Introduction
Prostate-specific antigen (PSA) has long been proven
to be effective as a tumor marker in prostate cancer cases
(1). In particular, PSA is most
effective when used as a post-treatment monitor. In cases of
radical prostatectomy (RP), the mere detection of PSA
postoperatively is highly likely to signify residual lesions
(2). In addition, postoperative
follow-up of prostate cancer cases is currently implemented mainly
by using PSA and rectal examination. Our institution also uses PSA
in the follow-up of postoperative RP cases and, from September,
2003 onwards, has been using ultra-sensitive PSA
(ARCHITECT®automated immunoassay analyser, Abbott
Laboratories; measurement threshold 0.008 ng/ml). Ultra-sensitive
PSA enables earlier detection of PSA recurrence compared to that
with conventional reagents (3–5).
However, the effectiveness of additional treatment in regard to PSA
recurrence postoperatively in RP cases remains debatable (6,7) and
unnecessary treatment should generally be avoided. It is
hypothesized that urologists may have experience with cases in
which, since PSA does not simply continue to increase
postoperatively, decision making as to the timing of additional
treatment may be challenging, even when pathological factors for
recurrence are taken into consideration. For this reason, we
investigated the effectiveness of ultra-sensitive PSA and its value
in averting the administration of additional treatment, using
transitions in ultra-sensitive PSA in patients who have undergone
RP.
Materials and methods
Patient characteristics
A total of 311 patients underwent prostate biopsy
and were diagnosed with prostate cancer at the National Kyushu
Cancer Center (Fukuoka, Japan) and additional associated
institutions. Tissue specimens, obtained from the 311 patients
between September, 2003 and March, 2009 were reviewed in embedded
whole-mount antegrade RP specimens with adenocarcinoma. All the
patients underwent pelvic lymph node dissection during the same
time period. A total of 111 patients were excluded from this study
due to prior hormonal therapy. The profile of the patients is
summarized in Table I. All the
patients were Japanese and the median follow-up after surgery was
52.2 months. The median age of the patients was 66 years (range,
47–77 years) and the preoperative PSA was 7.704 ng/ml (range,
0.959–39.413 ng/ml). The number of cases with clinical ≥T2 and
pathological ≥T3 disease was 102 (51.0%) and 64 (32.0%),
respectively. The number of patients with an RP Gleason score of ≥8
was 44 (22.0%). The number of patients with extraprostatic
extension (EPE), positive resection margin and positive lymph nodes
was 60 (30.0%), 32 (16.0%) and 3 (1.5%), respectively.
 | Table IPatient clinicopathological
profile. |
Table I
Patient clinicopathological
profile.
| Characteristics | Total, no.
(%)
(n=200) | BCF (−), no.
(%)
[n=183 (91.5)] | BCF (+), no.
(%)
[n=17 (8.5)] |
|---|
| Median age, years
(range) | 66 (47–77) | 66 (47–77) | 70 (57–75) |
| Median preoperative
PSA, ng/ml (range) | 7.704
(0.959–39.413) | 7.590
(0.959–39.413) | 8.115
(5.024–25.577) |
| Clinical stage, n
(%) |
| cT1c | 98 (49.0) | 94 (51.4) | 4 (23.5) |
| ≥cT2 | 102 (51.0) | 89 (48.6) | 13 (76.5) |
| Pathological stage, n
(%) |
| ≤pT2 | 136 (68.0) | 130 (71.0) | 6 (35.3) |
| ≥pT3 | 64 (32.0) | 53 (29.0) | 11 (64.7) |
| RP Gleason score, n
(%) |
| ≤7 | 156 (78.0) | 147 (80.3) | 9 (52.9) |
| ≥8 | 44 (22.0) | 36 (19.7) | 8 (47.1) |
| EPE, n (%) |
| 0 | 140 (70.0) | 134 (73.2) | 6 (35.3) |
| 1 | 60 (30.0) | 49 (26.8) | 11 (64.7) |
| RM, n (%) |
| 0 | 168 (84.0) | 156 (85.2) | 12 (70.6) |
| 1 | 32 (16.0) | 27 (14.8) | 5 (29.4) |
| pN, n (%) |
| 0 | 197 (98.5) | 182 (99.5) | 15 (88.2) |
| 1 | 3 (1.5) | 1 (0.5) | 2 (11.8) |
| PSA nadir, ng/ml [n
(%)] |
| <0.008 | 183 (91.5) | 173 (94.5) | 10 (58.8) |
| ≥0.008 | 17 (8.5) | 10 (5.5) | 7 (41.2) |
PSA nadir and biochemical failure
The PSA nadir was defined as the lowest PSA value
following RP and the measurement limit of serum PSA values was
<0.008 ng/ml in this study. In 183 patients (91.5%), the serum
PSA levels were decreased to <0.008 ng/ml. Biochemical failure
following RP was defined as two consecutive PSA values of ≥0.2
ng/ml (8).
Follow-up
The follow-up schedule following RP involved a PSA
assay every 3 months for the first 2 years, every 4 months for the
following 3 years and every 6 months thereafter. A pathologist
evaluated the degree of malignancy of the prostatectomy specimens,
according to the 2005 International Society of Urological Pathology
Consensus Conference on Gleason grading system (9), and the pathological stage, based on
the 2009 TNM classification (10).
Statistical analysis
Univariate and multivariate analyses were also
performed using the Cox proportional hazards regression model, in
order to identify the predictors associated with biochemical
failure following RP. The variables were as follows: age,
preoperative PSA, clinical T stage, pathological T stage, RP
Gleason score, EPE, resection margin status, lymph node metastasis
and PSA nadir. The biochemical failure-free rate was determined
using the Kaplan-Meier method. The log-rank test was used to
determine the differences among the cases in the PSA nadir group.
The Kruskal-Wallis test was used to determine the differences among
the cases in the PSA transition type group. The statistical
analyses were performed using the JMP® version 8
software program (SAS Institute, Inc., Cary, NC, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Correlation between clinicopathological
characteristics and biochemical failure
The correlation between the clinicopathological
characteristics and biochemical failure is presented in Table II. According to the Cox
proportional hazards analysis, preoperative characteristics, such
as age and clinical tumor stage, were significant predictors.
Postoperative characteristics, such as pathological tumor stage, RP
Gleason score, EPE, lymph node metastasis and PSA nadir were found
to be significant predictors based on the univariate analysis,
whereas according to the multivariate analysis, statistically
significant differences were found in the RP Gleason score, EPE,
lymph node metastasis and PSA nadir <0.008 ng/ml (P=0.0116,
0.0216, 0.0178 and <0.0001, respectively). PSA nadir <0.008
ng/ml exhibited the highest hazard ratio (HR) [HR=26.34, 95%
confidence interval (CI): 7.34–104.69].
 | Table IICorrelation between
clinicopathological characteristics and biochemical failure. |
Table II
Correlation between
clinicopathological characteristics and biochemical failure.
| Characteristics | Hazard ratio | P-value | 95% CI |
|---|
| Univariate
analysis |
| Age | 1.0954 | 0.0401 | 1.0039–1.2111 |
| Preoperative
PSA | 1.0608 | 0.1041 | 0.9859–1.1196 |
| cT1 vs. ≥cT2 | 3.5756 | 0.0157 | 1.2567–12.7632 |
| pT2 vs. pT3 | 4.1182 | 0.0041 | 1.5666–11.9563 |
| RP Gleason score ≤7
vs. ≥8 | 3.1470 | 0.0235 | 1.1760–8.2754 |
| EPE0 vs. EPE1 | 4.4515 | 0.0025 | 1.6934–12.9237 |
| RM0 vs. RM1 | 2.5284 | 0.1064 | 0.8023–6.8439 |
| pN0 vs. pN1 | 14.4627 | 0.0097 | 2.2538–52.7430 |
| PSA nadir (<0.008
vs. ≥0.008 ng/ml) | 11.7402 | <0.0001 | 4.2154–30.9738 |
| Multivariate
analysis |
| RP Gleason score ≤7
vs. ≥8 | 5.2407 | 0.0116 | 1.4613–19.6442 |
| EPE0 vs. EPE1 | 3.1760 | 0.0216 | 1.1835–9.4170 |
| pN0 vs. pN1 | 11.9323 | 0.0178 | 1.6884–55.7366 |
| PSA nadir (<0.008
vs. ≥0.008 ng/ml) | 26.3362 | <0.0001 | 7.3445–104.6937 |
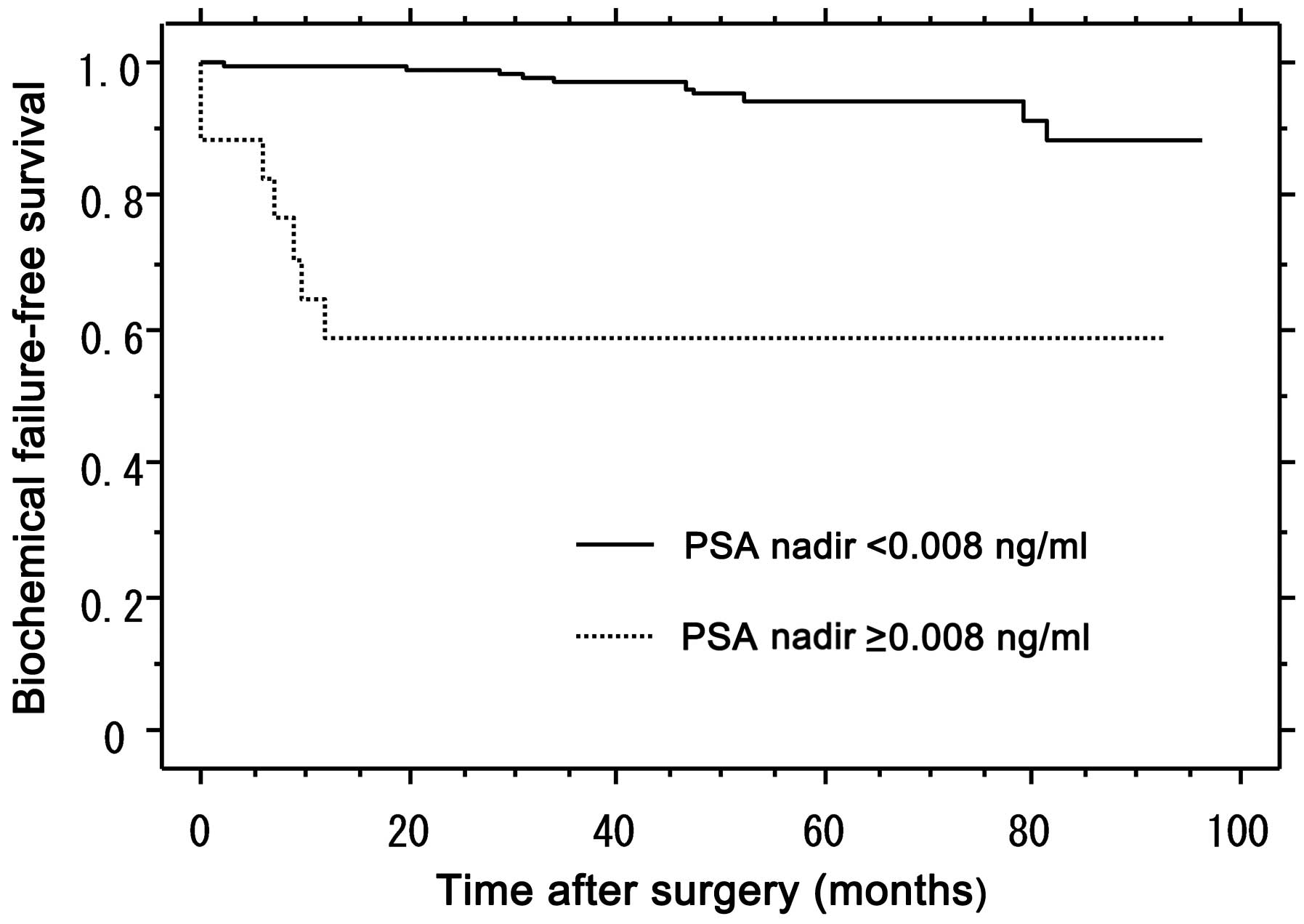
Biochemical failure-free survival of
patients stratified by PSA nadir value (<0.008 vs. ≥0.008
ng/ml)
After a median follow-up period of 52.2 months, the
biochemical failure-free rate in the PSA nadir <0.008 and ≥0.008
ng/ml groups was 94.3 and 58.8%, respectively (Fig. 1). The difference between the PSA
nadir <0.008 and ≥0.008 ng/ml groups was statistically
significant, according to the log-rank test (P<0.001; df=2).
Clinicopathological characteristics
according to biochemical failure group classificaction in the PSA
nadir <0.008 ng/ml group
The profile of the patients analyzed in the PSA
nadir <0.008 ng/ml group is presented in Table III. In the biochemical
failure-negative group, the number of cases with clinical ≥T2 and
pathological ≥T3 disease was 86 (49.7%) and 48 (27.7%),
respectively. The number of patients with a Gleason score of ≥8
were 36 (20.8%). The number of patients with EPE, positive
resection margin and positive lymph nodes was 44 (25.4%), 23
(13.3%) and 1 (0.6%), respectively. The number of patients with PSA
≥0.008 ng/ml after a PSA nadir of <0.008 ng/ml was 64
(35.0%).
 | Table IIIClinicopathological characteristics
according to BCF group classificaction in the PSA nadir <0.008
ng/ml group. |
Table III
Clinicopathological characteristics
according to BCF group classificaction in the PSA nadir <0.008
ng/ml group.
| Characteristics | Total, no.
(%)
(n=183) | BCF (−), no.
(%)
[n=173 (94.5)] | BCF (+), no.
(%)
[n=10 (5.5)] |
|---|
| Median age, years
(range) | 66 (47–77) | 66 (47–77) | 70 (63–73) |
| Median preoperative
PSA, ng/ml (range) | 7.491
(0.959–39.413) | 7.491
(0.959–39.413) | 7.652
(5.024–15.403) |
| Clinical stage, n
(%) |
| cT1c | 90 (49.2) | 87 (50.3) | 3 (30.0) |
| ≥cT2 | 93 (50.8) | 86 (49.7) | 7 (70.0) |
| Pathological stage,
n (%) |
| ≤pT2 | 129 (70.5) | 125 (72.3) | 4 (40.0) |
| ≥pT3 | 54 (29.5) | 48 (27.7) | 6 (60.0) |
| RP Gleason score, n
(%) |
| ≤7 | 141 (77.0) | 137 (79.2) | 4 (40.0) |
| ≥8 | 42 (33.0) | 36 (20.8) | 6 (60.0) |
| EPE, n (%) |
| 0 | 133 (72.7) | 129 (74.6) | 4 (40.0) |
| 1 | 50 (27.3) | 44 (25.4) | 6 (60.0) |
| RM, n (%) |
| 0 | 158 (86.3) | 150 (86.7) | 8 (80.0) |
| 1 | 25 (13.7) | 23 (13.3) | 2 (20.0) |
| pN, n (%) |
| 0 | 180 (98.4) | 172 (99.4) | 8 (80.0) |
| 1 | 3 (1.6) | 1 (0.6) | 2 (20.0) |
| PSA ≥0.008 ng/ml
after PSA nadir, n (%) | 64 (35.0) | 54 (31.2) | 10 (100) |
Correlation between clinicopathological
characteristics and biochemical failure in the PSA nadir <0.008
ng/ml group
The correlation between the clinicopathological
characteristics and biochemical failure in the PSA nadir <0.008
ng/ml group is presented in Table
IV. According to the Cox proportional hazards analysis of the
PSA nadir <0.008 ng/ml group, age, RP Gleason score, EPE and
lymph node metastasis were found to be significant predictors based
on the univariate analysis (P=0.0436, 0.0127, 0.0394 and 0.0020,
respectively). However, in the multivariate analysis, statistically
significant differences were only observed in pathological nodal
stage (pN) (P=0.0107).
 | Table IVCorrelation between
clinicopathological characteristics and biochemical failure in the
PSA nadir <0.008 ng/ml group. |
Table IV
Correlation between
clinicopathological characteristics and biochemical failure in the
PSA nadir <0.008 ng/ml group.
|
Characteristics | Hazard ratio | P-value | 95% CI |
|---|
| Univariate
analysis |
| Age | 1.1296 | 0.0436 | 1.0032–1.3040 |
| Preoperative
PSA | 1.0079 | 0.9009 | 0.8635–1.1061 |
| cT1 vs. ≥cT2 | 2.6392 | 0.1439 | 0.7252–12.3618 |
| pT2 vs. pT3 | 3.4898 | 0.0506 | 0.9967–13.6517 |
| RP Gleason score
≤7 vs. ≥8 | 5.0577 | 0.0127 | 1.4269–20.0230 |
| EPE0 vs. EPE1 | 3.7401 | 0.0394 | 1.0676–14.6372 |
| RM0 vs. RM1 | 1.7780 | 0.4933 | 0.2680–7.1155 |
| pN0 vs. pN1 | 33.1399 | 0.0020 |
4.8130–146.0192 |
| Multivariate
analysis |
| pN0 vs. pN1 | 17.5434 | 0.0107 |
2.1922–111.5099 |
PSA transition with PSA ≥0.008 ng/ml
after a PSA nadir of <0.008 ng/ml (Fig. 2)
i) Temporary elevation, defined as cases
demonstrating temporary PSA elevation followed by a decrease to
<0.008 ng/ml, was observed in 18 cases (28.1%); ii) peaking out,
defined as 0.008≤PSA<0.05 ng/ml, was observed in 33 cases
(51.6%); and iii) consecutive elevation, defined as PSA ≥0.05
ng/ml, was observed in 13 cases (20.3%).
Clinicopathological characteristics
according to PSA transition types in the group with PSA ≥0.008
ng/ml after a PSA nadir of <0.008 ng/ml
The profile of the patients who were analyzed in the
PSA ≥0.008 ng/ml after a PSA nadir of <0.008 ng/ml group is
presented in Table V. The number
of cases with temporary elevation, peaking out and consecutive
elevation type of transition was 18 (28.1%), 33 (51.6%) and 13
(20.3%), respectively. According to the Kruskal-Wallis test
analysis, statistically significant differences were observed in
pathological stage and EPE (both P=0.0003).
 | Table VClinicopathological characteristics
according to PSA transition types in the group with PSA≥0.008 after
a PSA nadir of <0.008 ng/ml. |
Table V
Clinicopathological characteristics
according to PSA transition types in the group with PSA≥0.008 after
a PSA nadir of <0.008 ng/ml.
| | Type of
transition |
|---|
| |
|
|---|
|
Characteristics | Total
(n=64) | Temporary
elevation, no. (%)
[n=18 (28.1)] | Peaking outa, no. (%)
[n=33 (51.6)] | Consecutive
elevationb, no. (%)
[n=13 (20.3)] | P-value |
|---|
| Median age, years
(range) | 67 (50–78) | 63 (50–74) | 70 (63–73) | 70 (63–73) | 0.3431 |
| Median
preoperative | 7.951 | 6.562 | 8.116 | 7.919 | 0.6013 |
| PSA, ng/ml
(range) | (1.477–22.632) | (3.232–11.410) | (1.477–22.632) | (5.024–13.388) | |
| Pathological stage,
n (%) | | | | | 0.0003 |
| ≤pT2 | 38 (59.4) | 17 (94.4) | 18 (54.5) | 3 (23.1) | |
| ≥pT3 | 26 (40.6) | 1 (5.6) | 15 (45.5) | 10 (76.9) | |
| RP Gleason score, n
(%) | | | | | 0.8242 |
| ≤7 | 45 (70.3) | 12 (66.7) | 23 (69.7) | 10 (76.9) | |
| ≥8 | 19 (29.7) | 6 (33.3) | 10 (30.3) | 3 (23.1) | |
| EPE, n (%) | | | | | 0.0003 |
| 0 | 41 (64.1) | 17 (94.4) | 21 (63.7) | 3 (23.1) | |
| 1 | 23 (35.9) | 1 (5.6) | 12 (36.3) | 10 (76.9) | |
| RM, n (%) | | | | | 0.1950 |
| 0 | 50 (78.1) | 16 (88.9) | 26 (78.8) | 8 (61.5) | |
| 1 | 14 (21.9) | 2 (11.1) | 7 (21.2) | 5 (38.5) | |
Discussion
Thompson et al (11) reported that adjuvant radiotherapy
following RP for stage pT3N0M0 prostate cancer significantly
reduces the risk of metastasis and increases survival (P=0.016 and
0.023, respectively). Furthermore, according to Bolla et al
(12), the results at a median
follow-up of 10.6 years indicated that conventional postoperative
irradiation significantly improves biochemical progression-free
survival and local control compared to a wait-and-see policy,
supporting the results at 5-years of follow-up (P=0.001); however,
the improvements in clinical progression-free survival were not
maintained. Late adverse effects (any type of any grade) were more
frequent in the postoperative irradiation compared to the
wait-and-see group (P=0.001) (12). In addition, Shen et al
(13) reported that, even in cases
with a positive resection stump, there were at least a few in which
PSA was decreased below the measurement threshold or in which no
PSA was detected. The authors of the present study have had a
similar experience, in which decision making regarding additional
treatment has often been challenging, in light of clinical factors
such as age and overall physical condition, even when based on
pathological recurrence factors. Therefore, even in pT3 cases, it
is possible that, under certain conditions, adjuvant radiotherapy
may be unnecessary. If the adverse events associated with adjuvant
therapy in post-RP cases are taken into consideration, it is
obvious that, from the patient point of view, unnecessary treatment
should be avoided.
It was previously reported that the lower the
ultra-sensitive PSA nadir value postoperatively, the lower the risk
of PSA recurrence (13,14). Our institution has been using
ultra-sensitive PSA since September, 2003 to implement follow-up in
RP cases, which, compared to the conventional methods, facilitates
PSA measurement at significantly lower levels, thereby facilitating
monitoring the extent of the decrease in postoperative PSA levels
and the early identification of PSA recurrence. Therefore, we aimed
to investigate the usefulness of ultra-sensitive PSA in Japanese
prostate cancer patients and its potential value in averting
unnecessary adjuvant therapy, using transitions in ultra-sensitive
PSA among postoperative RP cases.
As shown in Table
I, the PSA nadir was reduced to <0.008 ng/ml in 183 patients
(91.5%) who had undergone RP, while biochemical failure was
observed in 17 cases (8.5%). Ellis et al (3) reported the PSA value to be below the
measurement sensitivity level of 0.008 ng/ml in 86.2% of
cystoprostatectomy cases in which prostate cancer was not detected,
indicating that RP is efficiently performed in this institution. In
our group of cases, when factors affecting biochemical failure were
considered, the RP Gleason score, EPE, lymph node metastasis and
PSA nadir exhibited significant difference in the multivariate
analysis (P=0.0116, 0.0216, 0.0178 and <0.0001, respectively)
(Table II). Of these factors, PSA
nadir <0.008 ng/ml exhibited the highest HR (HR=26.34; 95% CI:
7.34–104.69). For this reason, we next considered biochemical
failure-free survival in terms of PSA nadir value in patients who
underwent RP. After a median follow-up of 52.2 months, the
biochemical failure-free rate in the PSA nadir <0.008 and ≥0.008
ng/ml groups was 94.3 and 58.8%, respectively (Fig. 1), with a statistically significant
difference according to the log-rank test (P<0.001; df=2). From
these results, we may hypothesize that the group with a PSA nadir
of <0.008 ng/ml following RP was significantly less likely to
experience biochemical failure compared to the group with PSA
≥0.008 ng/ml. Kinoshita et al (2) reported that cases where the PSA nadir
value does not decrease to ≤0.01 ng/ml postoperatively using the
ultra-sensitive method, are at a significant risk of PSA recurrence
(>0.1 ng/ml), which is consistent with our findings. Yu et
al (4) classed reagents with
an ultra-sensitive PSA detection threshold value of 0.1–0.3 ng/ml
as first-generation, those of 0.02–0.1 ng/ml as second-generation
and those of 0.001–0.02 ng/ml as third-generation. The advances in
these ultra-sensitive PSA reagents have enabled the selection of
postoperative RP cases in which the PSA values have decreased below
even the lowest measurement thresholds, indicating that unnecessary
additional treatment may be avoidable, regardless of the clinical
and pathological recurrence factors.
Even among patients in whom the PSA nadir value was
decreased to <0.008 ng/ml, biochemical failure was observed in
10 cases (5.5%) (Table II). A
possible explanation as to why these cases did not decrease to
below the theoretically predicted measurement threshold following
RP, i.e., did not decrease to <0.008 ng/ml using our reagents,
may be that surgery was unsuccessful (residual prostate cancer
tissue or presence of a small metastasis that was not detected
preoperatively). Furthermore, in addition to potential problems
with the reagents and measurement methods, there exists the
possibility of ‘noise’ created by residual benign prostate tissue
during measurement, or the production of PSA by the periurethral
glands or other organs. Therefore, we considered the factors
affecting biochemical failure only in cases in which such ‘noise’
had been removed as much as possible. As shown in Table II, of the 183 RP cases in which
the postoperative PSA nadir value was reduced to <0.008 ng/ml,
173 cases (94.5%) did not experience biochemical failure; of note,
among these cases, 48 (27.7%) were pT3. In addition, the group
experiencing biochemical failure comprised 10 cases (5.5%), of
which only 6 were pT3. Therefire, with pT3 used as the only
criterion for determining additional treatment, in this study, 44
out of 54 cases (81.5%) would have been administered unnecessary
treatment. The correlation between clinicopathological
characteristics and biochemical failure in the PSA nadir <0.008
ng/ml group is shown in Table IV.
In the multivariate analysis, statistically significant differences
were only observed in pN (P=0.0107). In this study, however, the pN
frequency was low, occurring in only 1.5% of the cases and not all
such cases developed recurrence. Conventionally, metastasis to the
lymph nodes is considered to reflect a poor prognosis and the
addition of whole-body endocrine therapy following RP is considered
the treatment of choice. Bader et al (15), however, reported a 78%
cause-specific survival rate in patients treated with RP and
extended pelvic lymph node dissection and who did not undergo any
adjuvant therapy until disease progression. Of note, among those
patients with 1 positive lymph node, 39% remained free of clinical
or biochemical progression, compared to 12% of patients with ≥2
positive nodes.
As a result, it is currently fairly standard for
transitions in postoperative PSA values to be monitored and in
several cases the need for additional treatment is determined based
on PSA transitions, regardless of clinical or pathological
factors.
Therefore, we considered the possibility of avoiding
additional treatment based on PSA transitions using ultra-sensitive
PSA. A total of 64 cases (35.0%) among those with a PSA nadir value
of <0.008 ng/ml increased to ≥0.008 ng/ml at least once. The
transitions in ultra-sensitive PSA among all cases are shown in
Table V. It was determined that
there are three trends in ultra-sensitive PSA and, as a result, the
cases were divided into three groups as follows: the temporary
elevation type group, in which PSA levels increased temporarily,
followed by a subsequent decrease to <0.008 ng/ml. According to
Shimizu et al (16),
determining PSA recurrence based on a definition of the number of
consecutive times ultra-sensitive PSA increases may be challenging.
The remaining cases were divided into two further groups based on
the tendency for PSA to rise consecutively to a boundary of PSA
0.05 ng/ml: the consecutive elevation type group (PSA≥0.05 ng/ml)
and the peaking out type group (0.008≤PSA<0.05 ng/ml). The
consecutive elevation type group, which is closest to our
perception of reccurrence, comprised only 20.3% of the entire
group, with 51.6% of the cases falling into the peaking out
category. With a single exception, the consecutive elevation type
group transited to <0.05 ng/ml. As shown in Table V, when the three groups were
compared, statistically significant differences were found in
pathological stage (≥pT3) and EPE (both P=0.0003). Therefore, we
may hypothesize that cases with the pathological factors ≥pT3 and
EPE are mainly following the transition of the consecutive
elevation type group. However, while only one case of each pT3 and
EPE (5.6%) was found in the transient group, there were 15 cases of
pT3 (45.5%) and 12 cases of EPE (36.3%) in the peaking out type
group. Therefore, unnecessary additional treatment may be avoided
if PSA transition patterns are taken into consideration alongside
pathological recurrence factors in the peaking out type group, as
ongoing observation, rather than treatment, is implemented until
PSA levels are at least 0.05 ng/ml. Terai et al (17) reported that the lower a patient’s
PSA prior to curative radiotherapy, the better the response. In
that study, 92% of the patients underwent curative radiotherapy at
PSA levels ≤0.5 ng/ml. As a result, it may be safe to hypothesize
that it is not to the patients’ disadvantage to hold off additional
treatment until the PSA levels rise to 0.05 ng/ml, the level the
authors consider appropriate, rather than implementing treatment
immediately after surgery. Since the consecutive elevation type
group, which is considered to require additional treatment, is
relatively small, the use of ultra-sensitive PSA to detect
transition facilitates the selection of cases that do not belong to
the continuous elevation type, thereby facilitating the avoidance
of unnecessary additional treatment.
The European Association of Urology guidelines state
that patients with a PSA level of 0.1–0.2 ng/ml following RP do not
exhibit either clinical or biochemical disease progression.
Therefore, the use of an ultra-sensitive PSA assay may not
justified for routine follow-up following RP (8). However, in this study, the
observation of PSA transition using ultra-sensitive PSA following
RP exhibited potential in enabling the selection of cases in which
additional treatment may be avoided, even where pathological
recurrence factors are present and, as such, is considered to be
useful.
We retrospectively assessed the usefulness of
ultra-sensitive PSA following RP in Japanese prostate cancer
patients. The biochemical failure-free survival in RP cases with
PSA nadir <0.008 ng/ml was found to be significantly lower
comapred to those with PSA nadir ≥0.008 ng/ml and using
ultra-sensitive PSA to confirm that postoperative values have
decreased to below the measurement threshold may avert
administering unnecessary additional treatment. Furthermore, in
cases in which PSA levels were reduced to below the measurement
threshold but increased subsequently, maintaining observation
without treatment until the levels reach at least 0.05 ng/ml may
also enable avoiding unnecessary additional treatment.
References
|
1
|
Polascik TJ, Oesterling JE and Partin AW:
Prostate specific antigen: a decade of discovery - what we have
learned and where we are going. J Urol. 162:293–306. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kinoshita H, Kamoto T, Nishiyama H,
Nakamura E, Matsuda T and Ogawa O: Prostate specific antigen nadir
determined using ultra-sensitive prostate specific antigen as a
predictor of biochemical progression after radical prostatectomy in
Japanese males. Int J Urol. 14:930–934. 2007. View Article : Google Scholar
|
|
3
|
Ellis WJ, Vessella RL, Noteboom JL, Lange
PH, Wolfert RL and Rittenhouse HG: Early detection of recurrent
prostate cancer with an ultrasensitive chemiluminescent
prostate-specific antigen assay. Urology. 50:573–579. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu H, Diamandis EP, Prestigiacomo AF and
Stamey TA: Ultrasensitive assay of prostate-specific antigen used
for early detection of prostate cancer relapse and estimation of
tumor-doubling time after radical prostatectomy. Clin Chem.
41:430–434. 1995.
|
|
5
|
Haese A, Huland E, Graefen M, Hammerer P,
Noldus J and Huland H: Ultrasensitive detection of prostate
specific antigen in the followup of 422 patients after radical
prostatectomy. J Urol. 161:1206–1211. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sandler HM and Eisenberger MA: Assessing
and treating patients with increasing prostate specific antigen
following radical prostatectomy. J Urol. 178:S20–S24. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Naito S: Evaluation and management of
prostate-specific antigen recurrence after radical prostatectomy
for localized prostate cancer. Jpn J Clin Oncol. 35:365–374. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mottet N, Bellmunt J, Bolla M, et al: EAU
guidelines on prostate cancer. Part II: Treatment of advanced,
relapsing, and castration-resistant prostate cancer. Eur Urol.
59:572–583. 2011. View Article : Google Scholar
|
|
9
|
Epstein JI, Allsbrook WC Jr, Amin MB and
Egevad LL; ISUP Grading Committee. The 2005 International Society
of Urological Pathology (ISUP) consensus conference on Gleason
grading of prostatic carcinoma. Am J Surg Pathol. 29:1228–1242.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM Classification of Malignant Tumors. 7th edition.
Wiley-Blackwell; Oxford: 2009
|
|
11
|
Thompson IM, Tangen CM, Paradelo J, et al:
Adjuvant radiotherapy for pathological T3N0M0 prostate cancer
significantly reduces risk of metastases and improves survival:
long-term followup of a randomized clinical trial. J Urol.
181:956–962. 2009. View Article : Google Scholar
|
|
12
|
Bolla M, van Poppel H, Tombal B, et al:
Postoperative radiotherapy after radical prostatectomy for
high-risk prostate cancer: long-term results of a randomised
controlled trial (EORTC trial 22911). Lancet. 380:2018–2027. 2012.
View Article : Google Scholar
|
|
13
|
Shen S, Lepor H, Yaffee R and Taneja SS:
Ultrasensitive serum prostate specific antigen nadir accurately
predicts the risk of early relapse after radical prostatectomy. J
Urol. 173:777–780. 2005. View Article : Google Scholar
|
|
14
|
Nakamura M, Hasumi H, Miyoshi Y, Sugiura
S, Fujinami K, Yao M, Kubota Y and Uemura H: Usefulness of
ultrasensitive prostate-specific antigen assay for early detection
of biochemical failure after radical prostatectomy. Int J Urol.
12:1050–1054. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bader P, Burkhard FC, Markwalder R and
Studer UE: Disease progression and survival of patients with
positive lymph nodes after radical prostatectomy. Is there a chance
of cure? J Urol. 169:849–854. 2003. View Article : Google Scholar
|
|
16
|
Shimizu F, Tanaka S, Matsuyama Y, Tominaga
T, Ohashi Y and Fujime M: Efficiency of ultrasensitive
prostate-specific antigen assay in diagnosing biochemical failure
after radical prostatectomy. Jpn J Clin Oncol. 37:446–451. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Terai A, Matsui Y, Yoshimura K, Arai Y and
Dodo Y: Salvage radiotherapy for biochemical recurrence after
radical prostatectomy. BJU Int. 96:1009–1013. 2005. View Article : Google Scholar : PubMed/NCBI
|