Introduction
Nasopharyngeal carcinoma (NPC) exhibits an extremely
high incidence in Southern China and Southeast Asia, particularly
among Cantonese individuals living in Guangdong province, with an
incidence of up to 20 per 100,000 individuals (1–4). NPC
is highly radiosensitive and radiotherapy (RT) is currently the
mainstay of treatment. Previous studies reported that the 5-year
survival rate was 66–83% with RT (5–7). For
conventional 2-dimensional (2D) RT, the survival rates of stage
T1–2/N0-1 NPC patients reached 75–90%; however, the survival rates
of stage T3–4/N2–3 patients were decreased to 50–75% (8). With the development of the RT
technique, including 3D conformal RT (CRT) and intensity-modulated
RT (IMRT), a local control of >90% was achieved in stage I/II
NPC patients (9–11). Furthermore, the improvement in the
survival of NPC patients may also be attributed to the application
of chemotherapy. Concurrent chemoradiotherapy was considered as the
standard of care for patients with locally advanced NPC, with 3-
and 5-year overall survival (OS) rates of ∼87% and ∼75%,
respectively (12–15).
A long-term cure is the most important outcome for
NPC. Over the last few decades, the aim to improve the long-term
outcome translated into the use of OS as the primary endpoint for
NPC prognostic studies and clinical trials (6–8).
Improved local control has been achieved by IMRT treatment and
resulted in the use of locoregional control (LRC) as the primary
endpoint for NPC treated with IMRT (11,13).
Historically, the 5-year survival rate has been the most commonly
used measurement for the comparison of the prognosis and the
assessment of the success of any particular treatment. The 5-year
survival endpoint is simple to measure, easy to interpret,
clinically meaningful and straightforward to explain; however, it
requires extended follow-up. In order to overcome this
disadvantage, an endpoint reached in <5 years is required. The
new endpoint shares the advantages of the 5-year survival mentioned
above, but may also provide answers to the questions posed by the
study more rapidly. Therefore, the purpose of our study was to
investigate OS and LRC at <5 years as a possible alternative to
the 5-year survival endpoint for NPC.
Materials and methods
Patient population
We reviewed the medical records of 2,820 patients
who were newly diagnosed with NPC which had been confirmed by
biopsy without distant metastasis in the Sun Yat-Sen University
Cancer Center (Guangzhou, China), in the period between November,
2000 and December, 2004. An ethical approval was provided by the
Sun Yat-set University Cancer Center. The patients with missing
information or who were lost to follow-up within 5 years were
excluded (n=370). A total of 2,450 patients were finally included
in our study. Taking into consideration that the RT technique may
alter the survival outcome and affect the results of the study, we
further analyzed patients who had received either conventional 2D
RT (n=1,842) or IMRT (n=157).
A receiver operating characteristic curve was used
to determine the optimal threshold difference value of age, with a
cut-off value of 49.5 years (sensitivity, 54.5% and specificity,
65.6%) in this study. Tumor staging was performed according to the
Union for International Cancer Control (UICC, 2002) staging system.
All patients completed the prescribed radical RT treatment, course
with or without chemotherapy.
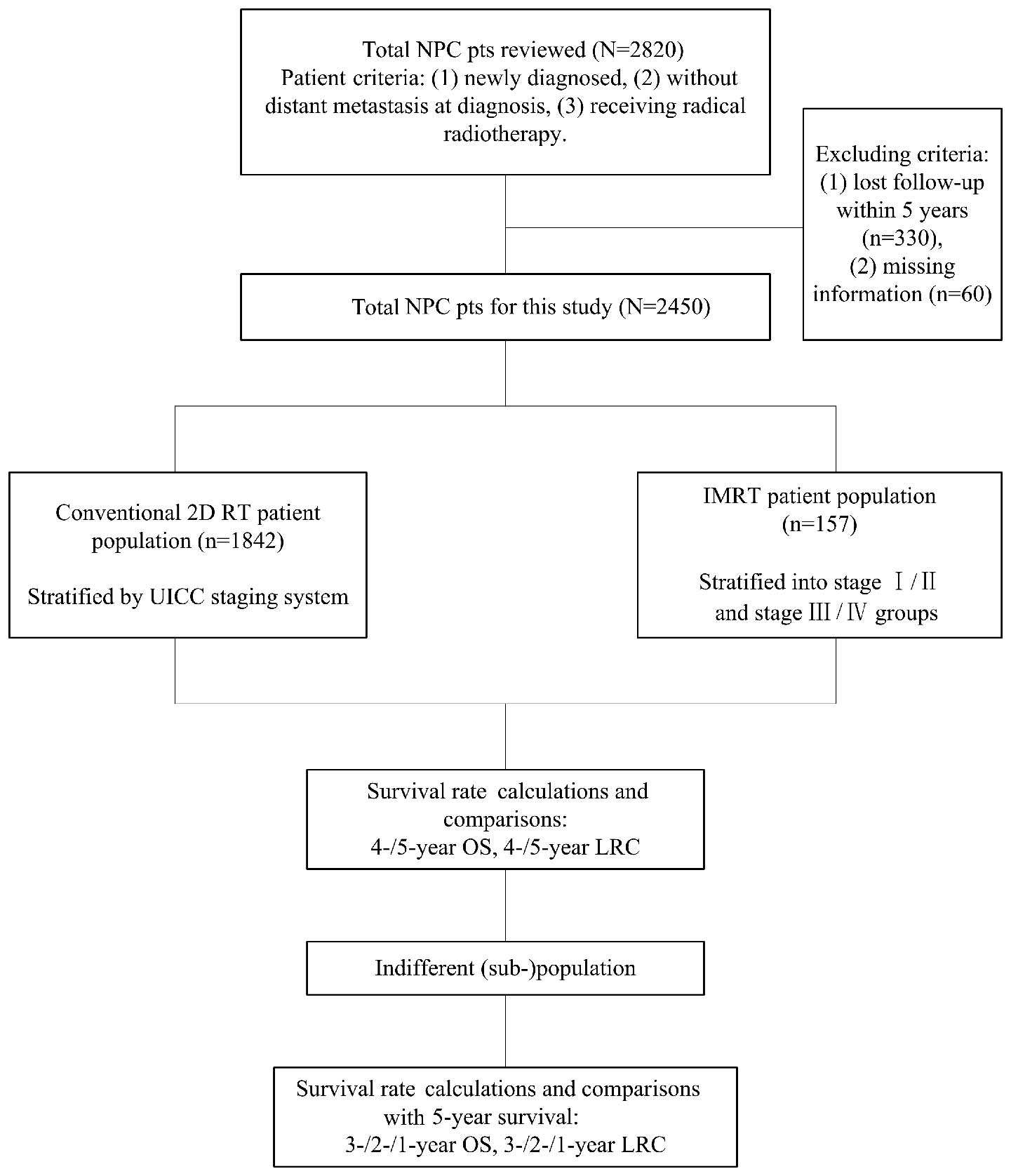
Study design. The flowchart of our study design is
presented in Fig. 1. We calculated
and compared the survival rates of the three patient populations at
4 and 5 years. Tumor stage was found to significantly affect
survival outcome; therefore, we repeated the analysis in patients
stratified by the UICC staging system in the three populations. If
a population or sub-population exhibited no significant difference
between the 4- and 5-year survival, we further calculated the 3-,
2- and 1-year survival rates and compared these to the 5-year
survival rate. The survival rate of the last indifferent comparison
was considered as the alternative endpoint to the 5-year
survival.
Treatment
RT alone was administered to stage I/II NPC patients
and RT combined with chemotherapy was administered to stage III/IV
NPC patients. RT was a conventional fractionation with a
high-energy 6–8 MV X-ray from a linear accelerator. Facial-cervical
field isocenter radiation with a low-melting point lead block was
used; the irradiation field included the skull base, nasopharynx
and neck. The facial-cervical and lower cervical anterior tangent
fields were used first, with the addition of the anterior nasal
field in cases with invasion of the nasal cavity, to a dose of 36
Gy, followed by the bilateral preauricular fields plus the anterior
tangent field, to a total dose of 60–78 Gy. Chemotherapy included
induction, concomitant and adjuvant chemotherapy. The
chemotherapeutic regimen was mainly cisplatin plus 5-fluorouracil
for 1–3 cycles.
Follow-up
The patients were followed up by phone and/or in the
outpatient clinic. The follow-up items included survival status,
LRC failure and distant metastasis. All the events were confirmed
by pathological examination and/or imaging. The last date of
follow-up was February, 2011.
Endpoints and statistical analysis
Two endpoints were selected, OS and LRC. OS was
defined as time from diagnosis to death from any cause. LRC was
defined as time from diagnosis to the first occurrence of tumor
growth at the primary site or regional lymph nodes.
The survival rates were calculated using a life
table. Survival curves were drawn using the Kaplan-Meier method
with the two-sided log-rank test. Survival rate comparisons were
performed with the McNemar’s test. All the tests were two-tailed
and P<0.05 was considered to indicate a statistically
significant difference. The statistical analyses were performed
with Statistical Product and Service Solutions software, version
17.0 (SPSS Inc., Chicago, IL, USA).
Results
Demographics
The baseline characteristics of the patients are
summarized in Table I. The most
common pathological type was non-keratinizing undifferentiated
carcinoma, accounting for 86.7% of the cases.
 | Table I.Baseline characteristics of the entire
patient population (n=2,450). |
Table I.
Baseline characteristics of the entire
patient population (n=2,450).
| Characteristics | No. | Percentage |
|---|
| Age (years) | | |
| ≤49 | 1502 | 61.3 |
| >50 | 948 | 38.7 |
| Gender | | |
| Female | 585 | 23.9 |
| Male | 1865 | 76.1 |
| UICC clinical
stage | | |
| I | 127 | 5.2 |
| II | 864 | 35.3 |
| III | 986 | 40.2 |
| IV | 473 | 19.3 |
| UICC T stage | | |
| T1 | 396 | 16.2 |
| T2 | 1032 | 42.1 |
| T3 | 626 | 25.5 |
| T4 | 396 | 16.2 |
| UICC N stage | | |
| N0 | 641 | 26.2 |
| N1 | 981 | 40.0 |
| N2 | 738 | 30.1 |
| N3 | 90 | 3.7 |
| Treatment | | |
| RT | 1095 | 44.7 |
|
Chemoradiotherapy | 1355 | 55.3 |
| RT modality | | |
| Conventional 2D
RT | 1842 | 75.2 |
| 3D CRT | 451 | 18.4 |
| IMRT | 157 | 6.4 |
Survival rate comparisons in the entire
patient population
The 4- and 5-year OS and LRC rates are presented in
Table II. The differences between
the rates were found to be statistically significant
(P<0.001).
 | Table II.Survival rates at 4 and 5 years and
their comparisons. |
Table II.
Survival rates at 4 and 5 years and
their comparisons.
| Stratification | OS (%)
| LRC (%)
| P-value
|
|---|
| 4-year | 5-year | 4-year | 5-year | 4- vs. 5-year
OS | 4- vs. 5-year
LRC |
|---|
| Total patients | 78.8 | 74.6 | 83.3 | 80.5 | <0.001 | <0.001 |
| UICC clinical
stage | | | | | | |
| I | 95.0 | 93.0 | 97.6 | 93.7 | 0.063 | 0.063 |
| II | 83.0 | 79.0 | 87.3 | 84.1 | <0.001 | <0.001 |
| III | 76.0 | 72.0 | 82.2 | 79.8 | <0.001 | <0.001 |
| IV | 64.0 | 59.0 | 74.8 | 71.7 | <0.001 | <0.001 |
| UICC T
stage | | | | | | |
| T1 | 86.0 | 83.0 | 89.4 | 86.9 | <0.001 | 0.002 |
| T2 | 78.0 | 74.0 | 84.1 | 81.0 | <0.001 | <0.001 |
| T3 | 76.0 | 73.0 | 83.4 | 81.3 | <0.001 | <0.001 |
| T4 | 66.0 | 60.0 | 75.3 | 71.5 | <0.001 | <0.001 |
| UICC N
stage | | | | | | |
| N0 | 88.0 | 84.0 | 90.3 | 86.6 | <0.001 | <0.001 |
| N1 | 76.0 | 72.0 | 83.1 | 80.4 | <0.001 | <0.001 |
| N2 | 71.0 | 67.0 | 78.7 | 76.2 | <0.001 | <0.001 |
| N3 | 55.0 | 55.0 | 74.4 | 73.3 | 0.500 | 1.000 |
| 2D RT patients | 76.4 | 72.0 | 81.8 | 78.7 | <0.001 | <0.001 |
| UICC clinical
stage | | | | | | |
| I | 98.9 | 94.4 | 98.9 | 94.4 | 0.125 | 0.125 |
| II | 82.5 | 77.9 | 86.1 | 82.8 | <0.001 | <0.001 |
| III | 74.1 | 70.2 | 80.2 | 77.8 | <0.001 | <0.001 |
| IV | 63 | 57.7 | 71.8 | 68 | <0.001 | <0.001 |
| UICC T
stage | | | | | | |
| T1 | 85 | 80.9 | 88.1 | 85 | <0.001 | 0.004 |
| T2 | 77.8 | 73.4 | 82.9 | 79.8 | <0.001 | <0.001 |
| T3 | 75.7 | 72.3 | 81.7 | 79.6 | <0.001 | 0.004 |
| T4 | 65.1 | 59 | 72.5 | 68.1 | <0.001 | <0.001 |
| UICC N
stage | | | | | | |
| N0 | 88.7 | 83.3 | 89.1 | 84.6 | <0.001 | <0.001 |
| N1 | 75.2 | 70.9 | 81.1 | 78.3 | <0.001 | <0.001 |
| N2 | 69.4 | 65.3 | 77.2 | 74.8 | <0.001 | <0.001 |
| N3 | 55.2 | 53.5 | 72.9 | 71.2 | 1.000 | 1.000 |
| IMRT patients | 90.4 | 87.9 | 92.4 | 91.7 | 0.125 | 1.000 |
| UICC clinical
stage | | | | | | |
| I | 100 | 100 | 100 | 100 | 1.000 | 1.000 |
| II | 91.2 | 91.2 | 94.1 | 94.1 | 1.000 | 1.000 |
| III | 88.9 | 87.5 | 91.7 | 90.3 | 1.000 | 1.000 |
| IV | 88.6 | 80 | 88.6 | 88.6 | 0.250 | 1.000 |
| UICC T
stage | | | | | | |
| T1 | 97.1 | 94.1 | 97.1 | 97.1 | 1.000 | 1.000 |
| T2 | 90.2 | 90.2 | 92.7 | 92.7 | 1.000 | 1.000 |
| T3 | 85.2 | 83.3 | 90.7 | 88.9 | 1.000 | 1.000 |
| T4 | 92.9 | 85.7 | 89.3 | 89.3 | 0.500 | 1.000 |
| UICC N
stage | | | | | | |
| N0 | 92.9 | 90.5 | 92.9 | 92.9 | 1.000 | 1.000 |
| N1 | 90.7 | 90.7 | 96.3 | 96.3 | 1.000 | 1.000 |
| N2 | 90.7 | 87 | 88.9 | 87 | 0.500 | 1.000 |
| N3 | 71.4 | 57.1 | 85.7 | 85.7 | 1.000 | 1.000 |
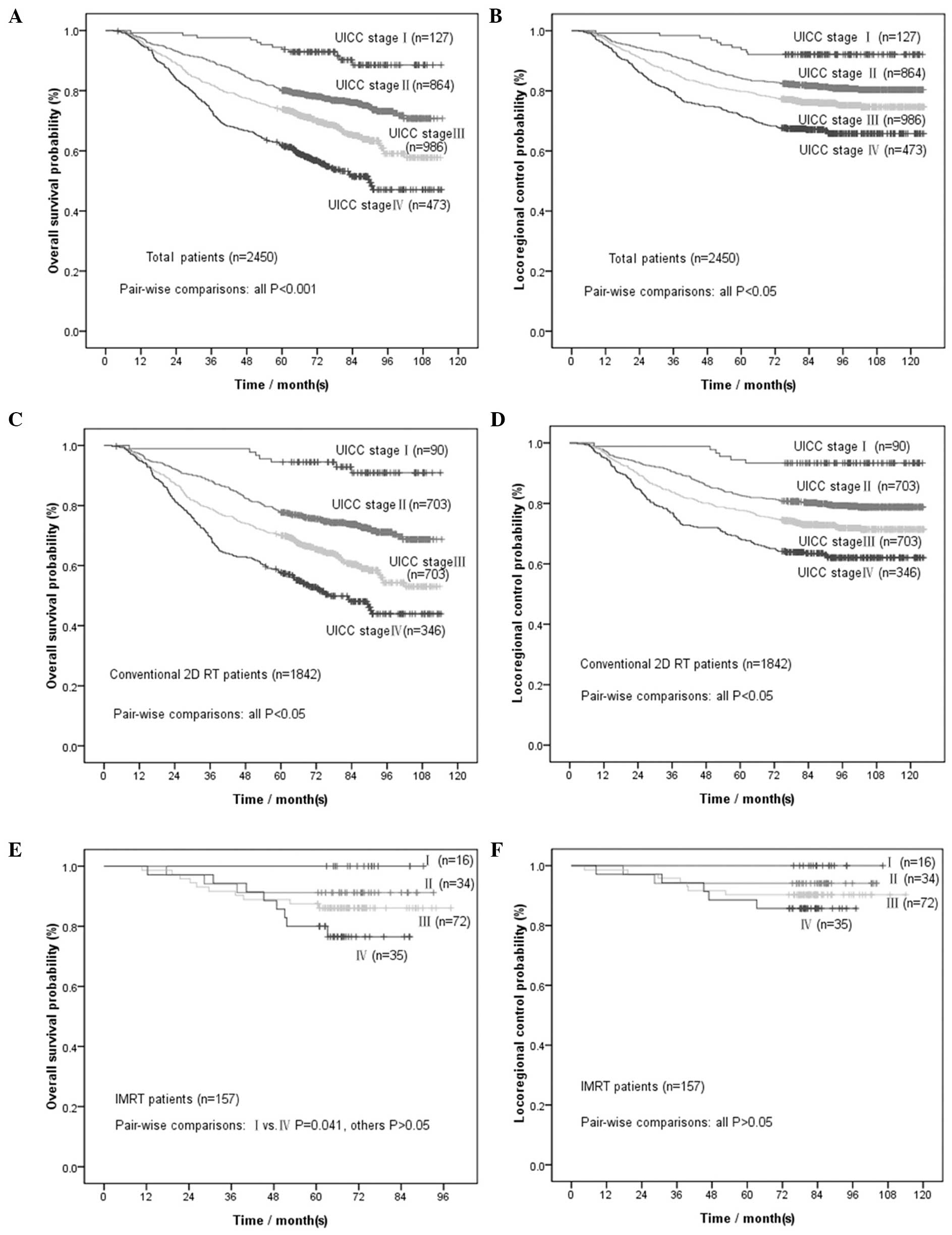
We further stratified patients according to the UICC
staging system. The corresponding survival rates are presented in
Table II. Survival rate
comparisons were performed for each stage (P-values shown in
Table II). The OS and LRC curves
by UICC clinical stage are presented in Fig. 2A and B. All the comparisons
exhibited statistically significant differences, except between
patients with UICC clinical stages I and N3. Further comparison of
the 3-, 2- and 1-year survival rates to the 5-year survival rate
for stages I and N3 (Table III)
revealed that, for patients with UICC clinical stage I, the 3-year
OS may be selected as an alternative endpoint to the 5-year OS
(P=0.063) and for patients with UICC stage N3 the 3-year LRC may be
selected as an alternative endpoint to the 5-year LRC
(P=0.063).
 | Table III.Survival rates at 1, 2 and 3 years
and comparisons with 5-year survival rates. |
Table III.
Survival rates at 1, 2 and 3 years
and comparisons with 5-year survival rates.
| Stratification | OS% (P-value)
| LRC% (P-value)
|
|---|
| 1-year | 2-year | 3-year | 1-year | 2-year | 3-year |
|---|
| Total patients | | | | | | |
| UICC clinical
stage I | 99.2 (0.016) | 99.2 (0.016) | 97.6 (0.063) | 99.2 (0.016) | 99.2 (0.016) | 98.4 (0.031) |
| UICC N3
stage | 94.4
(<0.001) | 75.3
(<0.001) | 66.3 (0.002) | 94.4
(<0.001) | 82.2 (0.008) | 78.9 (0.063) |
| 2D RT patients | | | | | | |
| UICC clinical
stage I | 98.9 (0.125) | 98.9 (0.125) | 98.9 (0.125) | 98.9 (0.125) | 98.9 (0.125) | 98.9 (0.125) |
| UICC N3
stage | 93.3
(<0.001) | 69.5 (0.002) | 62.8 (0.031) | 93.3
(<0.001) | 78.3 (0.125) | 76.7 (0.250) |
| IMRT patients | | | | | | |
| Stage I/II | 100 (0.250) | 98.0 (0.500) | 96.0 (1.000) | 100 (0.500) | 98.0 (1.000) | 96.0 (1.000) |
| Stage
III/IV | 99.1
(<0.001) | 96.3
(<0.001) | 92.5 (0.008) | 98.1 (0.004) | 97.2 (0.008) | 95.3 (0.031) |
Survival rate comparisons in patients
treated with 2D RT
The 4- and 5-year OS and LRC rates are presented in
Table II. The differences between
the rates were found to be statistically significant
(P<0.001).
We further stratified patients according to the UICC
staging system. The corresponding survival rates and P-values of
the comparisons for each stage are presented in Table II. The OS and LRC curves by UICC
clinical stage are presented in Fig.
2C and D. All comparisons exhibited statistically significant
differences, except between patients with UICC clinical stages I
and N3. Further comparison of the 3-, 2- and 1-year survival rates
to the 5-year survival rate for stages I and N3 (Table III) revealed that, for patients
with UICC stage I, the 1-year OS and LRC may be used as an
alternative endpoint to the 5-year OS and LRC (P=0.125), whereas
for patients with UICC stage N3, the 2-year LRC may be used instead
of the 5-year LRC (P=0.125).
Survival rate comparisons in patients
treated with IMRT
The 4- and 5-year OS and LRC rates are presented in
Table II. The differences between
the 4- and 5-year rates were not found to be statistically
significant (P=0.125 for OS and P=1.000 for LRC, Table II), whereas there were
statistically significant differences between the 3- and 5-year
rates (P=0.004 for OS and P=0.031 for LRC). We further stratified
patients according to the UICC staging system. The comparisons of
the 4- and 5-year rates for each stage are presented in Table II. All comparisons were found to be
of no statistical significance. The OS and LRC curves according to
UICC clinical stage are presented in Fig. 2E and F.
Considering the limited sample size for each
clinical stage in IMRT-treated patients (16 stage I, 34 stage II,
72 stage III and 35 stage IV patients), we divided the IMRT
patients into stage I/II and III/IV groups for further analysis.
For the 50 patients with stage I/II NPC, the 1-, 2-, 3-, 4- and
5-year OS rates were 100, 98, 96, 94 and 94%, whereas the LRC rates
were 100, 98, 96, 96 and 96%, respectively. Comparisons were
performed between the 4- and 5-year rates (P=1.00 for OS and P=1.00
for LRC); between the 3- and 5-year rates (P=1.00 for OS and P=1.00
for LRC); between the 2- and 5-year rates (P=0.50 for OS and P=1.00
for LRC); and between the 1- and 5-year rates (P=0.25 for OS and
P=0.50 for LRC) (Table III).
For the 107 patients with stage III/IV NPC, the 1-,
2-, 3-, 4- and 5-year OS rates were 99.1, 96.3, 92.5, 88.8 and
85.0%, whereas the LRC rates were 98.1, 97.2, 95.3, 90.7 and 89.7%,
respectively. Comparisons were performed between the 4- and 5-year
rates (P=0.125 for OS and P=1.00 for LRC); between the 3- and
5-year rates (P=0.008 for OS and P=0.031 for LRC); between the 2-
and 5-year rates (P<0.001 for OS and P=0.008 for LRC); and
between the 1- and 5-year rates (P<0.001 for OS and P=0.004 for
LRC) (Table III). Only the 4-year
survival rates were not significantly different from the 5-year
rates.
Discussion
The present study was conducted to investigate
whether the OS or LRC at <5 years are possible alternative
endpoints to the 5-year OS or LRC for NPC. The confirmation of such
a finding may enable clinical trials to be completed more quickly
with shorter alternative endpoints, meta-analyses may involve a
larger number of trials and potential novel therapeutic agents or
treatment modalities may be made available to patients more
rapidly. In the present study, we confirmed that OS and LRC at
<5 years may indeed be considered as alternative endpoints to
the 5-year OS and LRC for NPC.
Our results indicated that the 3-year OS and the
4-year LRC may be used as alternative endpoints for patients with
UICC clinical stage I. In addition, the 4-year OS and the 3-year
LRC may be used as alternative endpoints for patients with UICC
stage N3, regardless of the treatment technique. For patients
treated with 2D RT, the 1-year OS and LRC may be used as
alternative endpoints for stage I NPC patients, whereas the 4-year
OS and the 2-year LRC may be used as alternative endpoints for N3
stage patients. For patients treated with IMRT, the 1-year OS and
LRC may be used as alternative endpoints for stage I/II patients,
whereas the 4-year OS and LRC may be used as alternative endpoints
for stage III/IV patients. Shorter endpoints were not established
by our study.
For patients treated with 2D RT, only those with
UICC clinical stages I and N3 exhibited alternative endpoints at
<5 years, which is associated with the survival trend in each
UICC stage. As shown in Fig. 2C and
D, the survival curve for stage I was smooth prior to 5 years,
whereas the other curves exhibited a downward trend until 7 or 8
years. Similarly, N3 stage also presented a smooth curve prior to 5
years. The same phenomenon was also observed for 1- to 5-year
survival rates (Tables II and
III). Therefore, we investigated
the possibility of alternative endpoints for UICC stages I and
N3.
Although alternative endpoints were identified for
stages I and N3, they were the two extremes of survival. The
alternative endpoint for stage I represented stable and good
curative effects and that for stage N3 represented stable but poor
curative effects. The two stages quickly reached a plateau. This
finding was closely associated with the treatment technique.
Previous studies reported that control of stage I NPC with
conventional 2D RT is usually successful, but the response of
locoregionally advanced NPC, such as stage N3 NPC, remains poor
(5,7,16–19).
In our study, conventional 2D RT alone was successful in increasing
OS and LRC in >90% of stage I patients during the 5-year
follow-up. Patients with stage I NPC had a significantly low risk
of mortality and LRC failure. However, for stage N3 patients, the
rates of OS and LRC were decreased from 90% in the 1st year to
60–70% in the 2nd year, indicating that the patients were at high
risk, particularly short-term risk, of mortality, LRC failure and,
potentially, distant metastasis. Although the alternative endpoint
for stage I appears to be encouraging, as regards the OS and LRC
for stage N3 cases, there is still room for improvement.
As regards patients treated with IMRT, we identified
alternative endpoints for all the patients at <5 years, due to
the improvement in survival. IMRT has the advantage of dose
conformity, delivering high-radiation dose to the primary tumor,
while sparing critical organ/tissues at risk, which results in
enhancing the therapeutic ratio (20–24).
A number of previous studies reported encouraging results with
>90% LRC in patients treated with IMRT (9,25–29).
In our study, patients treated with IMRT also exhibited higher OS
and LRC rates compared to conventional 2D RT techniques (>85%
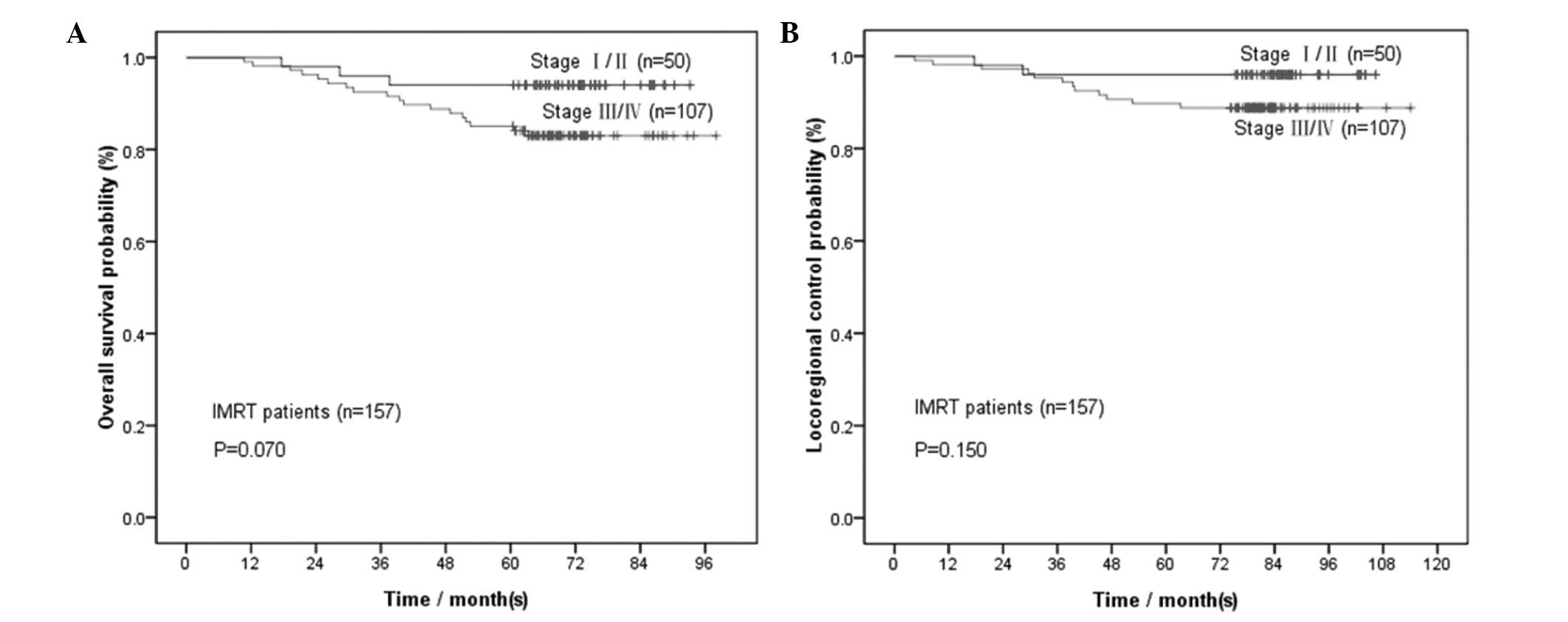
for OS and >90% for LRC). As shown in Fig. 3A and B, the OS and LRC curves for
stages I/II were almost smooth from 1 to 5 years, whereas those of
stages III/IV started to become smooth from the 4th year onwards.
The same trends were also indicated by the 1- to 5-year survival
rates of patients treated with IMRT (Tables II and III).
The 1-year OS and LRC as alternative endpoints for
stage I/II NPC patients treated with IMRT indicated that these
patients suffered from few tumor-related events, such as mortality
and locoregional control failure; thus, good and stable curative
effects were achieved. However, no shorter endpoint, other than the
4-year OS and LRC, was confirmed for stage III/IV patients treated
with IMRT, possibly due to the fact that stage III/IV NPC patients
are more prone to develop distant metastases compared to those with
stage I/II disease, due to either T3/T4 or N2/N3 involvement.
Although excellent local control was achieved with IMRT, patients
still exhibited distant failure (13,25–28).
The alternative endpoints of IMRT were superior to
those of 2D RT, regarding universality and stabilization. In
patients treated with IMRT, the alternative endpoints of 4-year OS
and LRC were extended to all the patients and were even shortened
to 1-year OS and LRC for stage I/II patients, which indicated that
a significant improvement in OS and LRC was achieved by IMRT.
Over the last few years, an increasing number of
studies have focused on IMRT in NPC, either for stages I/II or III/
IV. However, a number of those studies only calculated OS and LRC
within a 2- to 4-year follow-up period (9,11,12,25–28,30–32),
giving rise to the question whether these OS and LRC rates were the
same as the 5-year OS and LRC rates. The results of our study
indicated that the rates were indeed comparable. Therefore, some of
those studies may be included in meta-analyses of 5-year
endpoints.
Our study had the following advantages and clinical
significance: first, we reviewed a large patient sample (n=2,450)
using different types of RT techniques, including conventional 2D
RT, 3D CRT and IMRT; second, the follow-up of our study was ≥7
years; third, to the best of our knowledge, our study was the first
to focus on investigating OS or LRC at <5 years as possible
alternative endpoints to the 5-year endpoint for NPC; and finally,
our study indicated that it may be feasible to use OS and LRC at
<5 years as the primary endpoints in NPC clinical trials and
shorten the trial period.
The main limitation of our study was its
retrospective nature. The majority of the patients in our study
were treated by 2D RT; thus, the results obtained from the entire
patient cohort were biased and closer to the results of 2D RT.
Furthermore, the number of patients treated with IMRT was limited
and they were only divided into stage I/II and III/ IV groups,
rather than being stratified by the UICC staging system.
In conclusion, our study provided sound evidence
supporting the use of OS and LRC endpoints at <5 years as an
alternative to the 5-year endpoint for NPC; however, our results
require further confirmation. Even shorter endpoints may be
expected in the future with the improvements in NPC patient
survival.
Acknowledgements
This study was supported by the
Hi-Tech Research and Development Program of China (grant no.
2006AA02Z4B4).
References
|
1.
|
Chang ET and Adami HO: The enigmatic
epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol
Biomarkers Prev. 15:1765–1777. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Busson P, Keryer C, Ooka T and Corbex M:
EBV-associated nasopharyngeal carcinomas: from epidemiology to
virus-targeting strategies. Trends Microbiol. 12:356–360. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
No authors listed: Cancer incidence in
five continents Volume VIII. IARC Sci Publ. 2002:1–781. 2002.
|
|
4.
|
Wei WI and Sham JS: Nasopharyngeal
carcinoma. Lancet. 365:2041–2054. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Yi JL, Gao L, Huang XD, et al:
Nasopharyngeal carcinoma treated by radical radiotherapy alone:
Ten-year experience of a single institution. Int J Radiat Oncol
Biol Phys. 65:161–168. 2006.PubMed/NCBI
|
|
6.
|
Chen CY, Han F, Zhao C, et al: Treatment
results and late complications of 556 patients with locally
advanced nasopharyngeal carcinoma treated with radiotherapy alone.
Br J Radiol. 82:452–458. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Lee AW, Sze WM, Au JS, et al: Treatment
results for nasopharyngeal carcinoma in the modern era: the Hong
Kong experience. Int J Radiat Oncol Biol Phys. 61:1107–1116. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Lee AW, Poon YF, Foo W, et al:
Retrospective analysis of 5037 patients with nasopharyngeal
carcinoma treated during 1976–1985 overall survival and patterns of
failure. Int J Radiat Oncol Biol Phys. 23:261–270. 1992.PubMed/NCBI
|
|
9.
|
Kwong DL, Pow EH, Sham JS, et al:
Intensity-modulated radiotherapy for early-stage nasopharyngeal
carcinoma: a prospective study on disease control and preservation
of salivary function. Cancer. 101:1584–1593. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Kam MK, Leung SF, Zee B, et al:
Prospective randomized study of intensity-modulated radiotherapy on
salivary gland function in early-stage nasopharyngeal carcinoma
patients. J Clin Oncol. 25:4873–4879. 2007. View Article : Google Scholar
|
|
11.
|
Lee N, Harris J, Garden AS, et al:
Intensity-modulated radiation therapy with or without chemotherapy
for nasopharyngeal carcinoma: radiation therapy oncology group
phase II trial 0225. J Clin Oncol. 27:3684–3690. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
He X, Ou D, Ying H, Zhu G, Hu C and Liu T:
Experience with combination of cisplatin plus gemcitabine
chemotherapy and intensity-modulated radiotherapy for
locoregionally advanced nasopharyngeal carcinoma. Eur Arch
Otorhinolaryngol. 269:1027–1033. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Xiao WW, Huang SM, Han F, et al: Local
control, survival, and late toxicities of locally advanced
nasopharyngeal carcinoma treated by simultaneous modulated
accelerated radiotherapy combined with cisplatin concurrent
chemotherapy: long-term results of a phase 2 study. Cancer.
117:1874–1883. 2011. View Article : Google Scholar
|
|
14.
|
Langendijk JA, Leemans CR, Buter J,
Berkhof J and Slotman BJ: The additional value of chemotherapy to
radiotherapy in locally advanced nasopharyngeal carcinoma: a
meta-analysis of the published literature. J Clin Oncol.
22:4604–4612. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Baujat B, Audry H, Bourhis J, et al:
Chemotherapy in locally advanced nasopharyngeal carcinoma: an
individual patient data meta-analysis of eight randomized trials
and 1753 patients. Int J Radiat Oncol Biol Phys. 64:47–56. 2006.
View Article : Google Scholar
|
|
16.
|
Chua DT, Sham JS, Kwong DL and Au GK:
Treatment outcome after radiotherapy alone for patients with stage
I–II nasopharyngeal carcinoma. Cancer. 98:74–80. 2003.PubMed/NCBI
|
|
17.
|
Yeh SA, Tang Y, Lui CC, Huang YJ and Huang
EY: Treatment outcomes and late complications of 849 patients with
nasopharyngeal carcinoma treated with radiotherapy alone. Int J
Radiat Oncol Biol Phys. 62:672–679. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Chow E, Payne D, O’Sullivan B, et al:
Radiotherapy alone in patients with advanced nasopharyngeal cancer:
comparison with an intergroup study. Is combined modality treatment
really necessary? Radiother Oncol. 63:269–274. 2002. View Article : Google Scholar
|
|
19.
|
Xiao WW, Han F, Lu TX, Chen CY, Huang Y
and Zhao C: Treatment outcomes after radiotherapy alone for
patients with early-stage nasopharyngeal carcinoma. Int J Radiat
Oncol Biol Phys. 74:1070–1076. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Xia P, Fu KK, Wong GW, Akazawa C and
Verhey LJ: Comparison of treatment plans involving
intensity-modulated radiotherapy for nasopharyngeal carcinoma. Int
J Radiat Oncol Biol Phys. 48:329–337. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Cheng JC, Chao KS and Low D: Comparison of
intensity modulated radiation therapy (IMRT) treatment techniques
for nasopharyngeal carcinoma. Int J Cancer. 96:126–131. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Kam MK, Chau RM, Suen J, Choi PH and Teo
PM: Intensity-modulated radiotherapy in nasopharyngeal carcinoma:
dosimetric advantage over conventional plans and feasibility of
dose escalation. Int J Radiat Oncol Biol Phys. 56:145–157. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Wu VW, Kwong DL and Sham JS: Target dose
conformity in 3-dimensional conformal radiotherapy and intensity
modulated radiotherapy. Radiother Oncol. 71:201–206. 2004.
View Article : Google Scholar
|
|
24.
|
Veldeman L, Madani I, Hulstaert F, De
Meerleer G, Mareel M and De Neve W: Evidence behind use of
intensity-modulated radiotherapy: a systematic review of
comparative clinical studies. Lancet Oncol. 9:367–375. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Lee N, Xia P, Quivey JM, et al:
Intensity-modulated radiotherapy in the treatment of nasopharyngeal
carcinoma: an update of the UCSF experience. Int J Radiat Oncol
Biol Phys. 53:12–22. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Kam MK, Teo PM, Chau RM, et al: Treatment
of nasopharyngeal carcinoma with intensity-modulated radiotherapy:
the Hong Kong experience. Int J Radiat Oncol Biol Phys.
60:1440–1450. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Wolden SL, Chen WC, Pfister DG, Kraus DH,
Berry SL and Zelefsky MJ: Intensity-modulated radiation therapy
(IMRT) for nasopharynx cancer: update of the Memorial
Sloan-Kettering experience. Int J Radiat Oncol Biol Phys. 64:57–62.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Ng WT, Lee MC, Hung WM, et al: Clinical
outcomes and patterns of failure after intensity-modulated
radiotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol
Phys. 79:420–428. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Su SF, Han F, Zhao C, et al: Long-term
outcomes of early-stage nasopharyngeal carcinoma patients treated
with intensity-modulated radiotherapy alone. Int J Radiat Oncol
Biol Phys. 82:327–333. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Lin S, Lu JJ, Han L, Chen Q and Pan J:
Sequential chemotherapy and intensity-modulated radiation therapy
in the management of locoregionally advanced nasopharyngeal
carcinoma: experience of 370 consecutive cases. BMC Cancer.
10:392010. View Article : Google Scholar
|
|
31.
|
Lin S, Pan J, Han L, Zhang X, Liao X and
Lu JJ: Nasopharyngeal carcinoma treated with reduced-volume
intensity-modulated radiation therapy: report on the 3-year outcome
of a prospective series. Int J Radiat Oncol Biol Phys.
75:1071–1078. 2009.PubMed/NCBI
|
|
32.
|
Tham IW, Hee SW, Yeo RM, et al: Treatment
of nasopharyngeal carcinoma using intensity-modulated radiotherapy
- the National Cancer Centre Singapore experience. Int J Radiat
Oncol Biol Phys. 75:1481–1486. 2009. View Article : Google Scholar : PubMed/NCBI
|