Introduction
Breast cancer is the most common malignancy among
females (1) and the second most
common cause of cancer-related mortality behind lung cancer
(2). Due to changes in lifestyle,
the incidence of breast cancer, which is currently on the increase
in developing countries, including China, has increased
significantly. Based on DNA microarray techniques, breast cancer is
classified into five subtypes: luminal A, luminal B, normal
breast-like, human epidermal growth factor receptor 2
(HER2/neu)-overexpressing and basal-like (3). The basal-like and normal breast-like
subtypes, which are immunohistochemically characterized by the lack
of expression of the estrogen receptor (ER), progesterone receptor
(PgR) and HER2, are defined as triple-negative breast cancer (TNBC)
(4).
TNBC is a distinct breast cancer subtype, which
accounts for ~10–17% of all breast carcinomas (5). TNBC, usually occurring in young
females, is generally considered to exhibit an aggressive clinical
behavior and poor prognosis, due to the fact that it is insensitive
to endocrine and targeted therapy (6). Furthermore, the TNBC subgroup is
associated with a higher risk of distant recurrence and mortality
compared to its non-triple-negative counterparts, particularly
during the first 3–5 years of follow-up (6). However, few studies have been
conducted among non-Western populations (7) and the information on the Asian TNBC
subtype remains confusing and limited (8). Kurebayashi et al (9) reported that Japanese patients with
TNBC are mostly superimposable for disease-free survival (DFS) and
overall survival (OS). Lin et al (10) indicated that Taiwanese TNBC
patients exhibited a better 5-year OS compared with HER2-positive
patients. Yin et al (7)
revealed that recurrence-free survival in Chinese TNBC patients was
superior to that of HER2-positive patients. In order to elucidate
whether there are regional differences among patients in different
Chinese cities and whether they differ from Western populations, it
is critical to further delve into the clinical characteristics and
prognosis of TNBC in mainland Chinese patients.
In the present study, a retrospective analysis was
perfomed on the clinicopathological characteristics of TNBC
patients who received conventional treatment at the Department of
Oncology, First Affiliated Hospital of Medical School of Xi'an
Jiaotong University and the Department of Breast Surgery, General
Surgery, First Hospital of China Medical University. In this study,
the aim was to determine the clinicopathological characteristics of
this breast cancer subtype, evaluate the survival of patients
treated by the currently available conventional methods and analyze
the prognostic factors. The internal information on clinical TNBC
cases may elucidate the implications of the underlying distinction
in tumor biology from other breast cancer subgroups.
Materials and methods
Patient characteristics
Between January 1, 2004 and December 31, 2007, a
total of 972 female patients with breast cancer confirmed by
surgery and pathological examination in the Department of Oncology,
First Affiliated Hospital of Medical School of Xi'an Jiaotong
University and the Department of Breast Surgery, General Surgery,
First Hospital of China Medical University, were retrospectively
investigated. The study protocol was approved by the Institutional
Review Board of the two participating hospitals. The baseline data
included age, tumor characteristics (including, tumor size, lymph
node metastasis, distant metastasis, tumor grade, pathological
stage, ER/PgR/HER2 expression and histological type) and treatment
modalities. The quality of the cancer registry database was
reviewed and approved by the Ethics Committee of the First
Affiliated Hospital of Medical School of Xi'an Jiaotong
University.
Tumor characteristics
The pathological diagnosis was in accordance with
the histological classification of tumors developed by the World
Health Organization and clinical staging was based on the TNM
staging of breast cancer developed by the American Joint Committee
on Cancer. The immunohistochemical expression of ER, PgR and HER2
was detected with the streptavidin peroxidase conjugated method (SP
method) using 5-μm serial sections. The primary antibosdies used
were as follows: rabbit monoclonal anti-human ER antibody, rabbit
monoclonal anti-human PgR antibody (Fuzhou Maixin Biotechnology
Development Co., Ltd., Fuzhou, China) and rabbit anti-human HER2
antibody (Roche Diagnostics, Shanghai, China). The secondary
antibody used was HRP-ploymer anti mouse/rabbit IgG (Fuzhou Maixin
Biotechnology Development Co., Ltd.). Cells accounting for ≥10%
were considered as positive expression. The size of the primary
breast tumor was determined by using dual-track measurement.
Treatment
Routine preoperative chemotherapy was administered
to 116 out of the 972 cases (11.9%). All patients underwent
surgical treatment of breast cancer. The postoperative adjuvant
therapy was administered based on the recommendations of the
National Comprehensive Cancer Network guidelines. In our study, the
majority of the patients received the CMF chemotherapy regimen:
cyclophosphamide, 600 mg/m2; methotrexate, 40
mg/m2; and 5-FU, 500 mg/m2. In certain
high-risk patients, taxanes (paclitaxel, docetaxel) were added to
5-FU, epirubicin and cyclophosphamide (FEC regimen).
Follow-up
The patients were followed-up from the first day
following surgery to tumor recrudescence, metastasis or death from
any cause. Our final follow-up deadline was December 31, 2011. The
required information on therapeutic effect and prognosis was
collected mainly through letters, telephonical communication or
outpatient review. Local recurrence was defined as clinical or
histological recurrence in the ipsilateral breast, chest wall or
axillary lymph nodes. Distant metastasis referred to the clinical
and imaging identification of distant metastatic lesions. DFS was
defined as the time period from the first day after surgery to the
first local recurrence or distant metastasis. OS was measured from
the first day of follow-up.
Statistical analysis
The statistical software SPSS 17.0 (SPSS Inc.,
Chicafo, IL, USA) was used to analyze the collected data. Data were
expressed as means ± SD for continuous variables. An independent
t-test was used for the comparison of continuous variables.
Categorical variables were assessed using the Chi-square test when
appropriate. P<0.05 was considered to indicate a statistically
significant difference. A cumulative survival analysis of breast
cancer patients was performed with the Kaplan-Meier method and the
log-rank test was used for single-factor analysis. The multivariate
analysis was performed using the Cox proportional hazards
regression model.
Results
Clinical characteristics
All included patients were followed up for 6–84
months, without any losses. The patients in the TNBC group
accounted for 16.05% (156/972). The average age of the 156 TNBC
patients was 51.7 years, with a median age of 52.5 years. The
patients with TNBC had a similar age at diagnosis with non-TNBC
patients (P=0.943). The TNBC patients prior to menopause accounted
for 43.59% (68/156), which was not different from the non-TNBC
group.
In the TNBC group, the major pathological type was
infiltrative ductal carcinoma (82.05%), followed by infiltrative
lobular (5.77%) and medullary carcinoma (5.13%), which was a
distribution similar to that of the non-TNBC group (P=0.995). The
tumor diameter in TNBC patients was commonly 2–5 cm, accounting for
60.26% (94/156), with an average tumor diameter of 3.1 cm. The
percentage of tumor grade III was 51.92% in TNBC, which was
significantly higher compared with that of the non-TNBC group
(P=0.002). The cases with tumor stage ≥II accounted for 85.90%
(134/156). The percentage of axillary lymph node-positive cases was
lower in the TNBC compared to that in the non-TNBC group (P=0.009).
The number of positive lymph nodes was most commonly 1–3,
accounting for 45.45% (25/156), with 4–9 accounting for 34.55%
(19/156) and ≥10 accounting for 20% of the cases (11/156).
Clinicopathological data are summarized in Table I. The Chi-square test revealed that
there was no correlation between axillary lymph node metastasis and
the tumor size in the TNBC group (P=0.536, Table II).
 | Table IClinicopathological characteristics of
patients with breast cancer according to tumor subgroup. |
Table I
Clinicopathological characteristics of
patients with breast cancer according to tumor subgroup.
| Characteristics | Total (n=972) | Subgroup, n (%) | P-value |
|---|
|
|---|
| TNBC (n=156) | Non-TNBC (n=816) |
|---|
| Mean age at diagnosis
(years) | | 51.7 | 51.5 | 0.943 |
| Menopausal status
(%) | | | | 0.887 |
| Prior to
menopause | 416 | 68 (43.59) | 348 (42.65) | |
| Following
menopause | 556 | 88 (56.41) | 468 (57.35) | |
| Pathological
type | | | | 0.995 |
| Invasive ductal
carcinoma | 790 | 128 (82.05) | 662 (81.13) | |
| Invasive lobular
carcinoma | 57 | 9 (5.77) | 48 (5.88) | |
| Medullary
carcinoma | 51 | 8 (5.13) | 43 (5.27) | |
| Other | 74 | 11 (7.05) | 63 (7.72) | |
| Tumor size
(cm) | | | | 0.971 |
| ≤2 | 241 | 38 (24.36) | 203 (24.88) | |
| 2–5 | 592 | 94 (60.26) | 498 (61.03) | |
| >5 | 139 | 24 (15.38) | 115 (14.09) | |
| Grade (%) | | | | 0.002 |
| I–II | 646 | 75 (48.08) | 571 (69.98) | |
| III | 326 | 81 (51.92) | 245 (30.02) | |
| TNM stage | | | | 0.752 |
| I | 144 | 22 (14.10) | 122 (14.95) | |
| II | 300 | 54 (34.62) | 246 (30.15) | |
| III–IV | 528 | 80 (51.28) | 448 (54.90) | |
| Axillary lymph node
status | | | | 0.009 |
| Negative | 534 | 109 (69.87) | 425 (52.08) | |
| Positive | 438 | 47 (30.13) | 391 (47.92) | |
| Metastatic lymph
nodes | | | | 0.891 |
| 1–3 | 206 | 25 (45.45) | 181 (48.14) | |
| 4–9 | 139 | 19 (34.55) | 120 (31.91) | |
| ≥10 | 86 | 11 (20.00) | 75 (19.95) | |
| Family history | | | | 0.969 |
| Negative | 883 | 141 (90.38) | 742 (90.93) | |
| Breast cancer | 63 | 10 (6.41) | 53 (6.50) | |
| Other tumor | 26 | 5 (3.21) | 21 (2.57) | |
 | Table IIAssociation between lymph node
metastasis and primary tumor size in the TNBC group. |
Table II
Association between lymph node
metastasis and primary tumor size in the TNBC group.
| | Lymph node
metastasis | | |
|---|
| |
| | |
|---|
| T | n | Positive | Negative | χ2 | P-value |
|---|
| T1 | 38 | 16 | 22 | - | - |
| T2 | 98 | 40 | 58 | 6.349 | 0.536 |
| T3 | 20 | 14 | 6 | - | - |
| Total | 156 | 70 | 86 | - | - |
Recurrence and metastasis
In the TNBC group, a DFS was reported in 120 cases
(76.92%), whereas 36 patients (36/156, 23.08%) developed recurrence
and metastasis. Common distant metastatic sites were the lung,
bone, brain, liver, supraclavicular lymph nodes and pleura. There
were significant differences in the metastatic sites between the
TNBC and non-TNBC groups (P<0.001). Compared with non-TNBC, TNBC
patients exhibited a higher propensity for visceral and brain
metastasis (42.11 vs. 6.25%) and a lower incidence of bone
metastasis (15.79 vs. 62.50%). Related data are presented in
Table III.
 | Table IIIRecurrence or metastatic status of
breast cancer patients according to tumor subgroup (TNBC vs.
non-TNBC). |
Table III
Recurrence or metastatic status of
breast cancer patients according to tumor subgroup (TNBC vs.
non-TNBC).
| Subgroup, n
(%) | | |
|---|
|
| | |
|---|
|
Characteristics | TNBC | Non-TNBC | Total (n) | P-value |
|---|
| Recurrence or
metastasis | | | | 0.866 |
| Yes | 36 (23.08) | 180 (22.06) | 216 | |
| No | 120 (76.92) | 636 (77.94) | 756 | |
| Metastatic
site | | | | <0.001 |
| Bone | 3 (15.79) | 50 (62.50) | 53 | |
| Lung | 1 (5.26) | 2 (2.50) | 3 | |
| Liver | 4 (21.05) | 10 (12.50) | 14 | |
| Brain | 8 (42.11) | 5 (6.25) | 13 | |
| Other | 1 (5.26) | 7 (8.75) | 8 | |
| Multiple | 2 (10.53) | 6 (7.50) | 8 | |
Survival analysis
The univariate Cox's regression analysis identified
tumor subgroup (TNBC or non-TNBC) as an independent prognostic
factor associated with 7-year DFS and OS. Furthermore, tumor size,
TNM stage, axillary lymph node status and recurrence or metastasis
were also found to exert a statistically significant effect on DFS
and OS; however, regarding other variables, including age,
menstrual status, pathological type, tumor grade, number of
metastatic lymph nodes, metastatic site and family history of
breast cancer, there were no significant differences in DFS and OS
(Table IV).
 | Table IVPrognostic factors for disease-free
survival and overall survival of breast cancer patients in
univariate Cox regression analysis. |
Table IV
Prognostic factors for disease-free
survival and overall survival of breast cancer patients in
univariate Cox regression analysis.
| Disease-free
survival | Overall
survival |
|---|
|
|
|
|---|
|
Characteristics | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (≤50 vs. >50
years) | 1.029 | 0.697–1.521 | 0.884 | 1.028 | 0.695–1.519 | 0.891 |
| Menopausal status
(prior to vs. after) | 1.016 | 0.684–1.509 | 0.937 | 1.017 | 0.685–1.511 | 0.933 |
| Pathological type
(invasive ductal carcinoma vs. other) | 1.019 | 0.822–1.263 | 0.865 | 1.018 | 0.821–1.261 | 0.873 |
| Tumor size (≤2 vs.
>2 cm) | 0.099 | 0.038–0.257 | <0.0001 | 0.099 | 0.038–0.257 | <0.0001 |
| Grade (I–II vs.
III) | 1.202 | 0.804–1.798 | 0.371 | 1.198 | 0.801–1.792 | 0.379 |
| TNM staging (I–II
vs. III–IV) | 1.374 | 1.024–1.841 | 0.034 | 1.371 | 1.023–1.838 | 0.035 |
| Axillary lymph node
status (negative vs. positive) | 2.466 | 1.636–3.715 | <0.0001 | 2.438 | 1.618–3.673 | <0.0001 |
| Metastatic lymph
nodes (<4 vs. ≥4) | 1.096 | 0.931–1.291 | 0.271 | 1.098 | 0.933–1.294 | 0.260 |
| Recurrence or
metastasis (yes vs. no) | 0.639 | 0.420–0.972 | 0.036 | 0.642 | 0.422–0.976 | 0.038 |
| Metastatic site
(single vs. multiple) | 0.976 | 0.784–1.215 | 0.828 | 0.976 | 0.784–1.215 | 0.825 |
| Family history
(positive vs. negative) | 0.948 | 0.568–1.582 | 0.837 | 0.947 | 0.567–1.580 | 0.834 |
| Tumor subgroups
(TNBC vs. non-TNBC) | 0.236 | 0.159–0.350 | <0.0001 | 0.232 | 0.157–0.344 | <0.0001 |
To further analyze the possible factors affecting
the prognosis of patients with breast cancer, the multifactor Cox
proportional hazards regression model was used. The incorporated
factors included tumor size, TNM stage, axillary lymph node status,
tumor subgroups and metastatic or recurrence status. The
multivariate analysis demonstrated that tumor subgroup (TNBC or
non-TNBC) was statistically significant in 7-year DFS and OS.
Furthermore, tumor size and axillary lymph node status were
independent prognostic factors for TNBC and non-TNBC (Table V).
 | Table VPrognostic factors for disease-free
survival and overall survival of breast cancer patients in
multivariate Cox regression analysis. |
Table V
Prognostic factors for disease-free
survival and overall survival of breast cancer patients in
multivariate Cox regression analysis.
| Disease-free
survival | Overall
survival |
|---|
|
|
|
|---|
|
Characteristics | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Tumor size (≤2 vs.
>2 cm) | 0.115 | 0.044–0.302 | <0.0001 | 0.113 | 0.043–0.298 | <0.0001 |
| TNM stage (I–II vs.
III–IV) | 1.252 | 0.939–1.668 | 0.126 | 1.243 | 0.932–1.658 | 0.138 |
| Axillary lymph node
status (negative vs. positive) | 2.897 | 1.897–4.423 | <0.0001 | 2.859 | 1.873–4.362 | <0.0001 |
| Recurrence or
metastasis (yes vs. no) | 0.649 | 0.426–0.988 | 0.044 | 0.661 | 0.434–1.006 | 0.054 |
| Tumor subgroups
(TNBC vs. non-TNBC) | 0.190 | 0.127–0.283 | <0.0001 | 0.188 | 0.126–0.280 | <0.0001 |
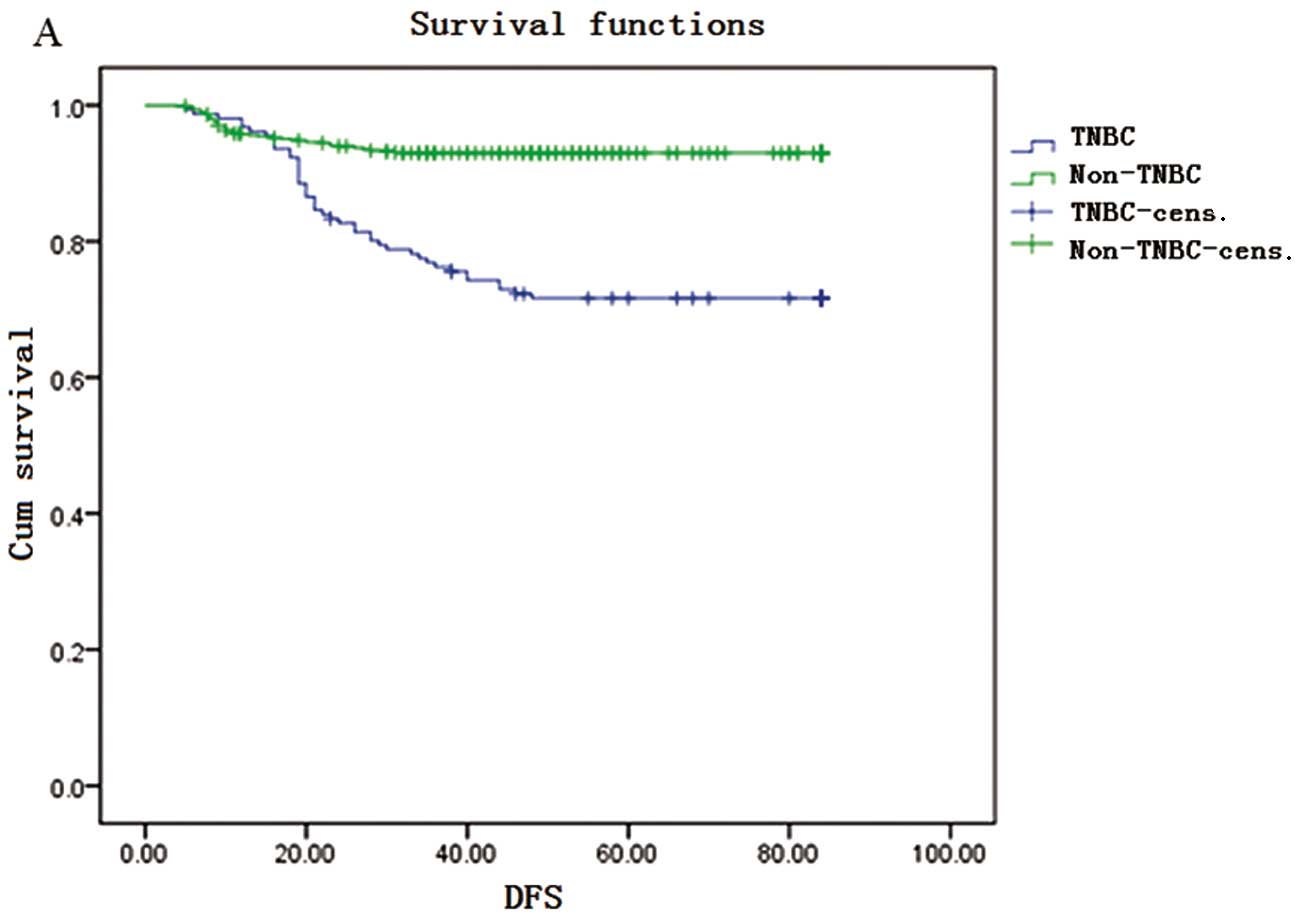
The Kaplan-Meier survival curves are shown in
Fig. 1. The DFS rate for TNBC
patients was 98, 76, 67 and 64% in the 1st, 3rd, 5th and 7th years,
respectively. The OS rate for TNBC patients was 97, 85, 72 and 68%
in the 1st, 3rd, 5th and 7th years, respectively. DFS and OS rates
were significantly lower in the TNBC compared to those in the
non-TNBC group in the 3rd, 5th and 7th years; however, in the 1st
year, the DFS rate of the TNBC was higher compared with that in the
non-TNBC group (98 vs. 95%).
Discussion
TNBC is a special subtype of breast cancer
classified according to cell morphology and cell surface receptors.
Patients with TNBC exhibit a poor prognosis due to its aggressive
biological behavior and lack of effective treatment, as this type
of cancer is insensitive to targeted and endocrine therapy. In
order to improve the outcomes for TNBC patients, ongoing studies on
TNBC are currently conducted clinically and experimentally. In this
study, we selected a cohort of 972 patients in order to analyze the
clinicopathological characteristics and prognosis of TNBC patients
in China. The included patients were selected from two different
hospitals in different cities, in order to enhance the reliability
of our results.
Numerous studies have demonstrated that
premenopausal African-American females were more prone to develop
TNBC. Carey et al (11)
reported that the morbidity rate of the TNBC subtype among
African-American breast cancer patients <50 years old may be as
high as 39%, whereas it is only 16% among Caucasian females and 14%
among post-menopausal African females. In our study, 16.5% of the
included patients had TNBC and it was demonstrated that age and
menopausal status did not significantly affect the TNBC incidence
in China, which is in accordance with the findings of previous
studies, reporting that the prevalence of TNBC among non-African
female breast cancer patients ranges between 10 and 17% and it is
lower compared to that among pre-menopausal African females
(12–14).
Kandel et al (15) demonstrated that the average tumor
size of TNBC is 2 cm and 50% of TNBC patients develop lymph node
metastases. With regard to pathological characteristics, this type
of breast cancer may exhibit three histological grades. However, in
the present study, we observed that a tumor size of 2–5 cm
represented the largest percentage in the TNBC group and axillary
lymph node metastases accounted for 30.13%. Histological grade III
accounted for the largest percentage in the TNBC group (P=0.002).
It is hypothesized that TNBC in China may differ from that
encountered in other countries.
Haffty et al (16) reported that TNBC exhibits a higher
proportion of positive family history of breast cancer. In this
study, 10 of the 156 TNBC patients (6.41%) had a family history of
breast cancer, which was not significantly higher when compared
with the non-TNBC subgroup. Furthermore, single- and multifactor
analyses did not suggest that TNBC was correlated with a family
history of breast cancer. Zhang et al (17) reported that, in China, family
history was not statistically different between the TNBC and
non-TNBC groups (P=0.180), which is in agreement with our findings.
Therefore, we may infer that triple-negative status is not
associated with an increased hereditary risk in Chinese
females.
The current investigation of the correlation between
tumor size and lymph node metastasis has produced varying results.
In a controlled study (13), it
was suggested that TNBC exhibits a higher tendency for lymph node
metastasis, compared to other types of breast cancer. By contrast,
other studies have demonstrated that there is no difference in the
frequency of lymph node metastasis in TNBC (12,16,18).
In an investigation of 292 cases of breast cancer specimens, Haffty
et al (16) suggested that
tumor size was not associated with lymph node metastasis. In this
study, for tumor sizes <2 cm, the lymph node metastatic rate was
as high as 42.1%, whereas it was 70.0% for tumor sizes >5 cm
(P>0.05). Therefore, tumor size was not found to correlate with
lymph node metastasis, which is in agreement with several studies
in China and abroad (12,16,18).
In the univariate Cox's regression analysis, our
results indicated that tumor size, TNM stage, axillary lymph node
status and recurrence or metastasis were prognostic factors for
7-year DFS and OS. Our multivariate Cox's regression analysis
demonstrated that tumor size and axillary lymph node status were
the main prognostic indicators for 7-year DFS and OS. These
findings are in accordance with those of previous studies (8,19).
Tumor subgroup (TNBC or non-TNBC) was identified as a significant
prognostic factor of breast cancer in the univariate and
multivariate survival analysis.
TNBC is prone to local recurrence and distant
metastasis. Dent et al (13) observed that, in the 5th year of
follow-up, the frequency of distant metastasis was significantly
higher among TNBC compared to that among non-TNBC patients (33.9
and 22.4%, respectively) and the risk of distant metastasis was
higher in the TNBC group (relative risk=2.6). There was a gradual
increase in the risk of distant metastasis in the TNBC group, with
a peak in the 2nd and 3rd years, followed by a rapid decline, with
a lower risk in the 5th year and no distant metastasis in the 8th
year of follow-up. In non-TNBC patients, the risk of distant
metastasis during follow-up appeared to remain constant over the
follow-up period. TNBC exhibits a propensity for organ-specific
metastasis. Rakha et al (12)reported that TNBC is more likely to
metastasize to the spinal cord, brain, meninges, liver and lungs,
but rarely to the bones. A study conducted at the M.D. Anderson
Cancer Center also reported that TNBC was associated with a higher
risk of visceral and a lower risk of bone metastases (20). TNBC has a higher risk of local
recurrence or distant metastasis following the final diagnosis.
This suggests that distant metastasis may exhibit a certain organ
tendency in TNBC (21–23) and the specific target organ
metastasis may be associated with its specific gene expression
(24–26). Consistently, our study demonstrated
that, compared to non-TNBC patients, TNBC patients had a higher
propensity for visceral metastasis (liver, 21.05 and lung, 5.26%),
as well as brain metastasis (42.11%), with a lower incidence of
bone metastasis (15.79 vs. 62.50%).
The TNBC subgroup exhibited a more aggressive
clinical course and a higher risk of recurrence and mortality when
compared to the non-TNBC group, with a 5- and 7-year survival rate
of 72 and 68%, respectively. Non-Hispanic females of African
descent exhibited the worst prognosis, with a 5-year survival rate
of only 14%. Khan et al (20) reported that of 282 African-American
female patients with a median age of 57 years, TNBC accounted for
30% of the cases. In this study, in the 1st year, the DFS rate for
TNBCs was 98%, which was higher compared with that in the non-TNBC
group. This may be attributed to the currently accepted view that
TNBC appears to be more sensitive to chemotherapy compared to
non-TNBC (27); thus, TNBC
patients may achieve higher short-term DFS rates. However, TNBC
patients have a worse OS (28) and
tend to relapse sooner compared to patients with other breast
cancer subtypes. This is mainly due to the shortened disease-free
period rendering the tumor more aggressive. Yin et al
(7) reported that, in multivariate
analysis, TNBC exhibited a significantly increased recurrence rate
within 2 years after surgery, which is inconsistent with our
findings. TNBC patients have a worse 7-year DFS and OS compared to
non-TNBC patients, with the risk of any recurrence increasing
sharply from the date of diagnosis, peaking at 1–2 years and
decreasing quickly thereafter, which was similar to the findings
reported by Dent et al (13).
The Cox model regression analysis results revealed
that axillary lymph node status was an independent prognostic
factor for TNBC (P=0.001). Our results were in accordance with the
majority of the previously published literature.
In conclusion, TNBC has its own unique clinical
pathological and molecular characteristics. Due to the lack of
specific treatment guidelines following surgery, despite the
administration of chemotherapy and radiotherapy, the prognosis of
TNBC patients remains poor. Efforts are currently focused on
developing a specific therapy for TNBC. It was reported that TNBC
may have a specific signal transduction pathway which is crucial in
the occurrence and development of breast cancer (29–31),
which may provide a novel approach for clinical treatment based on
molecular markers and investigation of this specific pathway.
Acknowledgements
The authors would like to thank Professor Borong
Pan, Outpatient Department of Oncology, Cancer Institute, Fourth
Military Medical University (Xi'an, China), for offering advice
regarding the modifications to this study.
Abbreviations:
|
TNBC
|
triple-negative breast cancer
|
|
ER
|
estrogen receptor
|
|
PgR
|
progesterone receptor
|
|
HER2
|
human epidermal growth factor receptor
2
|
References
|
1
|
Alkis N, Durnali AG, Arslan UY, et al:
Optimal timing of adjuvant treatment in patients with early breast
cancer. Med Oncol. 28:1255–1259. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jha K, Shukla M and Pandey M: Survivin
expression and targeting in breast cancer. Surg Oncol. 21:125–131.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Perou CM, Sorlie T, Eisen MB, et al:
Molecular portraits of human breast tumours. Nature. 406:747–752.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ono M, Tsuda H, Shimizu C, et al:
Tumor-infiltrating lymphocytes are correlated with response to
neoadjuvant chemotherapy in triple-negative breast cancer. Breast
Cancer Res Treat. 132:793–805. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hicks DG, Short SM, Prescott NL, et al:
Breast cancers with brain metastases are more likely to be estrogen
receptor negative, express the basal cytokeratin CK5/6, and
overexpress HER2 or EGFR. Am J Surg Pathol. 30:1097–1104. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oakman C, Viale G and Di Leo A: Management
of triple negative breast cancer. Breast. 19:312–321. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yin WJ, Lu JS, Di GH, et al:
Clinicopathological features of the triple-negative tumors in
Chinese breast cancer patients. Breast Cancer Res Treat.
115:325–333. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin C, Chien SY, Kuo SJ, et al: A 10-year
follow-up of triple-negative breast cancer patients in Taiwan. Jpn
J Clin Oncol. 42:161–167. 2012.PubMed/NCBI
|
|
9
|
Kurebayashi J, Moriya T, Ishida T, et al:
The prevalence of intrinsic subtypes and prognosis in breast cancer
patients of different races. Breast. 16(Suppl 2): S72–S77. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin C, Chien SY, Chen LS, Kuo SJ, Chang TW
and Chen DR: Triple negative breast carcinoma is a prognostic
factor in Taiwanese women. BMC Cancer. 9:1922009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Carey LA, Perou CM, Livasy CA, et al:
Race, breast cancer subtypes, and survival in the Carolina Breast
Cancer Study. JAMA. 295:2492–2502. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rakha EA, El-Sayed ME, Green AR, Lee AH,
et al: Prognostic markers in triple-negative breast cancer. Cancer.
109:25–32. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dent R, Trudeau M, Pritchard KI, et al:
Triple-negative breast cancer: clinical features and patterns of
recurrence. Clin Cancer Res. 13:4429–4434. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Calza S, Hall P, Auer G, et al: Intrinsic
molecular signature of breast cancer in a population-based cohort
of 412 patients. Breast Cancer Res. 8:R342006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kandel MJ, Stadler Z, Masciari S, et al:
Prevalence of BRCA1 mutations in triple negative breast cancer
(BC). J Clin Oncol. 24:5082006.
|
|
16
|
Haffty BG, Yang Q, Reiss M, et al:
Locoregional relapse and distant metastasis in conservatively
managed triple negative early-stage breast cancer. J Clin Oncol.
24:5652–5657. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang L, Hao C, Dong G and Tong Z:
Analysis of clinical features and outcome of 356 triple-negative
breast cancer patients in China. Breast Care (Basel). 7:13–17.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tischkowitz M, Brunet JS, Begin LR, et al:
Use of immunohistochemical markers can refine prognosis in triple
negative breast cancer. BMC Cancer. 7:1342007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nishimura R and Arima N: Is triple
negative a prognostic factor in breast cancer? Breast Cancer.
15:303–308. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Khan AM, Sabnani I, Tsang P, et al:
Triple-negative early-stage breast cancer in African American
women: A fuel to the fire. J Clin Oncol. 26:110392008.
|
|
21
|
Reis-Filho JS, Milanezi F, Steele D, et
al: Metaplastic breast carcinomas are basal-like tumours.
Histopathology. 49:10–21. 2006. View Article : Google Scholar
|
|
22
|
Abdulkarim BS, Cuartero J, Hanson J,
Deschenes J, Lesniak D and Sabri S: Increased risk of locoregional
recurrence for women with T1-2N0 triple-negative breast cancer
treated with modified radical mastectomy without adjuvant radiation
therapy compared with breast-conserving therapy. J Clin Oncol.
29:2852–2858. 2011. View Article : Google Scholar
|
|
23
|
Hernandez-Aya LF, Chavez-Macgregor M, Lei
X, et al: Nodal status and clinical outcomes in a large cohort of
patients with triple-negative breast cancer. J Clin Oncol.
29:2628–2634. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Minn AJ, Gupta GP, Siegel PM, et al: Genes
that mediate breast cancer metastasis to lung. Nature. 436:518–524.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bertucci F, Finetti P, Cervera N, et al:
Gene expression profiling shows medullary breast cancer is a
subgroup of basal breast cancers. Cancer Res. 66:4636–4644. 2006.
View Article : Google Scholar
|
|
26
|
Fadare O, Wang SA and Hileeto D: The
expression of cytokeratin 5/6 in invasive lobular carcinoma of the
breast: evidence of a basal-like subset? Hum Pathol. 39:331–336.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Carey LA, Dees EC, Sawyer L, et al: The
triple negative paradox: primary tumor chemosensitivity of breast
cancer subtypes. Clin Cancer Res. 13:2329–2334. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kaplan HG, Malmgren JA and Atwood M: T1N0
triple negative breast cancer: risk of recurrence and adjuvant
chemotherapy. Breast J. 15:454–460. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yin WJ, Lu JS, Di GH, Lin YP, Zhou LH, Liu
GY, Wu J, Shen KW, Han QX, Shen ZZ and Shao ZM: Clinicopathological
features of the triple-negative tumors in Chinese breast cancer
patients. Breast Cancer Res Treat. 115:325–333. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Thike AA, Cheok PY, Jara-Lazaro AR, Tan B,
Tan P and Tan PH: Triple-negative breast cancer:
clinicopathological characteristics and relationship with
basal-like breast cancer. Mod Pathol. 23:123–133. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shah SN, Cope L, Poh W, et al: HMGA1: a
master regulator of tumor progression in triple-negative breast
cancer cells. PLoS One. 8:e634192013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Maire V, Baldeyron C, Richardson M, et al:
TTK/hMPS1 is an attractive therapeutic target for triple-negative
breast cancer. PLoS One. 8:e637122013. View Article : Google Scholar : PubMed/NCBI
|