Introduction
Colorectal cancer (CRC) is the third most commonly
diagnosed cancer in males and the second in females, with >1.2
million new cancer cases and 608,700 deaths estimated to have
occurred in 2008 (1). Recent
advances in therapeutic strategies and surgical techniques have
significantly improved the prognosis of CRC patients with primary
disease; however, metastasis is a major concern for CRC patients
and physicians. Thirty-five percent of CRC patients have metastatic
tumors at the time of diagnosis and 33–50% of the patients without
metastases will progress to stage IV during the course of their
disease (1,2). Hence, it is necessary to develop
prominent biomarkers for detecting the presence of occult
metastasis, as well as for predicting the development of future
metastasis, which may provide a useful reference for the
therapeutic management of CRC patients.
Potential stem cell marker, Bmi1, is a member of the
polycomb-repressive complex 1, with a key role in gene silencing
through chromatin modifications (3,4). In
addition to the proposed role for Bmi1 as a key regulator of cell
growth control/senescence mechanisms, accumulating evidence
supports the role of Bmi1 in tumorigenesis. Bmi1 is overexpressed
in a variety of human cancers and its overexpression was found to
be correlated with tumor progression in CRC (5–7) and
other gastrointestinal cancers, including esophageal squamous cell
carcinoma (8,9), pancreatic cancer (10) and gastric cancer (11), indicating its functional and
prognostic role in gastrointestinal cancer patients. The Bmi1
autoantibody in the serum has also been suggested as a minimally
invasive biomarker for the prognosis of nasopharyngeal (12), esophageal squamous cell (8) and cervical carcinoma (13). More importantly, circulating Bmi1
mRNA has been detected in the plasma and was correlated with poor
prognosis of advanced breast (14)
and uterine cervical cancer patients (15). Those studies demonstrated the
potential of Bmi1 as a non-invasive surrogate marker for a variety
of cancer patients; however, such an application in CRC has not yet
been investigated.
In this study, the clinical significance of plasma
Bmi1 mRNA in CRC patients was investigated. The pre- and
postoperative plasma Bmi1 mRNA levels in CRC patients were
determined by quantitative polymerase chain reaction (PCR) and
correlated with the clinicopathological parameters, in order to
determine whether monitoring of plasma Bmi1 in CRC patients is
predictive of the development of distant metastasis.
Materials and methods
Patients and plasma samples
Blood samples were collected from 45 patients who
underwent surgical resection of primary CRC during their follow-up
at the Department of Surgery, Queen Mary Hospital, The University
of Hong Kong, between 2009 and 2013. Among these 45 patients, who
presented with no evidence of distant metastasis at the time of the
primary resection, 6 developed distant metastasis within 3 years,
whereas the remaining patients were recurrence-free at the last
follow-up visit to our clinic. The eligibility criteria for this
study included pre- and postoperative plasma sample availability
for quantitative PCR. Blood was anticoagulated by EDTA and
centrifuged at 1,500 g for 10 min. The plasma was collected,
divided into aliquots and snap-frozen at −80°C until use.
This study was approved by the Institutional Review
Board of our hospital and the patients provided written informed
consent prior to inclusion.
RNA isolation, reverse transcription and
quantitative PCR
RNA was isolated from 250 μl of plasma sample using
the mirVana™ miRNA isolation kit (Life Technologies, Carlsbad, CA,
USA), according to the manufacturer’s instructions. For the
synthesis of first-strand cDNA, 8 μl RNA was reversed transcribed
using PrimeScript™ RT Master mix (Takara Bio, Inc., Shiga, Japan)
in accordance with the manufacturer’s instructions. Quantitative
PCR was performed in a final volume of 15 μl containing 1.5 μl RT
transcript, 0.2 μM of each primer, 1X ROX reference dye and 7.5 μl
of FastStart Universal SYBR-Green Master (ROX) (Roche Applied
Science, Indianapolis, IN, USA). The Bmi1 forward and reverse
primer sequences (5′-3′) were ATCCCCACCTGATGTGTG and
AAAGCCCTGGAACTAATTTG, respectively. Quantitative PCR was performed
using the ABI 7900HT Fast Real-Time PCR system (Applied Biosystems,
Foster City, CA, USA) at 95°C for 10 min, followed by 40 cycles at
95°C for 15 sec and at 56°C for 1 min.
Statistical analysis
The plasma Bmi1 mRNA level was expressed as the
cycle threshold (Ct) detected by quantitative PCR using the same
threshold value of fluorescent signal intensity. The difference in
plasma Bmi1 mRNA level between the two different groups was
evaluated by the Student’s t-test, whereas the difference between
the pre- and postoperative blood levels in the same patient was
evaluated by the paired t-test. The differences in recurrence rates
among different groups of CRC patients were evaluated by the
Fisher’s exact test. The disease-free survival was analyzed using
the Kaplan-Meier product limit method and the log-rank test. All
the statistical analyses were performed with SigmaPlot software,
version 10.0 (Systat Software Inc., San Jose, CA, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Correlation of pre- and postoperative
plasma Bmi1 mRNA levels with clinicopathological parameters
We first compared the plasma Bmi1 transcript level
prior to and following surgical resection of primary colorectal
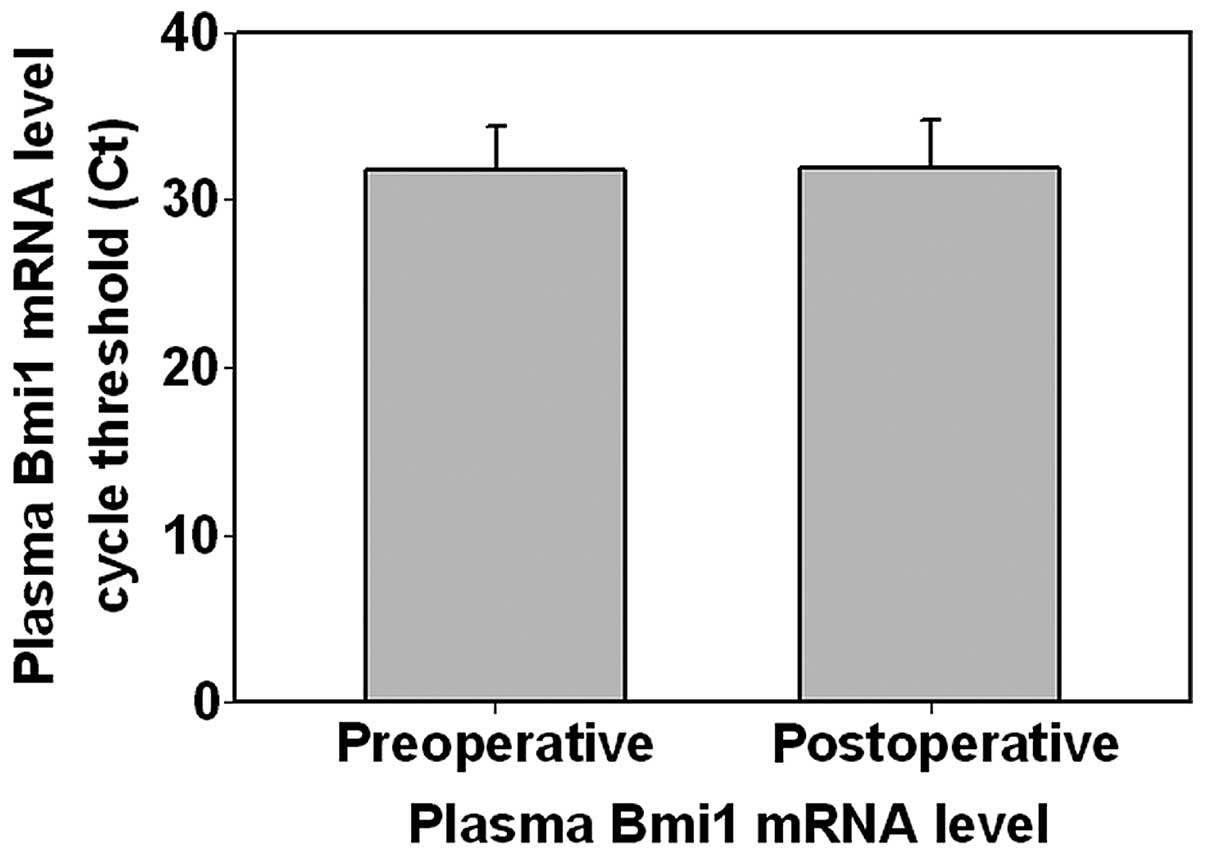
tumor in 45 patients. As shown in Fig.
1, the pre- and postoperative Bmi1 transcript levels (expressed
as mean Ct ± standard deviation, i.e., the higher the Ct, the lower
the transcript level) were 31.73±2.63 and 31.93±2.88, respectively,
without a statistically significant difference between the two
levels (P=0.614). We further compared the pre- and postoperative
Bmi1 transcript levels in patients with different
clinicopathological parameters (Table
I). There were no significant changes in pre- and postoperative
plasma Bmi1 transcript levels in CRC patients of different age,
gender, lymph node status and TNM stage. However, the changes were
significantly different between patients with metastatic and
non-metastatic CRC. In the 39 non-metastatic CRC patients, the
postoperative Bmi1 transcript level was significantly lower
compared to the preoperative level (32.13±2.677 vs. 31.44±2.764,
respectively; P=0.041). Furthermore, in the 6 CRC patients who
developed metastasis, there was a trend for higher postoperative
Bmi1 transcript level compared to the preoperative level
(30.85±3.916 vs. 33.27±0.718, respectively; P=0.164). These results
suggested that an increase in the postoperative Bmi1 level
correlated with the development of metastasis in CRC patients.
 | Table IClinicopathological correlation of
plasma Bmi1 mRNA levels in colorectal cancer patients. |
Table I
Clinicopathological correlation of
plasma Bmi1 mRNA levels in colorectal cancer patients.
| Clinicopathological
characteristics | No. of casesa | Plasma Bmi1 mRNA
level pre- vs. postoperarive (mean Ct ± SD) | P-value |
|---|
| Age (years) | | | |
| <65 | 12 | 29.82±1.76 vs.
30.07±2.48 | 0.753 |
| ≥65 | 24 | 30.36±2.22 vs.
30.18±2.97 | 0.718 |
| Gender | | | |
| Male | 22 | 30.33±2.01 vs.
30.04±3.04 | 0.657 |
| Female | 14 | 29.96±2.21 vs.
30.29±2.42 | 0.471 |
| Tumor size (cm) | | | |
| <5 | 15 | 30.48±2.32 vs.
29.99±3.20 | 0.523 |
| ≥5 | 9 | 30.18±2.16 vs.
30.49±2.70 | 0.589 |
| Lymph node
metastasis | | | |
| Absent | 17 | 30.04±2.14 vs.
30.57±2.34 | 0.224 |
| Present | 15 | 30.45±2.27 vs.
29.77±3.49 | 0.439 |
| TNM stage | | | |
| I–II | 15 | 30.68±1.76 vs.
30.90±2.14 | 0.524 |
| III–IV | 12 | 30.14±2.39 vs.
29.31±3.76 | 0.457 |
| Distant
metastasis | | | |
| Absent | 32 | 31.44±2.76 vs.
32.13±2.68 | 0.041 |
| Present | 6 | 33.27±0.72 vs.
30.85±3.92 | 0.164 |
Due to sampling limitations, the postoperative blood
plasma specimens were obtained from CRC patients at different time
points following surgery (range, 0.5–25 months; median, 5 months).
To determine whether the length of time post-operation affects the
plasma Bmi1 levels, we analyzed the association between the length
of time post-operation and the difference between post- and
preoperative plasma Bmi1 levels in CRC patients. Our results
demonstrated that there was no significant correlation (r=−0.0116,
P=0.945), suggesting that the length of time post-operation does
not affect the results obtained.
Association of higher postoperative
plasma Bmi1 mRNA with the development of distant metastasis
We next categorized the 45 patients into two groups
according to their plasma Bmi1 post- vs. preoperative level status
(Table II). Of the 45 patients,
29 exhibited a reduced postoperative Bmi1 level compared to the
preoperative level, whereas 16 displayed increased or unchanged
Bmi1 level following resection of the primary tumor. Of the 16
patients who exhibited no reduction in Bmi1 level, 5 developed
metastasis within 36 months post-operation. By contrast, only 1 of
the 29 patients exhibiting lower postoperative Bmi1 level developed
metastasis, suggesting that patients with reduced postoperative
Bmi1 levels were less likely to develop metastasis (P=0.017).
 | Table IIPlasma Bmi1 mRNA level changes
following curative resection of the primary tumor predict
development of metastasis. |
Table II
Plasma Bmi1 mRNA level changes
following curative resection of the primary tumor predict
development of metastasis.
| Plasma Bmi1 mRNA
level |
|---|
|
|
|---|
| Post-
>preoperative | Post-
<preoperative |
|---|
| Metastatic | 5 | 1 |
| Non-metastatic | 11 | 28 |
| Fisher’s exact test:
P=0.017 |
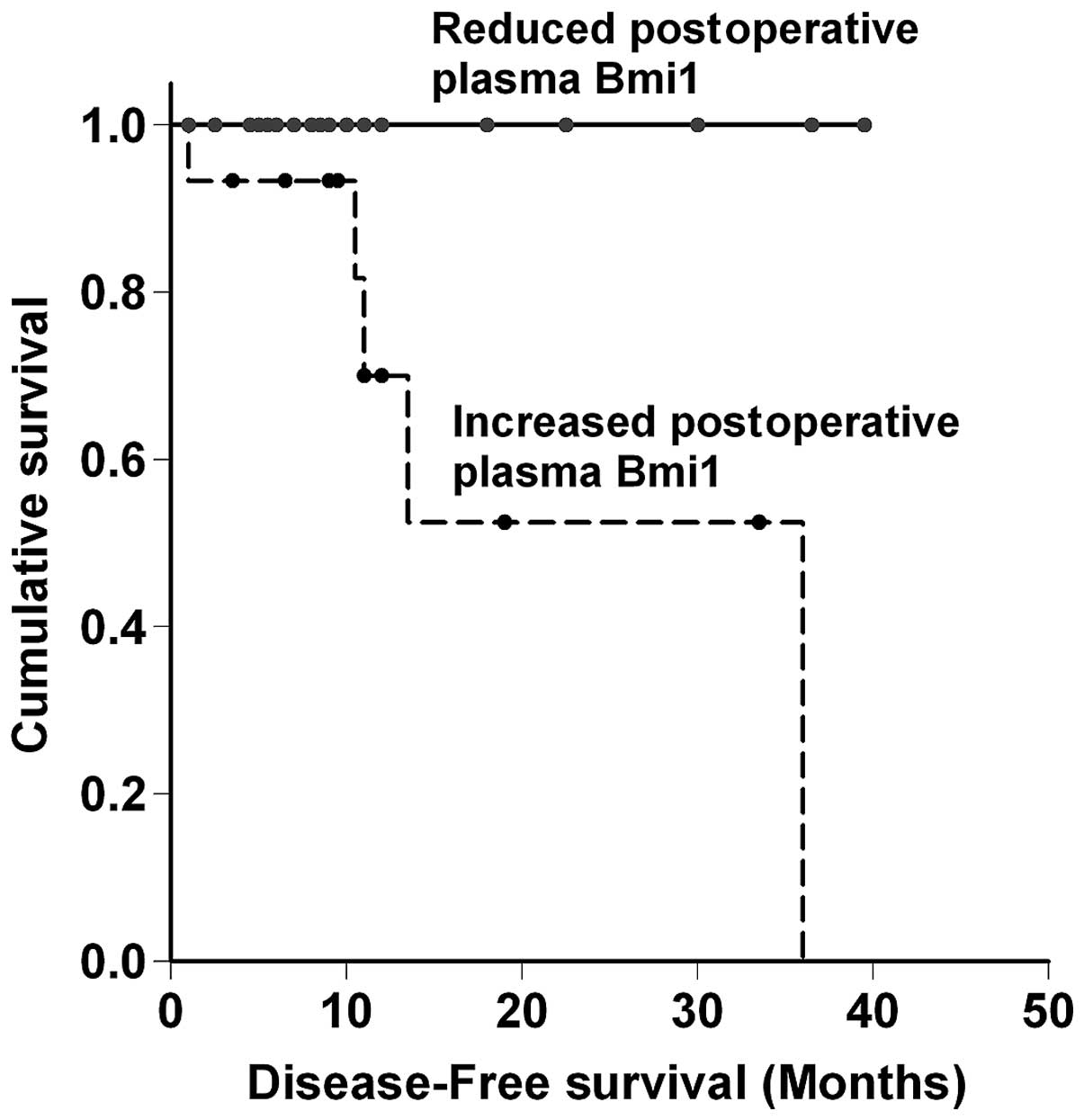
Finally, the prognostic significance of the changes
in the post- vs. preoperative Bmi1 level in CRC patients was
asessed (Fig. 2). The patients
with a reduced postoperative Bmi1 level (n=29) had a significantly
better prognosis (P=0.016) for disease-free survival when compared
to their increased postoperative Bmi1 level counterparts (n=16),
further demonstrating the correlation of changes in pre- and
postoperative Bmi1 levels with the development of metastasis.
Our results suggested that reduced plasma Bmi1
transcript levels following resection of the primary tumor was a
good prognostic factor for CRC patients, whereas patients with an
increased Bmi1 level were more likely to develop postoperative
metastasis. Therefore, monitoring the changes in the plasma Bmi1
level is a potential prognostic biomarker for the optimal
management of CRC patients following curative resection of the
primary tumor.
Discussion
Circulating Bmi1 mRNA prior to any treatment has
been suggested as a surrogate marker of poor prognosis in patients
with breast (14) and uterine
cervical cancer (15). In our
model, we observed that preoperative circulating Bmi1 mRNA did not
correlate with any clinical parameters of CRC patients (data not
shown), which may be explained by the relatively small number of
patients recruited in this study. However, we demonstrated the
significance of monitoring the changes in plasma Bmi1 transcript
levels prior to and following surgical resection of the primary
tumor in CRC patients, suggesting that monitoring the changes in
circulating Bmi1 mRNA levels may be a more accurate prognostic
biomarker in CRC patients.
Plasma Bmi1 mRNA levels in cancer patients were
found to be significantly higher compared to those in normal
subjects (14,15), suggesting that the increased
circulating Bmi1 mRNA may originate from the primary tumor and
surgical resection of the primary tumor should bring down the
levels of such transcripts. Of note, our study did not demonstrate
such an effect. We found no significant difference between the
overall pre- and postoperative plasma Bmi1 levels in CRC patients.
Therefore, we hypothesized that a subset of CRC patients whose Bmi1
levels remained high following primary tumor resection accounted
for such findings and we considered that those patients may be at
higher risk of developing metastasis, since high Bmi1 levels have
been associated with cancer metastasis (15–18).
To test our hypothesis, we divided the CRC patients into
non-metastatic and metastatic and found that the postoperative
circulating Bmi1 mRNA level in non-metastatic patients decreased to
a mean value of 0.6-fold of the preoperative level, confirming that
resection of the primary tumor removes the source of circulating
Bmi1 mRNA. However, metastatic patients exhibited a mean of 5-fold
induction in their circulating Bmi1 level postoperatively, which is
most likely the result of dissemination of CRC tumor cells that
accounted for the development of metastasis.
In the second part of our analysis, we found that
monitoring the changes of plasma Bmi1 mRNA prior to and following
curative resection was prognostic for the development of future
metastasis, suggesting that circulating plasma Bmi1 mRNA may be
used as a non-invasive biomarker for predicting and monitoring
occult metastasis in CRC patients. We consider this finding to be
useful for physicians in the postoperative treatment of CRC
patients, such as applying a more aggressive dosage of adjuvant
therapy to patients exhibiting no reduction of circulating Bmi1
mRNA after having their primary tumors removed.
Bmi1 mRNA and protein overexpression were previously
demonstrated in CRC tumor (5,7) and
were positively correlated with tumor stage, invasion and
metastasis (6), as well as with
poor disease-free and overall survival (6,19),
suggesting that Bmi1 is a promising prognostic biomarker in CRC
patients. To the best of our knowledge, this study was the first to
further demonstrate that the changes in plasma Bmi1 mRNA level
prior to and following resection of the primary tumor was
prognostic for the development of metastasis, indicating the
potential of circulating Bmi1 mRNA levels as a non-invasive,
convenient and relatively cost-effective surrogate biomarker for
the management of postoperative CRC patients and possibly patients
with other types of cancer in which Bmi1 is also overexpressed.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Garden OJ, Rees M, Poston GJ, et al:
Guidelines for resection of colorectal cancer liver metastases.
Gut. 55(Suppl 3): iii1–iii8. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Valk-Lingbeek ME, Bruggeman SW and van
Lohuizen M: Stem cells and cancer; the polycomb connection. Cell.
118:409–418. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Widschwendter M, Fiegl H, Egle D, et al:
Epigenetic stem cell signature in cancer. Nat Genet. 39:157–158.
2007. View
Article : Google Scholar
|
|
5
|
Kim JH, Yoon SY, Kim CN, et al: The Bmi-1
oncoprotein is overexpressed in human colorectal cancer and
correlates with the reduced p16INK4a/p14ARF proteins. Cancer Lett.
203:217–224. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li DW, Tang HM, Fan JW, et al: Expression
level of Bmi-1 oncoprotein is associated with progression and
prognosis in colon cancer. J Cancer Res Clin Oncol. 136:997–1006.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tateishi K, Ohta M, Kanai F, et al:
Dysregulated expression of stem cell factor Bmi1 in precancerous
lesions of the gastrointestinal tract. Clin Cancer Res.
12:6960–6966. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu WL, Guo XZ, Zhang LJ, et al:
Prognostic relevance of Bmi-1 expression and autoantibodies in
esophageal squamous cell carcinoma. BMC Cancer. 10:4672010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lv J, Cao XF, Ji L, et al: Association of
β-catenin, Wnt1, Smad4, Hoxa9, and Bmi-1 with the prognosis of
esophageal squamous cell carcinoma. Med Oncol. 29:151–160.
2012.
|
|
10
|
Song W, Tao K, Li H, et al: Bmi-1 is
related to proliferation, survival and poor prognosis in pancreatic
cancer. Cancer Sci. 101:1754–1760. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang XW, Sheng YP, Li Q, et al: BMI1 and
Mel-18 oppositely regulate carcinogenesis and progression of
gastric cancer. Mol Cancer. 9:402010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tong YQ, Liu B, Huang J, et al: BMI-1
autoantibody in serum as a new potential biomarker of
nasopharyngeal carcinoma. Cancer Biol Ther. 7:340–344. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tong YQ, Liu B, Zheng HY, et al: BMI-1
autoantibody as a new potential biomarker for cervical carcinoma.
PLoS One. 6:e278042011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Silva J, Garcia V, Garcia JM, et al:
Circulating Bmi-1 mRNA as a possible prognostic factor for advanced
breast cancer patients. Breast Cancer Res. 9:R552007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang X, Wang C, Wang L, et al: Detection
of circulating Bmi-1 mRNA in plasma and its potential diagnostic
and prognostic value for uterine cervical cancer. Int J Cancer.
131:165–172. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Meng X, Wang Y, Zheng X, et al:
shRNA-mediated knockdown of Bmi-1 inhibit lung adenocarcinoma cell
migration and metastasis. Lung Cancer. 77:24–30. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang J, Qiu Y, Chen G, Huang L and He J:
The relationship between Bmi-1 and the epithelial-mesenchymal
transition in lung squamous cell carcinoma. Med Oncol.
29:1606–1613. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo BH, Feng Y, Zhang R, et al: Bmi-1
promotes invasion and metastasis, and its elevated expression is
correlated with an advanced stage of breast cancer. Mol Cancer.
10:102011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Du J, Li Y, Li J and Zheng J: Polycomb
group protein Bmi1 expression in colon cancers predicts the
survival. Med Oncol. 27:1273–1276. 2010. View Article : Google Scholar : PubMed/NCBI
|