Introduction
Although there are various treatment protocols for
hepatocellular carcinoma (HCC), including liver resection and
radiofrequency ablation (RFA), >598,000 patients succumbed to
this disease worldwide in 2012 (1). Intrahepatic metastasis and
microvascular invasion (microsatellite lesions) are associated with
recurrence rate and prognosis (2–4). The
survival time of HCC patients with microsatellite lesions remains
unsatisfactory (5).
The mechanism of distant metastasis and recurrence
is often considered to be via the systemic circulation (6). Previously, Sasaki et al
(7) reported that the distance of
the microsatellite lesion from the main tumor (microsatellite
distance) was associated with prognosis. However, microsatellite
distance cannot be evaluated by computed tomography (CT) or
magnetic resonance imaging (MRI). If there remain microsatellite
lesion after treatment, recurrence is inevitable. When the strategy
for HCC treatment is being established, it is important to evaluate
the microsatellite distance. The prediction of preoperative
microsatellite distance is of particular importance for treatment
selection in HCC.
Fluorine-18 fluorodeoxyglucose positron emission
tomography/CT (18F-FDG PET/CT) is effective for
detecting and staging a variety of malignant diseases.
18F-FDG PET/CT is based on the fact that a number of
malignant tumors take up glucose. However, 18F-FDG
PET/CT was not found to be effective in detecting HCC, particularly
well-differentiated HCC, although FDG accumulation was found to
reflect malignant potential (8,9).
Patients with tumors exhibiting high FDG accumulation are
associated with a poor prognosis after therapy (10,11).
However, 18F-FDG PET/CT was found to be effective for
detecting extrahepatic metastases (12).
The aim of the present study was to investigate
whether 18F-FDG PET/CT is a useful preoperative imaging
modality for predicting the distance of the microsatellite lesion
from the main tumor and the postoperative recurrence pattern.
Patients and methods
Patients and follow-up
This was a retrospective study. All the study
protocols were approved by the Institutional Ethics Committee of
the Ehime Prefectural Central Hospital, Ehime University Graduate
School of Medicine (Ehime University Hospital) (approval number:
1106005 and UMIN ID number: 000008652) and all the patients
provided written informed consent. In this study, patients with
initial HCC who underwent hepatic resection (systematic
segmentectomy) at Ehime University Hospital and Ehime Prefectural
Central Hospital between April, 2006 and July, 2011 were enrolled.
Preoperatively, the patients underwent abdominal ultrasonography
(US), CT, MRI, or angiography. 18F-FDG PET/CT was
performed within 1 month prior to hepatic resection. Preoperative
transcatheter arterial chemoembolization was not performed. The
diagnosis of HCC was confirmed by pathological findings in all the
cases.
Adjuvant therapy was not administered in this study.
During follow-up, abdominal US or contrast-enhanced CT (CECT) was
performed every 3 months. The diagnosis of recurrence was based on
imaging findings. Extrahepatic metastasis was diagnosed with a
whole-body imaging study, including CECT or 18F-FDG
PET/CT. The period for disease recurrence was defined as the number
of days from hepatic resection to detection of disease
recurrence.
18F-FDG PET/CT imaging
protocol
The patients were fasted for a minimum of 6 h prior
to the injection of 222–370 MBq 18F-FDG. Images were
acquired at 60 min post-injection. Blood glucose levels were
measured prior to the injection and none of the patients were
withdrawn from the study due to high blood glucose levels; no
additional drugs for glucose control were administered.
18F-FDG PET/CT scans were obtained using
a multislice PET/CT camera (Discovery STE; GE Healthcare,
Milwaukee, WI, USA). The PET scanner contains bismuth germanate
detectors and reconstructs 35 axial images at 4.25-mm intervals,
with a field of view of 15.6 cm. Spatial resolution of this system
for 3-dimensional (3D) mode in full-width at half-maximum is 5.12
mm at 1-cm offset from the center (13). CT was performed on the same scanner
without contrast administration. The CT scan data were collected
with 160–280 mAs (adjusted to the patient’s body weight) and a
gantry rotation speed of 0.8 s. All the CT scans were obtained
using 3.75-mm axial sections for attenuation correction and
diagnosis. Whole-body acquisitions (head to mid-thigh) were
performed in 3D mode with the use of 6 or 7 bed positions and
emission images were acquired for 3 min per bed position. PET, CT
and fused PET/CT images were reconstructed and reviewed on
Advantage Workstations version 4.5 (GE Healthcare). The PET/CT and
CT diagnostic results were compared.
Interpretation and analysis of PET/CT
images
The evaluation of the 18F-FDG PET/CT
images was performed by a combined team of experienced nuclear
medicine physicians and radiologists, in consensus, who were
blinded to clinical and laboratory data and the final clinical
diagnosis. For each PET dataset, the tumor with the most intense
18F-FDG uptake of all foci was carefully identified for
the maximal count. A volumetric region of interest encompassing the
entire tumor was drawn to ensure correct identification of the
maximal count. The maximal standardized uptake value (SUVmax) was
calculated. In a region of interest, only the lesions that
exhibited the most substantial 18F-FDG uptake were
selected as the target lesions for evaluating response to therapy.
Semi-quantitative indices of the uptake of 18F-FDG were
calculated for each vascular segment. A volumetric region of
interest encompassing the entire tumor was drawn to ensure correct
identification of the maximal counts and its SUVmax.
Pathological evaluation
The resected specimens were divided at 10-mm
intervals and fixed in 20% formalin. The specimens were embedded in
paraffin, cut into 4-μm slices and stained with hematoxylin and
eosin. The histological assessment was performed by two experienced
pathologists (K.F. and Y.S.). The histological grade of the tumor
was evaluated according to the classification of the Liver Cancer
Study Group of Japan (14).
Satellite lesions in the resected specimens were first assessed
macroscopically and, if not identified, microscopic assessment was
performed. Satellite lesions were identified as microscopic portal
vein invasion or intrahepatic metastasis. To identify satellite
lesions, we applied the criteria defined by the Liver Cancer Study
Group of Japan: Tumors surrounding the main tumor with multiple
satellite nodules or small solitary tumors located near the main
tumor that are histologically similar or less differentiated than
the main tumor (14). The distance
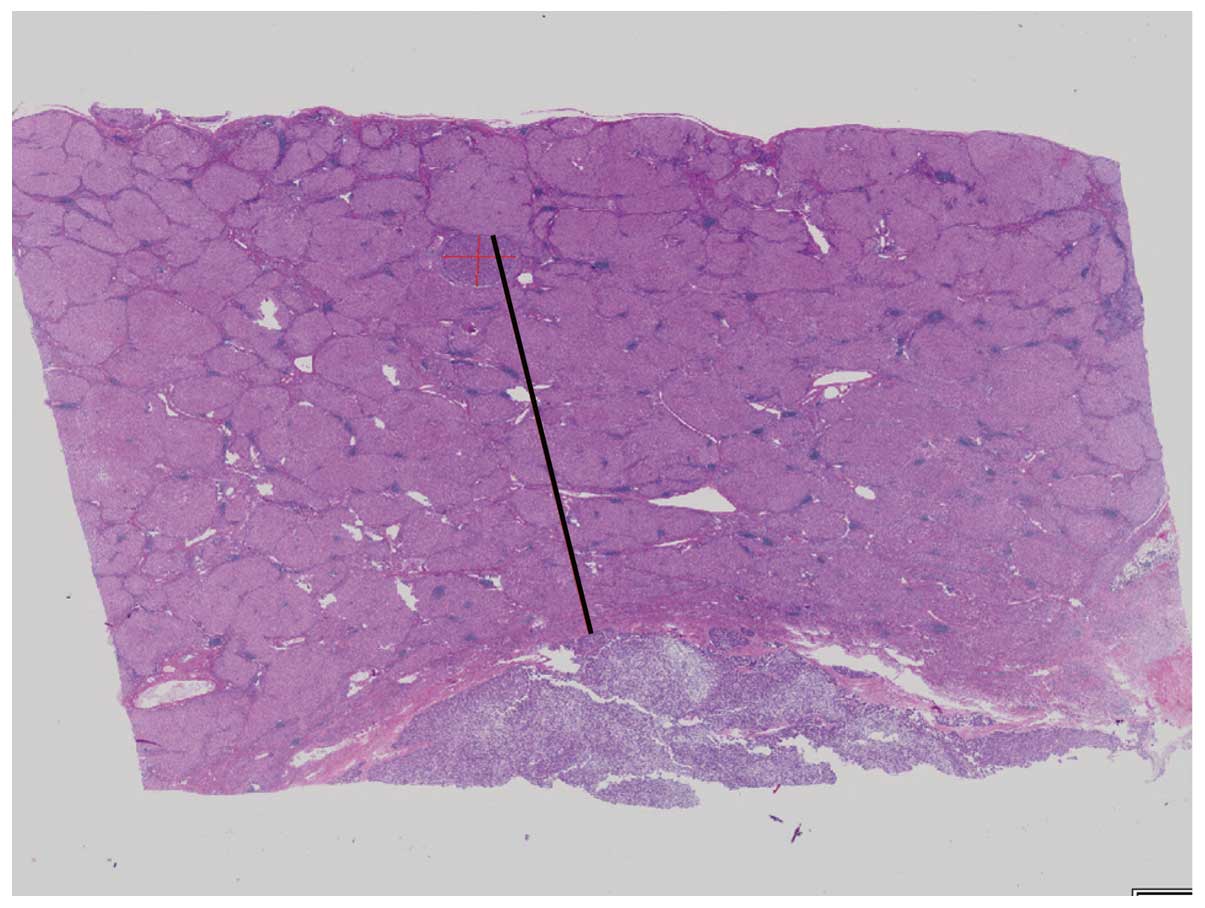
between the satellite lesion and the main tumor was measured using
the Virtual Slides system (Olympus Engineering Co., Ltd., Tokyo,
Japan) (Fig. 1).
Data analysis
The clinicopathological parameters were compared
between the groups using the Student’s t-test and the Chi-square
test, as appropriate. The significance of the differences was
assessed by non-parametric testing. The correlations between the
microsatellite distance and other parameters were assessed by the
Pearson’s product-moment correlation coefficient. Receiver
operating characteristic (ROC) curves were constructed and the area
under the ROC curve (AUC) was calculated by the trapezoidal rule.
Optimal cutoff values were selected to maximize sensitivity,
specificity and diagnostic accuracy. Sensitivity, specificity,
positive predictive value and negative predictive value (NPV) were
calculated using cutoffs obtained from the ROC curves. Variables
that were found to be significant on the univariate analysis of
factors affecting microsatellite distribution were included in a
subsequent multivariate analysis using Cox’s proportional hazards
model. P<0.05 was considered to indicate a statistically
significant difference. The statistical analysis was performed
using JMP statistical software package, version 9 (SAS Institute
Japan Ltd., Tokyo, Japan). All the values are presented as means ±
standard deviation.
Results
Clinical and pathological
characteristics
A total of 89 patients (70 men and 19 women; median
age, 68.4 years) were enrolled in this study. The patients’
clinical characteristics are presented in Table I. The median follow-up period was
12.1 months (range, 1–126.7 months). The median disease-free
survival was 12.0 months and 45 patients (50.5%) developed
postoperative recurrence. Intrahepatic recurrence occurred in 36
patients and extrahepatic recurrence occurred in 9. The locations
of extrahepatic recurrence were the lungs (n=4), lymph nodes (n=3),
bone (n=2) and adrenal gland (n=1). The median tumor SUVmax was 3.9
(range, 1.0–20.5). Of the 29 patients (32.6%) with microsatellite
lesions in the resected specimens, 19 developed recurrence after
resection.
 | Table IClinical characteristics of patients
in this study (n=89). |
Table I
Clinical characteristics of patients
in this study (n=89).
| Variables | Values |
|---|
| Patient
characteristics |
| Gender
(male/female) | 70/19 |
| Age, years [median
(range)] | 70 (38–87) |
| BMI,
kg/m2 [median (range)] | 23.0 (17.5–42.1) |
| Etiology |
| Hepatitis
B/hepatitis C/other | 14/48/27 |
| Biochemical value,
median (range) |
| AST (IU/l) | 43 (5–134) |
| ALT (IU/l) | 34 (9–161) |
| PLT
(104/μl) | 14.1
(3.6–31.3) |
| TBil (mg/dl) | 0.6 (0.2–2.6) |
| Alb (g/dl) | 4.0 (2.4–4.9) |
| PT (%) | 80.2
(58.4–117.3) |
| AFP (ng/dl) | 26.1
(1.4–75,000) |
| PIVKA-II
(mAU/ml) | 160
(4.5–75,000) |
| HCC
characteristics, median (range) |
| Tumor size
(cm) | 4.0 (1.2–21) |
| Number of tumors
in one patient (range) | 1 (1–3) |
| Tumor stage
(I/II/III/IVa) | 4/54/24/7 |
| SUVmax of
tumor | 3.9 (1.0–20.5) |
| Number of
satellite lesions | 29 |
| Follow-up |
| Mean follow-up
period (months) | 12.1 |
| Number of patients
with recurrence | 45 |
| Location of
extrahepatic metastasis (lung/bone/lymph nodule/adrenal gland) | 4/2/3/1 |
Parameters associated with microsatellite
distance
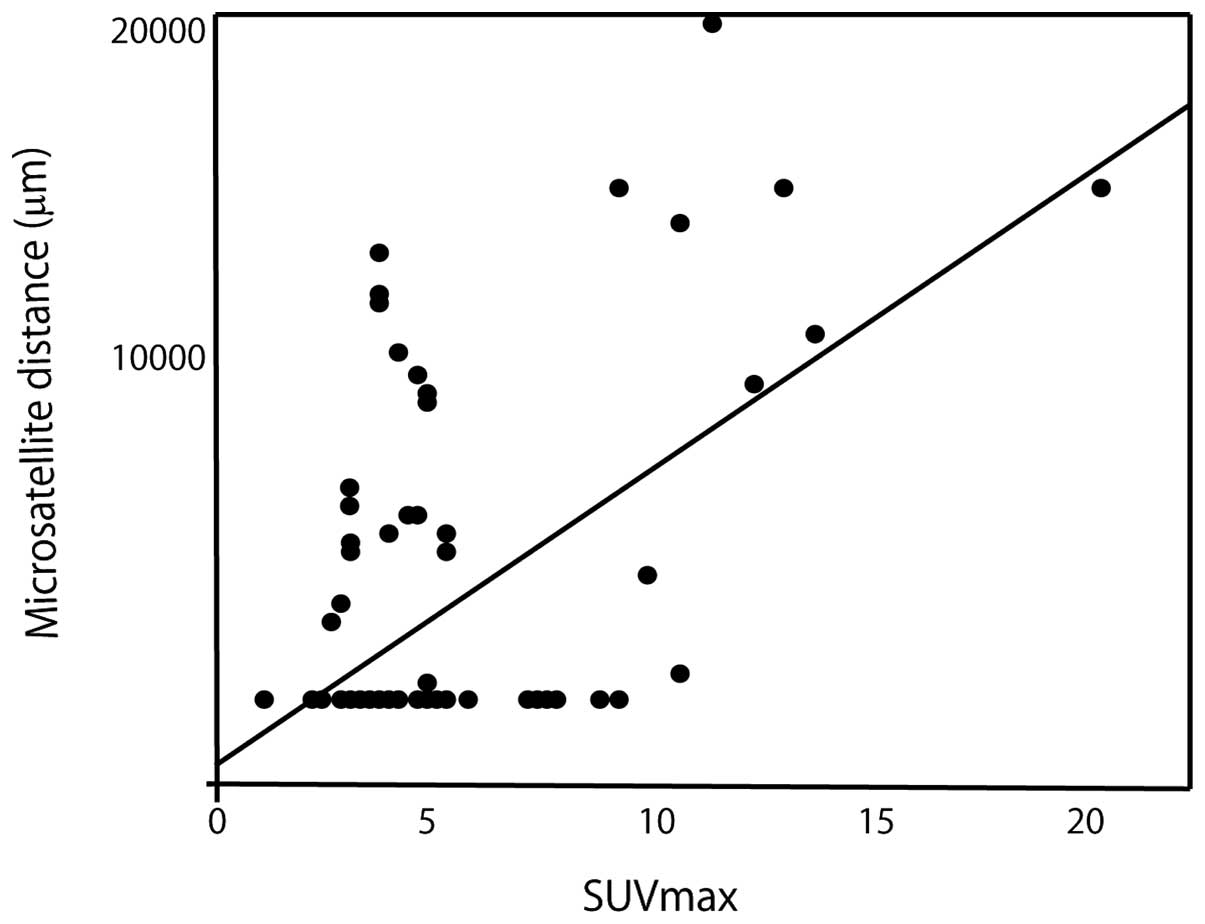
In a linear regression model, the significant
parameters included α-fetoprotein (AFP) (P<0.0003, r=0.37, 95%
CI: 0.18–0.54), protein induced by vitamin K absence or
antagonist-II (PIVKA-II) (P<0.0001, r=0.40, 95% CI: 0.20–0.56),
tumor diameter (P<0.0001, r=0.44, 95% CI: −0.26–0.60) and tumor
SUVmax (P<0.0001, r=0.58, 95% CI: 0.41–0.70) (Table II).
 | Table IILinear regression model for
parameters associated with microsatellite distance. |
Table II
Linear regression model for
parameters associated with microsatellite distance.
| Parameters | r value | 95% CI | t value | P-value |
|---|
| Age (years) | −0.04 | −0.30–0.18 | −0.35 | 0.727 |
| BMI
(kg/m2) | −0.12 | −0.32–0.09 | −1.10 | 0.274 |
| AST (IU/l) | −0.14 | −0.07–0.34 | 1.32 | 0.192 |
| ALT (IU/l) | −0.005 | −0.21–0.20 | −0.01 | 0.988 |
| PLT
(x104/μl) | −0.17 | −0.04–0.37 | 1.47 | 0.145 |
| TBil (mg/dl) | −0.06 | −0.27–0.14 | −0.56 | 0.579 |
| Alb (g/dl) | −0.06 | −0.26–0.16 | −0.45 | 0.651 |
| PT (%) | −0.04 | −0.30–0.18 | 0.08 | 0.935 |
| AFP (ng/ml) | 0.37 | 0.18–0.54 | 3.37 | 0.0003 |
| PIVKA-II
(mAU/ml) | 0.40 | 0.20–0.56 | 4.16 | <0.0001 |
| HbA1c (%) | −0.15 | −0.33–0.08 | −1.47 | 0.145 |
| Diameter of tumor
(cm) | 0.44 | −0.26–0.60 | 4.63 | <0.0001 |
| SUVmax | 0.58 | 0.41–0.70 | 6.53 | <0.0001 |
SUVmax exhibited the most significant correlation
with microsatellite distance. The linear regression model is shown
in Fig. 2.
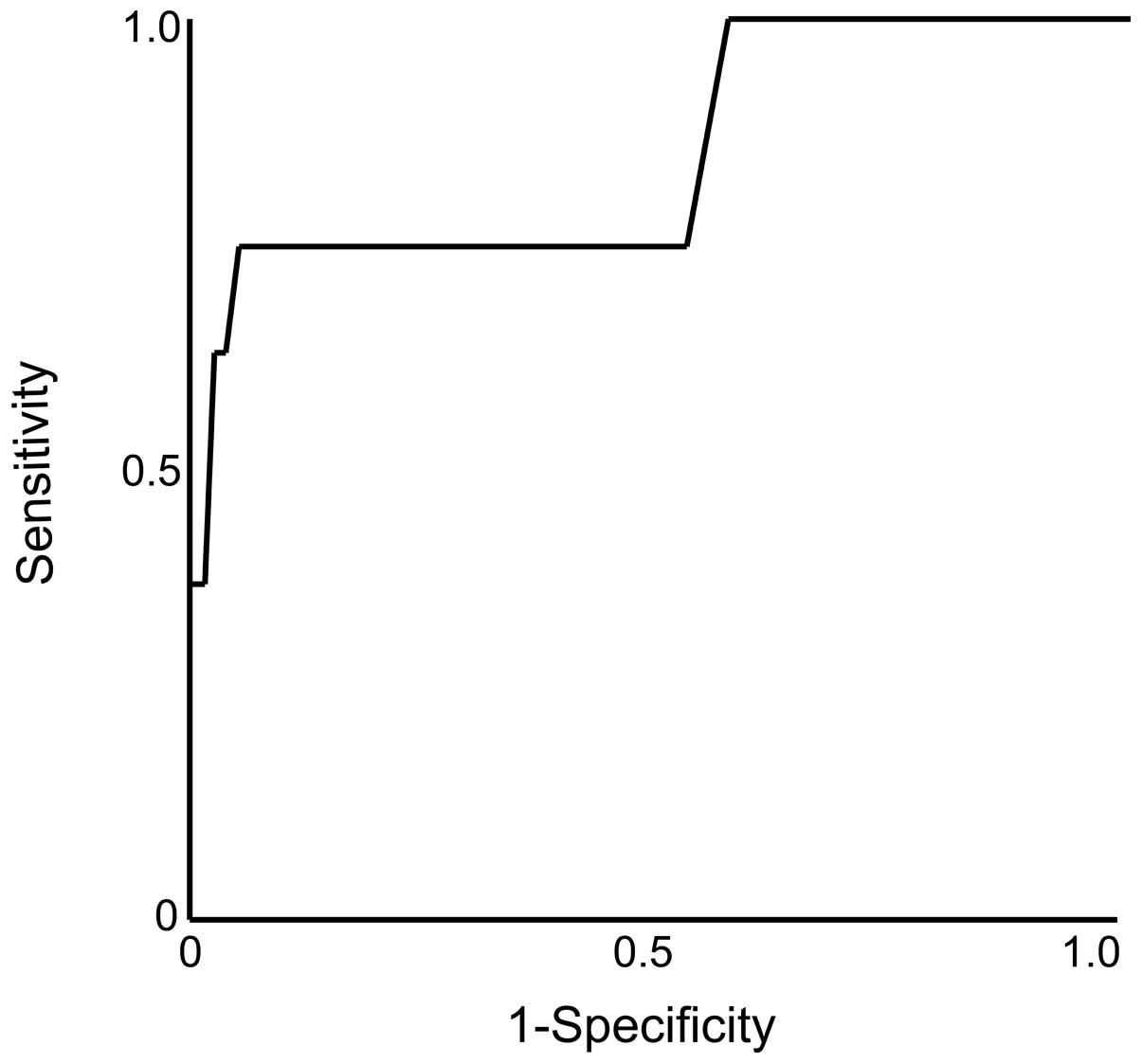
Independent risk factors for
microsatellite distance >1 cm
On the univariate analysis of the independent risk
factors for microsatellite distance >1 cm, the significant
factors were preoperative tumor diameter (P=0.017; hazard
ratio=1.23; 95% CI: 1.04–1.39) and SUVmax (P=0.0003; hazard
ratio=1.65; 95% CI: 1.30–2.29) (Table III). On the multivariate
analysis, the only significant factor was SUVmax (P=0.002; hazard
ratio=1.60; 95% CI: 1.23–2.26). ROC curves were constructed and are
presented in Fig. 3.
 | Table IIIPreoperative predictive factors for
microsatellite distance >1 cm on univariate and multivariate
regression models. |
Table III
Preoperative predictive factors for
microsatellite distance >1 cm on univariate and multivariate
regression models.
| Univariate
analysis | Multivariate
analysis | |
|---|
|
|
| |
|---|
| Parameters | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value | AUC |
|---|
| Age (years) | 0.97
(0.91–1.13) | 0.273 | | | |
| BMI
(kg/m2) | 0.86
(0.65–1.07) | 0.251 | | | |
| AST (IU/l) | 1.00
(0.98–1.03) | 0.501 | | | |
| ALT (IU/l) | 0.99
(0.96–1.02) | 0.863 | | | |
| PLT
(x104/μl) | 1.07
(0.96–1.19) | 0.180 | | | |
| TBil (mg/dl) | 0.26
(0.01–2.11) | 0.327 | | | |
| Alb (g/dl) | 1.34
(0.35–6.40) | 0.819 | | | |
| PT (%) | 1.00
(0.94–1.07) | 0.868 | | | |
| AFP (ng/ml) | 1.00
(0.99–1.00) | 0.182 | | | |
| PIVKA-II
(mAU/ml) | 1.00
(0.99–1.00) | 0.122 | | | |
| HbA1c (%) | 0.41
(0.09–1.34) | 0.182 | | | |
| Diameter of tumor
(cm) | 1.23
(1.04–1.39) | 0.017 | 1.06
(0.82–1.32) | 0.591 | |
| SUVmax | 1.65
(1.30–2.29) | 0.003 | 1.60
(1.23–2.26) | 0.002 | 0.854 |
The predictive accuracy for microsatellite distance
>1 cm was highest for SUVmax (0.854). The optimal cutoff value
was 8.8. The sensitivity and specificity were 75.0 and 95.0%,
respectively. The NPV was also found to be high (97.3%).
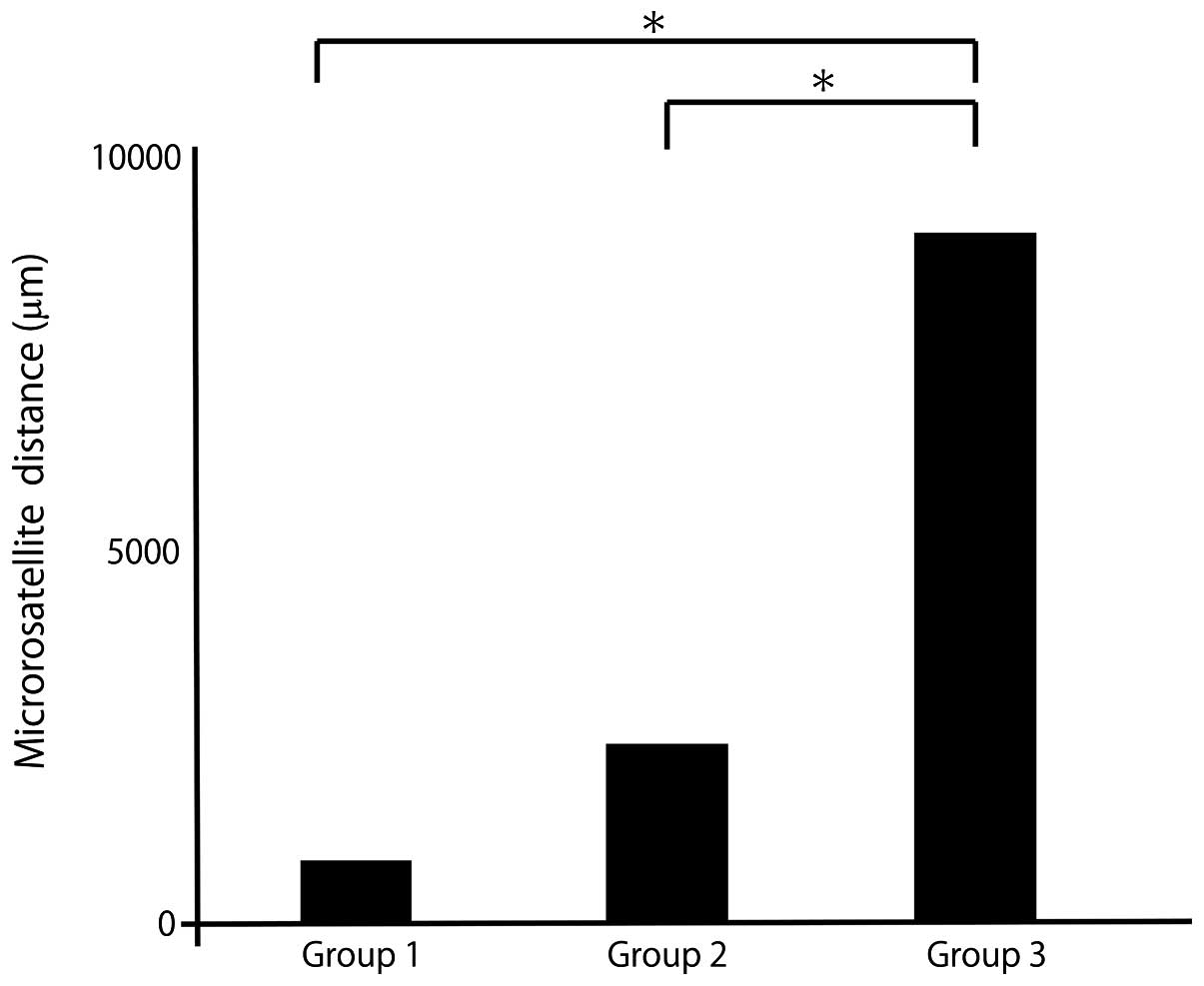
Microsatellite distance and SUVmax by
postoperative recurrence pattern
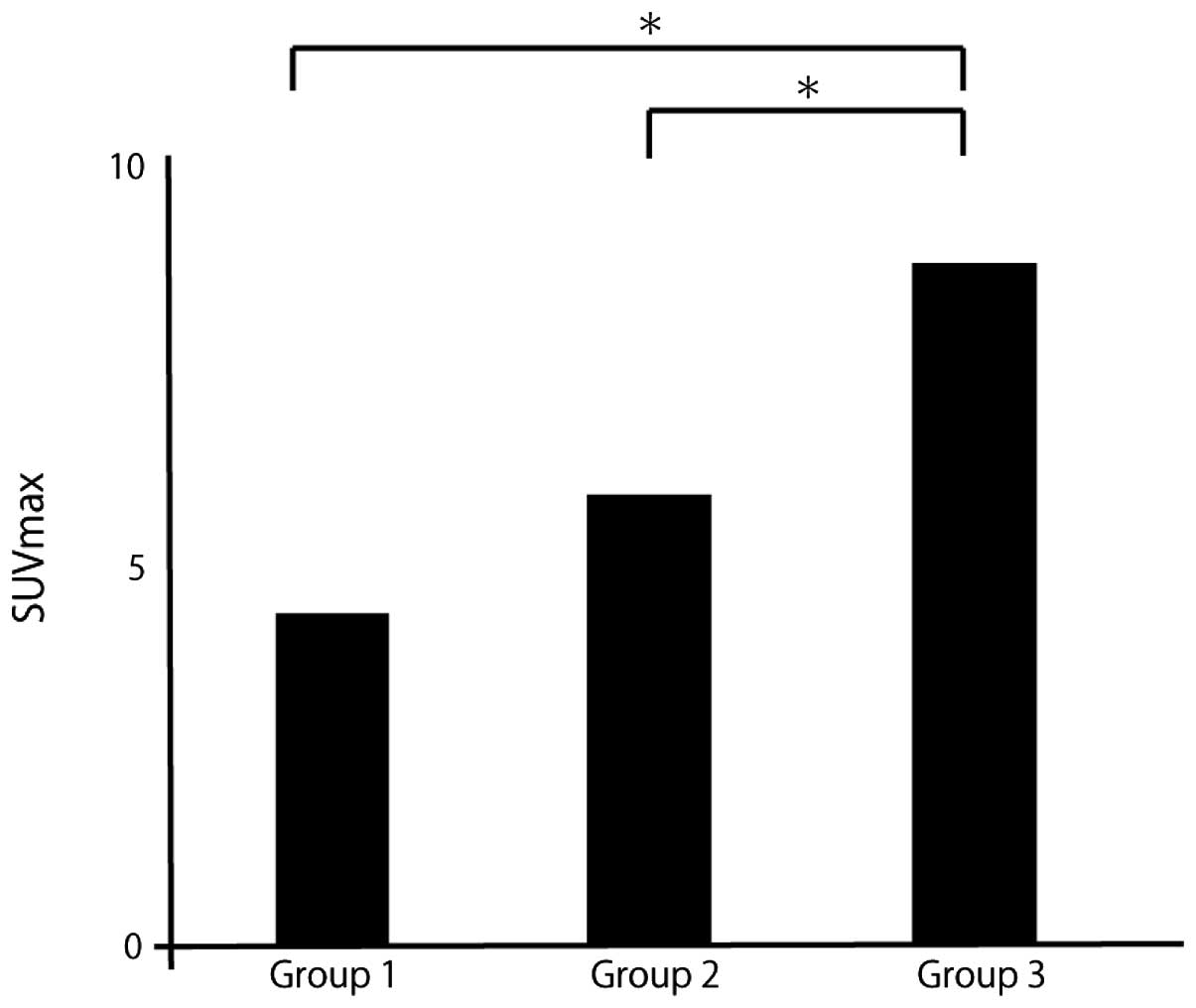
The patients were divided into 3 groups according to
the initial recurrence pattern: No recurrence (group 1),
intrahepatic recurrence (group 2) and extrahepatic recurrence
(group 3).
The mean distance was 841 μm in group 1, 2,098 μm in
group 2 and 9,691 μm in group 3 (Fig.
4). The mean SUVmax was 4.0 in group 1, 5.1 in group 2 and 8.6
in group 3 (Fig. 5). The mean
microsatellite distance and mean SUVmax were significantly higher
with extrahepatic recurrence compared with other recurrence
patterns.
Independent risk factors for prediction
of the postoperative recurrence pattern
On the univariate analysis for the independent risk
factors for postoperative extrahepatic recurrence, the significant
factors were preoperative AFP level (P=0.019, hazard ratio 1.00,
95% CI: 1.00–1.00), PIVKA-II level (P=0.012, hazard ratio 1.00, 95%
CI:1.00–1.00), SUVmax (P=0.001, hazard ratio 1.26, 95% CI:
1.03–1.53), and Diameter of tumor (P=0.033, hazard ratio 1.19, 95%
CI: 1.01–1.42) (Table IV).
 | Table IVPreoperative predictive factors for
postoperative extrahepatic recurrence on univariate and
multivariate regression models. |
Table IV
Preoperative predictive factors for
postoperative extrahepatic recurrence on univariate and
multivariate regression models.
| Univariate
analysis | Multivariate
analysis | |
|---|
|
|
| |
|---|
| Parameters | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value | AUC |
|---|
| Age (years) | 1.01
(0.95–1.09) | 0.641 | | | |
| BMI
(kg/m2) | 1.04
(0.88–1.20) | 0.584 | | | |
| AST (IU/l) | 1.01
(0.99–1.04) | 0.122 | | | |
| ALT (IU/l) | 1.01
(0.99–1.03) | 0.158 | | | |
| PLT
(x104/μl) | 1.04
(0.94–1.14) | 0.414 | | | |
| TBil (mg/dl) | 1.45
(0.28–5.22) | 0.593 | | | |
| Alb (g/dl) | 1.46
(0.42–6.08) | 0.573 | | | |
| PT (%) | 1.00
(0.95–1.04) | 0.854 | | | |
| AFP (ng/ml) | 1.00
(1.00–1.00) | 0.019 | 1.00
(1.00–1.00) | 0.125 | |
| PIVKA-II
(mAU/ml) | 1.00
(1.00–1.00) | 0.012 | 1.00
(0.99–1.00) | 0.387 | |
| HbA1c (%) | 0.55
(0.16–1.52) | 0.286 | | | |
| Diameter of tumor
(cm) | 1.19
(1.01–1.42) | 0.033 | 0.93
(0.64–1.20) | 0.620 | |
| SUVmax | 1.26
(1.03–1.53) | 0.001 | 1.24
(1.01–1.55) | 0.033 | 0.846 |
Discussion
The rate of HCC recurrence following treatment
remains high (15,16). In previous studies, the 5-year
disease-free survival rate following liver resection was reported
to be 72% (2). Therefore, it is
crucial to assess the risk of postoperative recurrence. The
presence of microsatellite lesions (intrahepatic metastasis and
microvascular invasion) is an independent risk factor for
postoperative recurrence (15,17–19).
Sumie et al (20) reported
that microvascular invasion was an independent risk factor for
recurrence-free survival. The 5-year recurrence-free survival rates
for patients with and without microvascular invasion were 20.8 and
52.6%, respectively. Microvascular invasion and intrahepatic
metastases were identified as independent predictors of
disease-specific survival.
Determining the extent of the treatment margin is
critical, as an insufficient treatment margin causes disease
recurrence after treatment. It is crucial to determine the
treatment margin within the microsatellite lesion to prevent
postoperative recurrence as much as possible. In previous studies,
the microsatellite distance was associated with prognosis. Sasaki
et al (7) reported that the
overall survival rate of patients with a microsatellite distance
>5 mm was lower compared to that of patients with a
microsatellite distance <5 mm.
Even if the presence of a microsatellite lesion can
be predicted preoperatively, it is difficult to predict the
microsatellite distance. The extent of the treatment margin remains
controversial. However, US, CT, or MRI cannot predict the presence
of microsatellite lesions and the microsatellite distance
preoperatively.
The use of 18F-FDG PET/CT, which is based
on the fact that a number of malignant tumors exhibit facilitated
glucose uptake, was found to be effective for detecting and staging
a variety of malignant diseases. However, in HCC,
18F-FDG PET/CT is not effective for detecting tumors,
particularly well-differentiated HCC. However, FDG accumulation
reflects tumor aggressiveness (8,9).
Patients with tumors with a high accumulation of FDG have a poor
prognosis after therapy (10,11).
In other malignant tumors (e.g., malignant lymphoma or breast
cancer), it is possible to diagnose not only the localization but
also disease activity and biological characteristics.
18F-FDG PET/CT was found to be useful for predicting
disease activity and biological characteristics in HCC, including
pathological evaluation. 18F-FDG accumulation in HCC
also reflects malignant potential. Hiraoka et al (21) demonstrated that high accumulation
of FDG in HCC increased the risk of early postoperative recurrence
following curative resection and the appearance of microsatellite
lesions.
In the present study, the efficacy of
18F-FDG PET/CT for the prediction of microsatellite
distance was investigated. To the best of our knowledge, this is
the first study to demonstrate the correlation between
microsatellite distance and median SUVmax; microsatellite distance
correlated with SUVmax (r=0.58). On the multivariate analysis, the
only significant factor was SUVmax (P=0.002; hazard ratio=1.60; 95%
CI: 1.23–2.26). When the SUVmax was >9.3, the microsatellite
distance was >1 cm.
There are various patterns of postoperative
recurrence of HCC, including intrahepatic recurrence (within the
same or a different segment of the primary tumor) or extrahepatic
recurrence. The patterns of postoperative recurrence are associated
with the prognosis of HCC. Intrahepatic metastasis may be
satisfactorily treated and controlled by resection or RFA. Shiina
et al (22) reported that
the 5-year survival rate in patients treated by RFA was 60.2%. In
addition, Ng et al (23)
reported that the overall survival rates were significantly higher
in patients with same segment recurrence of the primary tumor
compared to different segment recurrence. Among the various
recurrence patterns, extrahepatic recurrence has the worst
prognosis following curative therapy. In previous reports, the
1-year survival rate was 40–70% in patients with extrahepatic
recurrence (24,25). It is crucial to predict the
postoperative recurrence pattern. However, the number of available
studies investigating the imaging modalities useful for predicting
the postoperative recurrence pattern is currently limited. In the
present study, the SUVmax was found to be significantly higher in
patients with postoperative extrahepatic recurrence of HCC compared
to that in other patients.
It was previously demonstrated that HCCs with a
higher SUVmax exhibit a higher malignant potential. There are two
patterns of metastasis in HCC, via the portal veins or the systemic
circulation. The mechanism of distant metastasis is based on
various factors, according to previous studies (26,27).
In microsatellite lesions with a microsatellite distance >1 cm,
the pattern of metastasis is via the systemic circulation in almost
all cases. Epithelial-to-mesenchymal transition (EMT) has been
proposed as playing an important role in distant metastasis in
cancer. During the process of EMT, epithelial cells lose polarity
and intercellular junctions and acquire mesenchymal-like
characteristics, such as motility, becoming able to detach from the
original tissue. The activation of EMT induces more distant
metastases as microsatellite distance is >1 cm. In a previous
study, FDG uptake was found to reflect the activation of glucose
metabolism. Amann et al (28) reported that HCC with a higher
uptake of glucose via glucose transporter 1 (GLUT-1) into cancer
cells exhibited higher proliferative activity and that
18F-FDG PET/CT reflects proliferative activity in HCC.
The uptake of 18F-FDG and the expression of GLUT-1 were
increased during the process of EMT, stimulated by transforming
growth factor β (29). Therefore,
it was suggested that high FDG accumulation may primarily reflect
activation of EMT, tumor aggressiveness and distant metastasis in
the present study.
There were several limitations to the present study.
First, the design was retrospective. Second, the mean HCC diameter
was relatively large; therefore, a validation study for
microsatellite distance in small HCCs would be required as a
further experiment. Third, our sample size was small; therefore,
further investigations, including a larger patient sample, are
required.
In conclusion, SUVmax may be a valid predictor of
microsatellite distance and postoperative recurrence pattern.
SUVmax may also be an indicator for determining the treatment
protocol. There is currently no standard safety margin for liver
resection or RFA. Generally, if the SUVmax is >8.8, we select
anatomic liver resection with a margin >1 cm. In the future, if
the prediction of extrahepatic metastasis is feasible using the
SUVmax, SUVmax may become the standard for the introduction of
systemic chemotherapy.
Acknowledgements
The authors would like to thank Dr Yoshiko Soga
(Department of Pathology, Ehime University Graduate School of
Medicine) and Mr Kenji Tanimoto (Department of Gastroenterology and
Metabology, Ehime University Graduate School of Medicine) for their
valuable technical assistance.
This study was supported in part by Grants-in-Aid
for Scientific Research (Japan Society for the Promotion of
Science, KAKENHI no. 24590980 to Y.H. and KAKENHI no. 25860541 to
Y.K.).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2
|
Shah SA, Cleary SP, Wei AC, et al:
Recurrence after liver resection for hepatocellular carcinoma: risk
factors, treatment, and outcomes. Surgery. 141:330–339. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Uchino K, Tateishi R, Shiina S, et al:
Hepatocellular carcinoma with extrahepatic metastasis: clinical
features and prognostic factors. Cancer. 117:4475–4483. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kanda M, Tateishi R, Yoshida H, et al:
Extrahepatic metastasis of hepatocellular carcinoma: incidence and
risk factors. Liver Int. 28:1256–1263. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nagasue N, Uchida M, Makino Y, et al:
Incidence and factors associated with intrahepatic recurrence
following resection of hepatocellular carcinoma. Gastroenterology.
105:488–494. 1993.PubMed/NCBI
|
|
6
|
Miyazaki K, Soyama A, Hidaka M, et al: Ex
vivo hepatic venography for hepatocellular carcinoma in livers
explanted for liver transplantation. World J Surg Oncol. 9:1112011.
View Article : Google Scholar
|
|
7
|
Sasaki A, Kai S, Iwashita Y, Hirano S,
Ohta M and Kitano S: Microsatellite distribution and indication for
locoregional therapy in small hepatocellular carcinoma. Cancer.
103:299–306. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Delbeke D, Martin WH, Sandler MP, Chapman
WC, Wright JK Jr and Pinson CW: Evaluation of benign vs. malignant
hepatic lesions with positron emission tomography. Arch Surg.
133:510–515. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iwata Y, Shiomi S, Sasaki N, et al:
Clinical usefulness of positron emission tomography with
fluorine-18-fluorodeoxyglucose in the diagnosis of liver tumors.
Ann Nucl Med. 14:121–126. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee JW, Paeng JC, Kang KW, et al:
Prediction of tumor recurrence by 18F-FDG PET in liver
transplantation for hepatocellular carcinoma. J Nucl Med.
50:682–687. 2009.PubMed/NCBI
|
|
11
|
Kornberg A, Freesmeyer M, Barthel E, et
al: 18F-FDG-uptake of hepatocellular carcinoma on PET
predicts microvascular tumor invasion in liver transplant patients.
Am J Transplant. 9:592–600. 2009. View Article : Google Scholar
|
|
12
|
Kawaoka T, Aikata H, Takaki S, et al: FDG
positron emission tomography/computed tomography for the detection
of extrahepatic metastases from hepatocellular carcinoma. Hepatol
Res. 39:134–142. 2009. View Article : Google Scholar
|
|
13
|
Teras M, Tolvanen T, Johansson JJ,
Williams JJ and Knuuti J: Performance of the new generation of
whole-body PET/CT scanners: Discovery STE and Discovery VCT. Eur J
Nucl Med Mol Imaging. 34:1683–1692. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kanai T, Hirohashi S, Upton MP, et al:
Pathology of small hepatocellular carcinoma. A proposal for new
gross classification. Cancer. 60:810–819. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Adachi E, Maehara S, Tsujita E, et al:
Clinicopathologic risk factors for recurrence after a curative
hepatic resection for hepatocellular carcinoma. Surgery. 131(Suppl
1): S148–S152. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lam CM, Lo CM, Yuen WK, et al: Prolonged
survival in selected patients following surgical resection for
pulmonary metastasis from hepatocellular carcinoma. Br J Surg.
85:1198–1200. 1998. View Article : Google Scholar
|
|
17
|
Shimada K, Sakamoto Y, Esaki M, et al:
Analysis of prognostic factors affecting survival after initial
recurrence and treatment efficacy for recurrence in patients
undergoing potentially curative hepatectomy for hepatocellular
carcinoma. Ann Surg Oncol. 14:2337–2347. 2007. View Article : Google Scholar
|
|
18
|
Yang Y, Nagano H, Ota H, et al: Patterns
and clinicopathologic features of extrahepatic recurrence of
hepatocellular carcinoma after curative resection. Surgery.
141:196–202. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cha C, Fong Y, Jarnagin WR, Blumgart LH
and DeMatteo RP: Predictors and patterns of recurrence after
resection of hepatocellular carcinoma. J Am Coll Surg. 197:753–758.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sumie S, Kuromatsu R, Okuda K, et al:
Microvascular invasion in patients with hepatocellular carcinoma
and its predictable clinicopathological factors. Ann Surg Oncol.
15:1375–1382. 2008. View Article : Google Scholar
|
|
21
|
Hiraoka A, Ochi H, Hidaka S, et al: FDG
positron emission tomography/computed tomography findings for
prediction of early recurrence of hepatocellular carcinoma after
surgical resection. Exp Ther Med. 1:829–832. 2010. View Article : Google Scholar
|
|
22
|
Shiina S, Tateishi R, Arano T, et al:
Radiofrequency ablation for hepatocellular carcinoma: 10-year
outcome and prognostic factors. Am J Gastroenterol. 107:569–577.
2012.PubMed/NCBI
|
|
23
|
Ng KK, Poon RT, Lo CM, et al: Analysis of
recurrence pattern and its influence on survival outcome after
radiofrequency ablation of hepatocellular carcinoma. J Gastrointest
Surg. 12:183–191. 2008. View Article : Google Scholar
|
|
24
|
Uka K, Aikata H, Takaki S, et al: Clinical
features and prognosis of patients with extrahepatic metastases
from hepatocellular carcinoma. World J Gastroenterol. 13:414–420.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Natsuizaka M, Omura T, Akaike T, et al:
Clinical features of hepatocellular carcinoma with extrahepatic
metastases. J Gastroenterol Hepatol. 20:1781–1787. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang GT, Lee HS, Chen CH, et al:
Correlation of E-cadherin expression and recurrence of
hepatocellular carcinoma. Hepatogastroenterology. 46:1923–1927.
1999.PubMed/NCBI
|
|
27
|
Sakon M, Nagano H, Nakamori S, et al:
Intrahepatic recurrences of hepatocellular carcinoma after
hepatectomy: analysis based on tumor hemodynamics. Arch Surg.
137:94–99. 2002. View Article : Google Scholar
|
|
28
|
Amann T, Maegdefrau U, Hartmann A, et al:
GLUT1 expression is increased in hepatocellular carcinoma and
promotes tumorigenesis. Am J Pathol. 174:1544–1552. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li W, Wei Z, Liu Y, et al: Increased
18F-FDG uptake and expression of Glut1 in the EMT
transformed breast cancer cells induced by TGF-beta. Neoplasma.
57:234–240. 2010.
|