Introduction
Lung cancer is the most common type of malignancy
and constitutes the leading cause of cancer death worldwide.
Non-small cell lung cancer (NSCLC) comprises 85% of all lung cancer
cases, and ∼55–65% of patients present with advanced or stage
III/IV disease at the time of diagnosis (1). The prognosis in patients with
untreated advanced NSCLC is extremely poor, with a median survival
time of ≤6 months.
Among the patients who are newly diagnosed with
NSCLC, 30–40% of them are aged ≥70 years (2). The management of this cohort of
patients constitutes a challenge for medical oncologists. Elderly
patients are a specific population that requires special care, due
to the fact that they have metabolic changes and increased
likelihood of comorbidities. They are characterised by relatively
inferior immune system and functions of the major organs, which
should be taken into consideration when selecting the appropriate
chemotherapy in the clinical setting. Clinical trials on the
treatment of advanced NSCLC in the elderly are limited, and no
optimal regimen has been identified. The Multicenter Italian Lung
Cancer in the Elderly Study (MILES) phase III randomized trial
(3) compared the efficacy and
toxicity of the combination of vinorelbine plus gemcitabine with
sinlge-drug treatment in elderly patients with advanced NSCLC.
Tumor response and overall survival were not observed after
combination therapy, while increased toxicity was reported. Thus,
monochemotherapy is believed to be a priority in the treatment of
elderly patients with advanced NSCLC.
Pemetrexed is a novel antifolate cytotoxic
chemotherapy agent that targets multiple folate-dependent enzymatic
pathways, which inhibit multiple enzymes involved in purine and
pyrimidine synthesis, thereby effectively inhibiting both DNA and
RNA synthesis (4). As a promising
drug, pemetrexed has demonstrated good antitumor activity in the
treatment of various solid tumors in previous clinical studies
(5). The aim of this retrospective
study was to evaluate the efficacy of pemetrexed vs. vinorelbine in
elderly patients with previously untreated advanced non-squamous
NSCLC.
Patients and methods
Eligibility criteria
Consecutive patients aged ≥70 years with
histologically- or cytologically-diagnosed stage IIIB/IV
non-squamous NSCLC were included in this study. Detailed inclusion
criteria were the following: at least one measurable lesion, life
expectancy of >3 months, Eastern Cooperative Oncology Group
(ECOG) performance status 0–2; adequate marrow reserve (leukocyte
count ≥3.8×109/l and absolute neutrophil
≥1.5×109/l, platelets ≥90×109/l and
hemoglobin ≥90 g/l) and hepatic [alanine aminotransferase (ALT) and
aspartate aminotransaminase (AST) <1.5-fold of the upper limit
of normal value, total bilirubin <1-fold of the upper limit of
normal value), as well as renal (<45 ml/min in calculated
creatinine clearance rate) functions. This study was conducted
according to ICH Good Clinical Practice guidelines and written
informed consent was obtained from the patients or their
families.
Treatment plan
Patients in the pemetrexed group received pemetrexed
500 mg/m2 as >10-min intravenous infusion on day 1 of
a 3-week cycle. Premedication was as follows: 1,000 μg
vitamin B12 injected at the beginning of 1 week prior to day 1 of
cycle 1 and repeated every 3 cycles, 400 μg folic acid
ingested orally on a daily basis starting 1 week prior to the first
cycle of chemotherapy and continued until 3 weeks after the therapy
completion, 4 mg dexamethasone ingested orally twice per day from
the day before to the day after each dose of pemetrexed. Patients
in the vinorelbine group were administered tri-weekly vinorelbine
25 mg/m2 intravenous infusion on days 1 and 8.
Vinorelbine treatment was delayed on day 8 when
leukocyte count, platelet count and hemoglobin level were
<2.0×109/l, <50×109/l and <60 g/l,
respectively, and was withheld until the patient had a minimum
leukocyte count of 3.8×109/l, a minimum platelet count
of 90×109/l and a hemoglobin level of ≥90 g/l,
respectively. Patients were withdrawn from the study when >5
weeks had elapsed from day 1 of any cycle until these criteria were
satisfied. The presence of grade 4 neutropenia led to a reduction
in the doses of pemetrexed and vinorelbine by 100 mg/m2
and 5 mg/m2, respectively, in the subsequent cycle.
Treatment was interrupted at any time in the event of progressive
disease. Treatment was discontinued when the patient experienced
unacceptable toxicity, withdrew consent or refused treatment.
Assessments
Prior to treatment, the patients underwent a
complete medical history and physical examination, fiberoptic
bronchoscopy, cervical to abdominal computed tomography (CT), a
brain magnetic resonance imaging (MRI), an electrocardiogram (ECG),
pulmonary function tests and a radionuclide bone scan. Laboratory
examinations included a routine blood, liver and renal function
tests, as well as routine electrolyte analysis. Baseline tumor
measurements were taken ≤2 weeks before treatment. The physical and
laboratory examinations were performed weekly. Chest CT was
repeated every two cycles to evaluate tumor response and the
Response Evaluation Criteria in Solid Tumors (RECIST) were
recommended (6). Toxic-effect
grades were based on version 3.0 of the National Cancer Institute
Common Terminology Criteria (7).
Statistical analysis
The primary objective was to determine whether
pemetrexed improved survival compared to vinorelbine. Overall
survival was calculated from the initiation of treatment to the
date of death due to any cause or last follow-up. Progression-free
survival was calculated from the initiation of treatment to the
date of disease progression, recurrence or death due to any cause.
Survival curves were constructed according to the Kaplan-Meier
method and were compared using the log-rank test. The χ2
test was used in the response rate comparison and toxicity
analysis. Statistical analyses were performed using the Statistical
Package for Social Science (SPSS) 16.0 and two-sided P-values of
<0.05 were considered to indicate statistically significant
difference.
Results
Between January 2009 and March 2011, 62 patients
were included the study. Among them, 36 patients were treated with
pemetrexed monochemotherapy regimen, and 26 with the single-agent
vinorelbine. Demographic and clinical characteristics of patients
are summarized in Table I.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristics | Pemetrexed, n (%)
(n=36) | Vinorelbine, n (%)
(n=26) |
|---|
| Age, years | | |
| Median (range) | 75 (70–83) | 74.5 (70–84) |
| Gender | | |
| Male | 30 (83.3) | 21 (80.8) |
| Female | 6 (16.7) | 5 (19.2) |
| ECOG performance
status | | |
| 0–1 | 34 (94.4) | 25 (96.2) |
| 2 | 2 (5.6) | 1 (3.8) |
| Stage | | |
| IIIB | 11 (30.6) | 8 (30.8) |
| IV | 25 (69.4) | 18 (69.2) |
| Histologic type | | |
| Adenocarcinoma | 35 (97.2) | 24 (92.3) |
| Large cell | 1 (2.8) | 0 (0) |
| Other | 0 (0) | 2 (7.7) |
| Organs involved in
cancer | | |
| 1 | 1 (2.8) | 0 (0) |
| 2 | 13 (36.1) | 11 (42.3) |
| 3 | 11 (30.6) | 9 (34.6) |
| ≥4 | 11 (30.6) | 6 (23.1) |
| Comorbid illness | | |
| Presence | 15 (41.7) | 10 (38.5) |
| Absence | 21 (58.3) | 16 (61.5) |
The median number of cycles received was six in the
pemetrexed group (range, 3–10) and four in the vinorelbine group
(range, 2–6), which was significantly different (P=0.035). In
total, 20 (55.6%) of the 36 pemetrexed-treated patients completed
≥6 cycles and 14 (53.8%) of the 26 vinorelbine-treated patients
completed ≥4 cycles of chemotherapy.
Second-line treatment was administered to 57
patients (92.0%; 33 pemetrexed-treated and 24 vinorelbine-treated
patients). Among the patients initially treated with vinorelbine, 7
patients received second-line pemetrexed treatment; while 16
pemetrexed-treated and nine vinorelbine-treated patients received
albumin-bound paclitaxel as second-line therapy. Twenty-five
patients (40.3%) received second-line gefitinib or erlotinib
treatment: 17 patients (47.2%) in the pemetrexed group and 8
patients (30.8%) in the vinorelbine group. Optimum supportive care
was provided to the remaining 5 patients.
Response and survival
We did not observe complete response (CR) in the two
groups of patients. Disease control rate [(DCR = complete response
(CR) + partial response (PR) + stable disease (SD)] was
significantly increased in the pemetrexed compared to the
vinorelbine group (80.5 vs. 65.3%; P=0.011) (Table II). Progressive disease during
treatment occurred in 19.4% of pemetrexed-treated patients and in
34.6% of vinorelbine-treated patients, and a statistically
significant difference was observed (P=0.021).
 | Table IIPatient response to treatment. |
Table II
Patient response to treatment.
| Response | Pemetrexed, n (%)
(n=36) | Vinorelbine, n (%)
(n=26) |
|---|
| CR | 0 (0) | 0 (0) |
| PR | 8 (22.2) | 3 (11.5) |
| SD | 21 (58.3) | 14 (53.8) |
| Progressive
disease | 7 (19.4) | 9 (34.6) |
| DCR | 80.5 | 65.3 |
The follow-up ended on January 31, 2012, with the
median follow-up period being 16.5 months (range, 6.5–38).
Thirty-six (58.1%) of the 62 patients succumbed to the disease
(pemetrexed group, n=20; vinorelbine group, n=16). The median
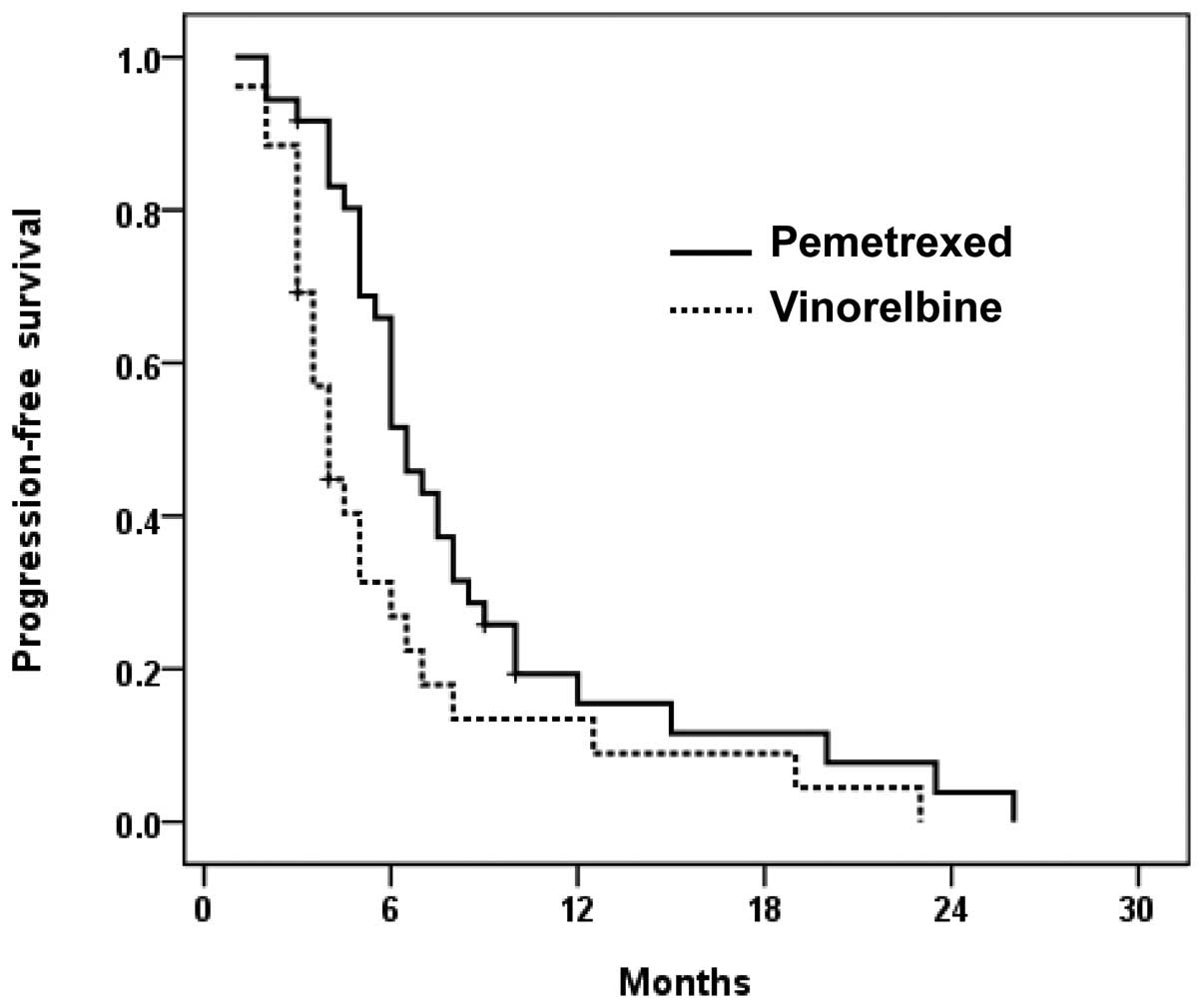
progression-free survival time with pemetrexed was longer compared
to vinorelbine (6.5 vs. 4.0 months, P=0.018) (Fig. 1). One-year survival rates were 71.5
and 53.3% for the pemetrexed and vinorelbine groups, respectively.
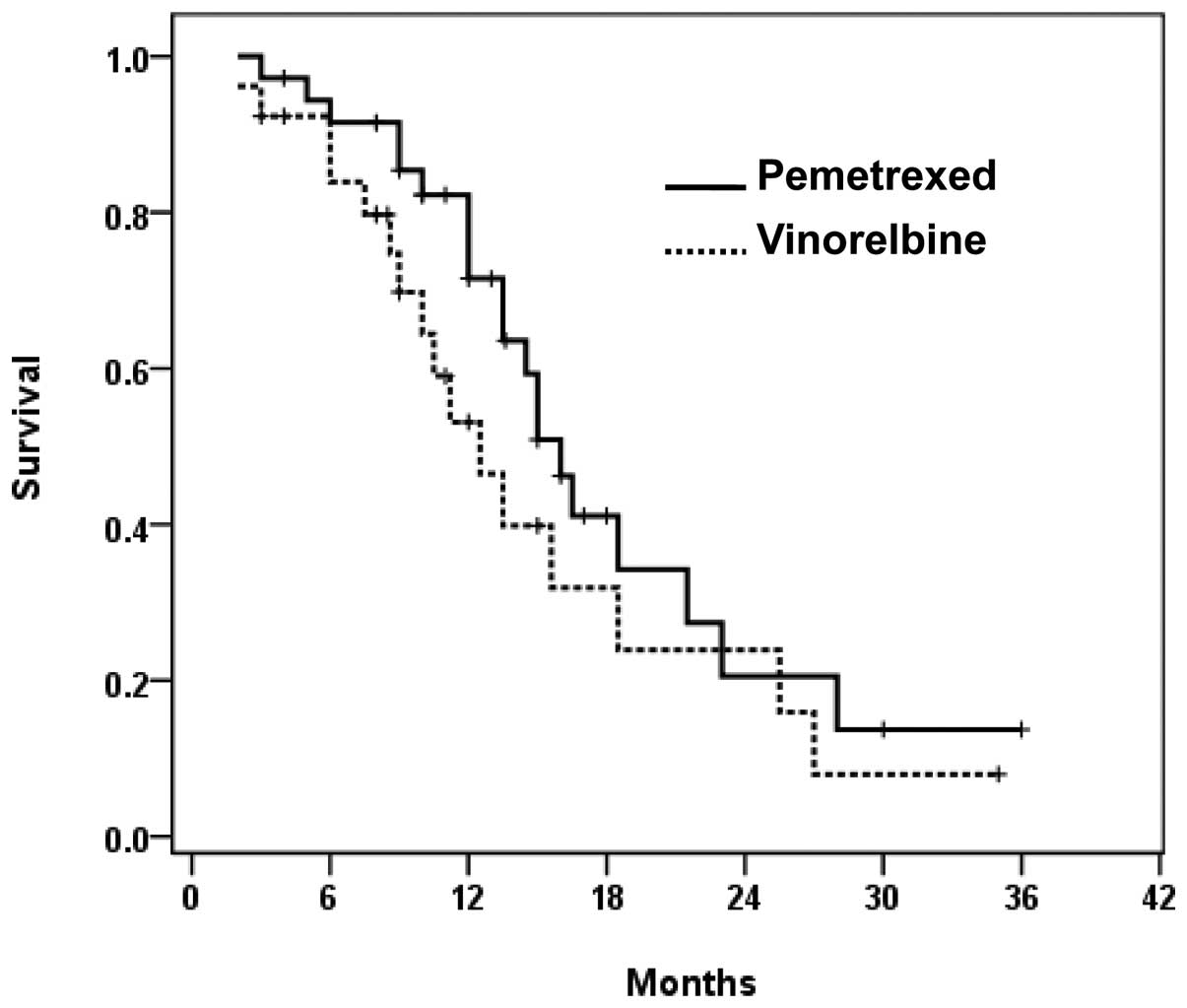
The median overall survival times were 16.0 months in the
pemetrexed group, and 12.5 months in the vinorelbine group,
indicating that pemetrexed prolonged median survival time by 3.5
months, while the overall survival distributions were not
statistically significant (P=0.191) (Fig. 2).
Toxicity
Toxicity was assessed in all the patients and
cycles, and the major toxicities are summarized in Table III. Neutropenia occurred in more
patients in the vinorelbine group compared to the pemetrexed group,
grade 3–4 neutropenia was noted in 53.8 and 11.1% of patients in
the two groups, respectively (P<0.001). The frequencies of
anemia and thrombocytopenia were higher in the vinorelbine group
compared to that in the pemetrexed group, however, no statistically
significant differences were identified (P>0.05).
Nausea/vomiting and infection occurred more frequently in the
vinorelbine group compared to the pemetrexed group (P<0.01).
Overall toxicity in the two treatment groups was generally mild and
well-tolerated in elderly patients with advanced NSCLC.
 | Table IIIPemetrexed and vinorelbine
toxicities. |
Table III
Pemetrexed and vinorelbine
toxicities.
| Pemetrexed, n (%)
(n=36) | Vinorelbine, n (%)
(n=26) | |
|---|
|
|
|---|
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | P-value |
|---|
| Hematologic | | | | | | | | | |
| Neutropenia | 13 (36.1) | 5 (13.9) | 3 (8.3) | 1 (2.8) | 1 (3.8) | 10 (38.5) | 9 (34.6) | 5 (19.2) | 0.000 |
| Anemia | 9 (25.0) | 4 (11.1) | 0 | 0 | 14 (53.8) | 8 (30.8) | 1 (3.8) | 0 | 0.355 |
|
Thrombocytopenia | 8 (22.2) | 2 (5.6) | 0 | 0 | 11 (42.3) | 5 (19.2) | 2 (7.7) | 0 | 0.106 |
| Non-hematologic | | | | | | | | | |
| Fatigue | 15 (41.7) | 1 (2.8) | 0 | 0 | 17 (65.4) | 2 (7.7) | 0 | 0 | 0.834 |
| Appetite loss | 7 (19.4) | 1 (2.8) | 0 | 0 | 14 (53.8) | 3 (11.5) | 0 | 0 | 0.623 |
|
Nausea/vomiting | 3 (8.3) | 0 | 1 (2.8) | 0 | 6 (23.1) | 3 (11.5) | 0 | 0 | 0.001 |
| Constipation | 2 (5.6) | 2 (5.6) | 0 | 0 | 5 (19.2) | 4 (15.4) | 0 | 0 | 0.725 |
| Diarrhea | 3 (8.3) | 1 (2.8) | 0 | 0 | 3 (11.5) | 0 | 0 | 0 | - |
| ALT/AST | 5 (13.9) | 0 | 0 | 0 | 6 (23.1) | 1 (3.8) | 0 | 0 | 0.130 |
| Creatinine | 1 (2.8) | 0 | 0 | 0 | 1 (3.8) | 0 | 0 | 0 | - |
| Infection | 2 (5.6) | 0 | 0 | 0 | 1 (3.8) | 2 (7.7) | 0 | 0 | 0.007 |
| Peripheral
neuropathy | 0 | 0 | 0 | 0 | 1 (3.8) | 0 | 0 | 0 | - |
Discussion
The population of elderly patients with NSCLC is on
the increase in China as well as in Western countries due to a
general increase in life expectancy. NSCLC is a common disease in
the elderly population and the provision of optimal treatment to
elderly NSCLC patients is becoming an important issue, since
age-related impairment of organ function and presence of
potentially complicating comorbid conditions should be taken into
consideration.
First-line chemotherapy treatment is not frequently
administered to elderly patients with advanced stage NSCLC.
However, it has become useful in recent years, due to the fact that
chemotherapy with a third-generation agent (gemcitabine, taxane or
vinorelbine) has significantly improved median survival and quality
of life in those patients (3,8–10).
In the USA, 28% of elderly stage IIIB/IV NSCLC patients diagnosed
in 1997 were administered chemotherapy, which increased to 36% of
patients diagnosed in 2002 (11).
Combination chemotherapy is used with caution in elderly patients
due to the high risk of adverse events and a lower ability to
tolerate the potential toxicity, thus single agents are generally
accepted by oncologists as first-line therapy. Single-agent
pemetrexed has been considered a standard treatment option in
previously-treated advanced or metastatic NSCLC, as an objective
response and symptomatic benefit in combination with a favorable
safety profile were provided (12). However, limited data are available
on the efficacy and toxicity of pemetrexed in elderly patients with
advanced non-squamous NSCLC.
Our findings show that pemetrexed demonstrated a
significantly higher DCR (80.5 vs. 65.3%; P=0.011), and an
improvement in progression-free survival (6.5 vs. 4.0 months;
P=0.018) compared to vinorelbine in elderly patients with advanced
non-squamous NSCLC. Pemetrexed-treated patients also exhibited an
increased 1-year survival rate (71.5 vs. 53.3%) and a longer median
survival time (16.0 vs. 12.5 months) compared to
vinorelbine-treated patients, while the differences were not
statistically significant. Limitations of this study were the
limited number of patients and the high proportion of patients
(92.0%) subsequently receiving second-line therapy.
In this study, the survival rates following
vinorelbine treatment were higher compared to those reported in
other studies; single-agent vinorelbine as first-line chemotherapy
in elderly NSCLC patients has previously indicated 1-year survival
rates of 13–38% and median survival times of 4.5–9.9 months
(3,10,13,14).
Notably, the median survival time of 16.0 months with pemetrexed
treatment in this study was comparable to that reported for
platinum-doublet chemotherapies assessed in recent studies in
chemotherapy-naive non-squamous NSCLC patients, where median
survival times of 11.4–17.3 months were reported (15,16).
There are at least three possible ways to explain the prolonged
median survival time in the two treatment groups in this study. One
possibility constitutes the relatively improved prognosis of the
included patients. A second possibility is that the increased
survival rates could have been a result of the significant
proportion of patients receiving second-line treatment. A third
possibility is that Asian patients are sensitive to epidermal
growth factor receptor-tyrosine kinase inhibitors (EGFR-TKI), 47.2
and 30.8% of patients who were treated with pemetrexed and
vinorelbine, respectively, received second-line treatment with
gefitinib or erlotinib.
Age is a pivotal factor in the treatment,
decision-making and cancer patient outcomes. The physiological
hematopoietic capacity affected by aging may lead to an increased
susceptibility to cytotoxic therapy. Several studies (?) have been
conducted to evaluate cytotoxic agents in elderly patients with
advanced NSCLC. The Elderly Lung Cancer Vinorelbine Italian Study
(ELVIS) investigated for the first time the effects of vinorelbine
on the quality of life and survival of elderly patients with
advanced NSCLC in 1999 (13).
Compared to optimum supportive care alone, the patients who
received vinorelbine had a longer median duration of survival (6.4
vs. 4.8 months) and were significantly more likely to survive up to
one year (32 vs. 14%). In addition, patients receiving vinorelbine
exhibited improved outcome compared to controls on measures related
to lung cancer symptoms and pain as well as on social, cognitive
and physical functioning. The conclusive results of the MILES study
also recommend that single-agent vinorelbine or gemcitabine should
be preferred over the combination therapy as palliative treatment
for elderly patients with advanced NSCLC (3). The WJTOG study investigated the
efficacy and safety of docetaxel vs. vinorelbine in elderly
patients with advanced NSCLC (10). Compared to vinorelbine, the
docetaxel elevated tumor response rate ranged from 9.9 to 22.7%
(P=0.019) and increased median progression-free survival from 3.1
to 5.5 months (P<0.001). There was no statistical difference in
the median overall survival with docetaxel vs. vinorelbine (14.3
vs. 9.9 months; P=0.138). Docetaxel monotherapy was therefore
considered a standard treatment option, while it was associated
with increased treatment-related toxicity; the incidence of grade
3–4 neutropenia and leucopenia was 82.9 and 58.0%,
respectively.
In the present study, the toxicity profiles for the
two treatment groups were mild and tolerable. However, severe
neutropenia occurred significantly less often with pemetrexed
treatment. The incidence of anemia and thrombocytopenia was also
lower in the pemetrexed group compared to the vinorelbine group,
while these differences were not statistically significant. In this
study, patients treated with pemetrexed also experienced a
relatively lower incidence of non-hematologic toxicities compared
with patients treated with vinorelbine, a fact indicating that
pemetrexed is well-tolerated among patients ≥70 years.
In conclusion, pemetrexed treatment improved DCR,
progression-free survival, and presented a lower incidence of
treatment-related adverse events compared with vinorelbine
treatment in elderly patients with advanced non-squamous NSCLC.
However, overall survival was not significantly improved. Based on
these findings, pemetrexed monotherapy might be considered a good
option in the treatment of elderly patients with advanced
non-squamous NSCLC.
References
|
1.
|
Ettinger DS, Bepler G, Bueno R, et al:
Non-small cell lung cancer. Clinical practice guidelines in
oncology. J Natl Compr Canc Netw. 4:548–582. 2006.PubMed/NCBI
|
|
2.
|
Extermann M: Measuring comorbidity in
older cancer patients. Eur J Cancer. 36:453–471. 2000. View Article : Google Scholar
|
|
3.
|
Gridelli C, Perrone F, Gallo C, et al:
Chemotherapy for elderly patients with advanced non-small-cell lung
cancer: the Multicenter Italian Lung Cancer in the Elderly Study
(MILES) phase III randomized trial. J Natl Cancer Inst. 95:362–372.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Hanauske AR, Eismann U, Oberschmidt O, et
al: In vitro chemosensitivity of freshly explanted tumor cells to
pemetrexed is correlated with target gene expression. Invest New
Drugs. 25:417–423. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Rollins KD and Lindley C: Pemetrexed: a
multitargeted anti-folate. Clin Ther. 27:1343–1382. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors European Organization for Research and Treatment of Cancer,
National Cancer Institute of the United States, National Cancer
Institute of Canada. J Natl Cancer Inst. 92:205–216. 2000.
View Article : Google Scholar
|
|
7.
|
National Cancer Institute: Cancer Therapy
Evaluation Program: Investigator’s handbook: A Handbook for
Clinical Investigators Conducting Therapeutic Clinical Trials
Supported by CTEP, DCTD, NCI. http://ctep.cancer.gov/investigatorResources/investigators_handbook.htm.
Accessed: January 24, 2012.
|
|
8.
|
Gridelli C: The ELVIS trial: a phase III
study of single-agent vinorelbine as first-line treatment in
elderly patients with advanced non-small cell lung cancer. Elderly
Lung Cancer Vinorelbine Italian Study Oncologist. 6:4–7.
2001.PubMed/NCBI
|
|
9.
|
Nakamura Y, Sekine I, Furuse K and Saijo
N: Retrospective comparison of toxicity and efficacy in phase II
trials of 3-h infusions of paclitaxel for patients 70 years of age
or older and patients under 70 years of age. Cancer Chemother
Pharmacol. 46:114–118. 2000.PubMed/NCBI
|
|
10.
|
Kudoh S, Takeda K, Nakagawa K, et al:
Phase III study of docetaxel compared with vinorelbine in elderly
patients with advanced non-small-cell lung cancer: results of the
West Japan Thoracic Oncology Group Trial (WJTOG 9904). J Clin
Oncol. 24:3657–3663. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Lang K, Marciniak MD, Faries D, et al:
Trends and predictors of first-line chemotherapy use among elderly
patients with advanced non-small cell lung cancer in the United
States. Lung Cancer. 63:264–270. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Cohen MH, Johnson JR, Wang YC, et al: FDA
drug approval summary: pemetrexed for injection (Alimta) for the
treatment of non-small cell lung cancer. Oncologist. 10:363–368.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Effects of vinorelbine on quality of life
and survival of elderly patients with advanced non-small-cell lung
cancer. The Elderly Lung Cancer Vinorelbine Italian Study Group. J
Natl Cancer Inst. 91:66–72. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Frasci G, Lorusso V, Panza N, et al:
Gemcitabine plus vinorelbine versus vinorelbine alone in elderly
patients with advanced non-small-cell lung cancer. J Clin Oncol.
18:2529–2536. 2000.PubMed/NCBI
|
|
15.
|
Kubota K, Nishiwaki Y, Ohashi Y, et al:
The Four-Arm Cooperative Study (FACS) for advanced non-small-cell
lung cancer (NSCLC). J Clin Oncol (ASCO Annual Meeting
Proceedings). 14S:abs. 7006. 2004.
|
|
16.
|
Mok TS, Wu YL, Thongprasert S, et al:
Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N
Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|