlooked in the
oral and maxillofacial region.
In the present case the impaired renal function was
addressed by physicians. Subsequently, the patient underwent
chemotherapy instead of surgery due to symptoms including swelling,
mass formation, non-healing ulcer, pain, bleeding and fracture of
the jawbone, tooth mobility and migration, macroglossia and
radiolucent lesions. Osteolytic lesions are reported more
frequently in the mandible as compared to the maxilla, particularly
in the posterior teeth region, ramus and condylar process,
presumably due to greater hematopoietic activity in these areas
(13,18). As for the image findings, results
of the CT provided detailed information regarding the extent of
cortical involvement of the tumor, whereas MRI revealed marrow
infiltration as well as diffuse patterns of infiltration that may
not be adequately visualized using radiographic imaging alone
(2).
An ulcerated, haemorhagic tissue mass of 3 × 5 cm,
arising from the maxillary left third molar to the maxillary left
seventh molar, was observed in the present case. Panoramic
radiography and MRI/CT revealed an osteolytic lesion in the left
posterior maxilla body, haziness of maxillary sinus and multiple
destruction and infiltration of the lumber spine. The patient had a
previous medical history of lumbar intervertebral disc prolapsed
over a period of five years, although the pain associated with the
prolapsed disc was not aggravated. The patient’s chief complaint
was the oral lesion that had shown rapid progression. Therefore, a
differential diagnosis between multiple myeloma and multiple
metastatic disease should be conducted, particularly in elderly
individuals. The differential diagnosis depends on the
identification of abnormal monoclonal plasma cells in the full
blood count, bone marrow and biopsy, M-protein in the serum or
urine and a clinical image consistent with multiple myeloma.
Serum eletrophoresis identifies myeloma protein
(M-protein) in ∼93% of the patients. Additionally, ∼70% of myelomas
secrete IgG, with κ light chains being more common (63%) (6). In the present case, serum protein
electrophoresis showed an IgG monoclonal spike of ∼72.4 g/l with
the λ light chain. Urine electrophoresis may identify M-protein in
∼60% of patients. Nevertheless, no myeloma protein was detected in
the urine of the patient of this study.
Immunohistochemical staining should be performed to
confirm plasmacytoma. Up to 85% of plasma cell neoplasms are
positive for EMA, an antibody against epithelial membrane antigen
that recognizes the breast epithelial mucin complex (2). CD138 immunostaining of trephine
sections is useful in determining the extent of infiltration in
selected cases. L26 antibody staining of CD20 molecule, which is
usually expressed on mature B cells and a subset of immature B
cells, adheres to the expression patterns in normal B-cell
development. As a result, plasmacytomas are usually negative for
this antibody. Light chain restriction for κ or λ is usually
observed and almost 70% of plasma cell neoplasms are κ-positive
(20,21).
In this case, the histological and
immunohistochemical results led to the diagnosis of malignant
plasma-cell lesion. The MRI/CT revealed the presence of multiple
oesteolytic lesions. The diagnosis of multiple myeloma was
subsequently confirmed by full blood count, incisional biopsy, bone
marrow biopsy and laboratory examinations.
The natural history of myeloma is heterogeneous with
survival times ranging from a few weeks to >20 years. Analysis
of prognostic factors is essential to compare outcomes within and
between clinical trials. The Durie/Salmon staging system was
published in 1975 (22) but has
been superseded by the ISS reproduced in Table II (9). The ISS defines three risk categories
determined by the serum concentration of β2-microglobulin and
albumin. The use of staging systems to determine choice of therapy
for individual patients remains unproven. As for the patient in
this study, the diagnosis was symptomatic myeloma with ROTI staging
III (Table II).
 | Table II.The International Staging System (ISS)
for multiple myeloma.a |
Table II.
The International Staging System (ISS)
for multiple myeloma.a
| Stage | Criteria | Median survival
(months) |
|---|
| I | Serum β2
microglobulin <3.5 mg/l (296 nmol/l) and serum albumin ≥3.5/dl
(35 g/l or 532 μmol/l) | 62 |
| II | Neither I or
IIIb | 45 |
| III | Serum β2
microglobulin ≥5.5 mg/l (465 nmol/l) | 29 |
Chemotherapy is only suggested for patients with
symptomatic myeloma based on the presence of ROTI (10). In this case, we used PMD combined
with thalidomide as an induction therapy prior to autologous
stem-cell transplantation. Therefore, the prognosis of this patient
is to continue be followed up.
In conclusion, although maxillofacial manifestation
in patients with multiple myeloma is not uncommon, multiple myeloma
is often overlooked and misdiagnosed. Therefore, findings of this
case report suggest that multiple myeloma should be considered as a
differential diagnosis and related laboratory/radiographic
evaluations should be administered when considering a patient whose
chief complaint is unusual maxillary pain.
References
|
1.
|
Seoane J, Aguirre-Urizar JM, Esparza-Gomez
G, Suarez-Cunqueiro M, Campos-Trapero J and Pomareda M: The
spectrum of plasma cell neoplasia in oral pathology. Med Oral.
8:269–280. 2003.(In English).
|
|
2.
|
Dinter DJ, Neff WK, Klaus J, Bohm C,
Hastka J, Weiss C, Schoenburg SO and Metzgeroth G: Comparison of
whole-body MR imaging and conventional X-ray examination in
patients with multiple myeloma and implications for therapy. Ann
Hematol. 88:457–464. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Mulligan ME and Badros AZ: PET/CT and MR
imaging in myeloma. Skeletal Radiol. 36:5–16. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Beers MH, Porter RS, Jones TV, Kaplan JL
and Berkwits M: The Merck Manual of Diagnosis and Therapy. 18th
edition. Merck Research Laboratories; Whitehouse Station, NJ: pp.
1129–1131. 2006
|
|
5.
|
Dores GM, Landgren O, McGlynn KA, Curtis
RE, Linet MS and Devesa SS: Plasmacytoma of bone, extramedullary
plasmacytoma, and multiple myeloma: incidence and survival in the
United States, 1992–2004. Br J Hematol. 144:86–94. 2009.
|
|
6.
|
Kyle RA, Gertz MA, Witzig TE, Lust JA,
Lacy MQ, Dispenzieri A, Fonseca R, Rajkumar V, Offord JR, Larson
DR, et al: Review of 1027 patients with newly diagnosed multiple
myeloma. Mayo Clin Proc. 78:21–33. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Mai Y, Qiu L and Li R: The clinical and
laboratory features of 432 patients with multiple myeloma. J Leuk
Lymphoma. 13:198–201. 2004.
|
|
8.
|
Durie BG, Kyle RA, Belch A, Bensinger W,
Blade J, et al: Myeloma management guidelines: a consensus report
from the Scientific Advisors of the International Myeloma
Foundation. Hematol J. 4:379–398. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Greipp PR, San Miguel J, Durie BG, et al:
International staging system for multiple myeloma. J Clin Oncol.
23:3412–3420. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
International Myeloma Working Group:
Criteria for the classification of monoclonal gammopathies,
multiple myeloma and related disorders: a report of the
International Myeloma Working Group. Br J Haematol. 121:749–757.
2003. View Article : Google Scholar
|
|
11.
|
Bird JM, Owen RG, D’Sa S, Snowden JA,
Pratt G, et al: Guidelines for the diagnosis and management of
multiple myeloma 2011. Br J Haematol. 154:32–75. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Nau KC and Lewis WD: Multiple myeloma:
diagnosis and treatment. Am Fam Physician. 78:853–859.
2008.PubMed/NCBI
|
|
13.
|
Ashcroft AJ, Davies FE and Morgan GJ:
Aetiology of bone disease and the role of bisphosphonates in
multiple myeloma. Lancet Oncol. 4:284–292. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Epstein JB, Emerton S, Guglietta A and Le
N: Assessment of epidermal growth factor in oral secretions of
patients receiving radiation therapy for cancer. Oral Oncol.
33:359–363. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Mozaffari E, Mupparapu M and Otis L:
Undiagnosed multiple myeloma causing extensive dental bleeding:
report of a case and review. Oral Surg Oral Med Oral Pathol Oral
Radiol Endod. 94:448–453. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Pinto LS, Campagnoli EB, Leon JE, et al:
Maxillary lesion presenting as a first sign of multiple myeloma:
case report. Med Oral Patol Oral Cir Bucal. 12:E344–E347.
2007.PubMed/NCBI
|
|
17.
|
Shah A, Ali A, Latoo S and Ahmad I:
Multiple Myeloma presenting as Gingival mass. J Maxillofac Oral
Surg. 9:209–212. 2010. View Article : Google Scholar
|
|
18.
|
Zachriades N, Papanicolaou S,
Papavassiliou D, Vairaktaris E, Triantafyllou D and Mezitis M:
Plasma cell myeloma of the jaws. Int J Oral Maxillofac Surg.
16:510–515. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Pisano JJ, Coupland R, Chen SY and Miller
AS: Plasmacytoma of the oral cavity and jaws: a clinicopathologic
study of 13 cases. Oral Surg Oral Med Oral Pathol Oral Radiol
Endod. 83:265–271. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Hsi ED and Yegappan S: Lymphoma
immunophenotyping: a new era in paraffin-section
immunohistochemistry. Adv Anat Pathol. 8:218–239. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Bayer-Garner IB, Prieto VG and Smoller BR:
Detection of clonality with κ and λ immunohistochemical analysis in
cutaneous plasmacytomas. Arch Pathol Lab Med. 128:645–648.
2004.
|
|
22.
|
Durie BG and Salmon SE: A clinical staging
system for multiple myeloma. Correlation of measured myeloma cell
mass with presenting clinical features, response to treatment, and
survival. Cancer. 36:842–854. 1975. View Article : Google Scholar : PubMed/NCBI
|