Introduction
In the central regions of solid tumors, the
extracellular pH falls below pH 6.5 as a consequence of lactate
accumulation, which is caused by hypoxic conditions produced by a
lack of sufficient vascularization (1,2) or
an increase in tumor-specific glycolysis combined with impaired
mitochondrial oxidative phosphorylation (3). Organ functions may be strongly
affected by the disruption of the pH homeostasis as all the organs
contain a large number of enzymes with pH-sensitive catalytic
activity. Therefore, it can be argued that alternative metabolic
processes are activated under acidic conditions to compensate for
the decline in processes functioning at alkaline pH.
When various metabolic processes are working under
different pH conditions, the efficacy of a number of inhibitors
under acidic conditions may be different to those observed in
conventional alkaline media. Impaired efficacy of paclitaxel,
mitoxantrone and topotecan has been previously reported at pH 6.5
as compared to their efficacy at pH 7.4 in murine EMT6 and human
MGH-U1 cells (4), and acidic
conditions induced daunorubicin resistance by increasing the
activity of p-glycoprotein via p38 activation in rat prostate
cancer cells (5).
Malignant pleural mesothelioma is an aggressive
tumor associated with asbestos exposure, and its prognosis is
extremely poor (6). Mesothelioma
shows resistance against numerous chemotherapeutic reagents
(7). Our previous study found that
statins inhibited the proliferation of mesothelioma cells strongly
in an acidic medium with a pH that was close to the pH of an area
of cancer in vivo (8).
Statins, which are inhibitors of mevalonate synthesis, are
prescribed for hyperlipidemia as the inhibition of mevalonate
synthesis reduces blood cholesterol levels. However, the
anti-cancer activity of statins has not been demonstrated in
vitro. Recently, clinical studies have revealed that stains are
effective at attenuating the growth of cancer cells in vivo
(9,10), in agreement with our previous in
vitro observations at acidic pH (8). A previous study has shown that the
anticancer activity is caused by the inhibition of geranylgeranyl
diphosphate, derived from mevalonate, indicated that the function
of certain geranylgeranylated proteins is essential for cell
proliferation under acidic conditions (8,11).
In addition to the investigations with inhibitors, our previous
studies found that different signal transduction pathways function
under acidic environments (12,13),
and that C-Terminus protein of IκB-β, which is an IκB-β variant,
acted as a critical transcriptional regulatory factor at pH 6.3
only, and not at pH 7.4 (14,15).
These previous findings indicate that numerous
proteins are functioning preferentially under low pH conditions.
DNA array analysis showed that the expression of ~700 genes was
elevated more than two-fold in mesothelioma cells under acidic
conditions compared to in cells cultured in an alkaline medium
(16). Numerous genes were also
found to be strongly expressed in breast cancer cells cultured in
an acidic medium (17). These gene
products may be good candidate therapeutic targets and/or
diagnostic markers of cancers. In the present study, the aim was to
confirm whether or not the genes with an increased expression in
cancer cells cultured in acidic medium are expressed in human
cancer nests. A total of 8 genes with an increased expression in
mesothelioma cells cultured under acidic conditions were selected
and the expression was examined in human specimens from patients
with cancer. The expression of the selected genes was demonstrated
to be higher in numerous human cancer specimens compared to those
in the specimens prepared from the surrounding normal areas.
Materials and methods
Human specimens from patients
Human tumor and the corresponding non-tumorous
tissues were obtained from the Chiba Cancer Center Tissue Bank
(Chiba, Japan) and used in the study with permission from the
Institutional Ethical Committees of Chiba Cancer Center and Chiba
University.
RNA extraction from human specimens
The human tissues that were stored at −80°C were
mixed with ice-cold TRI reagent (Sigma-Aldrich, St. Louis, MO,
USA). After 1 min on ice, the human tissues were homogenized on ice
with a homogenizer until the pellets were broken and cell lysis was
completed. Total RNA was isolated from the lysate according to the
manufacturer’s instructions for the TRI reagent.
Quantitative polymerase chain reaction
(qPCR)
Total RNA (1 μg), prepared as described above, was
reverse-transcribed using ReverTra Ace (Toyobo Co., Ltd., Osaka,
Japan) in a total volume of 20 μl containing the random primer for
18S rRNA or the polyT primer for the targeted genes. qPCR
amplification was performed with an ABI Prism 7000 Sequence
Detection System (Applied Biosystems, Foster City, CA, USA) using
the FastStart Universal SYBR Green Master (Rox) (Roche Diagnostics,
Basel, Switzerland) according to the manufacturer’s instructions.
The PCR reaction was carried out with a mixture containing 12.5 μl
PCR Master, 7.5 μM of each sense and antisense primer, 25 ng cDNA,
and nuclease-free water in a total volume of 25 μl. The standard
thermal profile for PCR amplification was 50°C for 2 min, 95°C for
10 min and 40 cycles of 95°C for 15 sec and 60°C for 60 sec. The
primers used are shown in Table
I.
 | Table IPrimers used in the present
study. |
Table I
Primers used in the present
study.
| Gene name | Sequence |
|---|
| 18S rRNA | F:
TAGAGTGTTCAAAGCAGGCCC
R: CCAACAAATAGAACCGCGGT |
| MnSOD | F: TGA ACG TCA CCG
AGG AGA AG
R: CGT GCT CCC ACA CAT CAA TC |
| IL-32 | F:
TCAAAGAGGGCTACCTGGAG
R: TTTCAAGTAGAGGAGTGAGCTCTG |
|
ATP6V0D2 | F:
GACCCAGCAAGACTATATCAACC
R: TGGAGATGAATTTTCAGGTCTTC |
| TNFRSF9 | F:
AAACGGGGCAGAAAGAAACT
R: CTTCTGGAAATCGGCAGCTA |
| AREG | F:
GGGAGTGAGATTTCCCCTGT
R: AGCCAGGTATTTGTGGTTCG |
| ErbB3 | F:
TGCAGTGGATTCGAGAAGTG
R: GGCAAACTTCCCATCGTAGA |
|
LOC553158 | F:
AGCCTCCCAGAGCACAACTA
R: ATGGCCAGATCAAATTCAGC |
| DMGDH | F:
GAGCTCACGGCTGGATCTAC
R: CCACCACCTGACCAGTTTCT |
A previous study has reported that the content of
ribosomes per cell is ~4×106 (18), and the amount of mRNA per cell can
be estimated using 18S rRNA as a control RNA with the following
equation, in which Ct is the threshold cycle number:
4×106×2{(Ct of 18S rRNA) − (Ct of sample
RNA)}.
Results
Quantification of mRNA levels in human
cancer specimens
Our previous study showed that the expression of 58
genes was elevated more than three-fold in mesothelioma cells
cultured for 24 h in an acidic medium (16). The 58 genes are listed in Table II. Seven genes were selected of
the 58 genes with various functions, which were interleukin 32
(IL-32), lysosomal H+ transporting ATPase, V0
subunit d2 (ATP6V0D2), tumor necrosis factor receptor
superfamily, member 9 (TNFRSF9), amphiregulin,
schwannoma-derived growth factor (AREG), v-erb-b2
erythroblastic leukemia viral oncogene homolog 3 (ErbB3),
PRR5-ARHGAP8 (LOC553158) and dimethylglycine dehydrogenase
(DMGDH), and the expression of these genes was examined in
human cancer specimens. In addition, the expression of the gene
encoding manganese superoxide dismutase (MnSOD) was examined
as MnSOD has been reported to participate in gastric and colorectal
tumor metastasis (19,20), although the expression of
MnSOD at acidic pH was 1.6-fold in mesothelioma cells. The
selected genes are shown in Table
II.
 | Table IIGenes with an elevated expression of
>3-fold at acidic pH. |
Table II
Genes with an elevated expression of
>3-fold at acidic pH.
| Gene | Expression at pH
6.7 (fold)a | Relative
amountb | Description |
|---|
| RHCE | 7.816 | 0.58 | Rh blood group,
CcEe antigens |
| RSPO3 | 7.346 | 0.70 | R-spondin 3 homolog
(Xenopus laevis) |
| ZSCAN4 | 6.346 | 1.06 | zinc finger and
SCAN domain containing 4 |
|
ErbB3c | 5.997 | 0.69 | v-erb-b2
erythroblastic leukemia viral oncogene homolog 3 (avian) |
| AREGc | 5.650 | 0.92 | amphiregulin
(schwannoma-derived growth factor) |
|
FLJ33706 | 5.579 | 1.75 | hypothetical
protein FLJ33706 |
|
TNFRSF9c | 5.464 | 2.58 | tumor necrosis
factor receptor superfamily, member 9 |
| BMP1 | 5.186 | 0.40 | bone morphogenetic
protein 1 |
| PIPOX | 5.069 | 0.66 | pipecolic acid
oxidase |
|
LOC653193 | 4.485 | 0.43 | similar to
Amphiregulin precursor (AR) (Colorectum cell-derived growth factor)
(CRDGF) |
|
DMGDHc | 4.310 | 0.39 | dimethylglycine
dehydrogenase |
|
LOC553158c | 4.306 | 0.44 | PRR5-ARHGAP8
fusion |
| KCTD19 | 4.231 | 0.33 | potassium channel
tetramerisation domain containing 19 |
| ZC3H6 | 4.220 | 0.15 | zinc finger
CCCH-type containing 6 |
| SIGLEC1 | 4.184 | 0.29 | sialic acid binding
Ig-like lectin 1, sialoadhesin |
| GRHL3 | 4.142 | 0.54 | grainyhead-like 3
(Drosophila) |
| FBXO32 | 4.117 | 1.49 | F-box protein
32 |
| BMP2 | 4.014 | 0.48 | bone morphogenetic
protein 2 |
| LXN | 3.987 | 9.88 | latexin |
| INPP5D | 3.967 | 0.49 | inositol
polyphosphate-5-phosphatase, 145kDa |
| RARRES1 | 3.882 | 0.49 | retinoic acid
receptor responder (tazarotene induced) 1 |
|
NYD-SP14 | 3.847 | 0.48 | NYD-SP14
protein |
| RRAD | 3.827 | 4.50 | Ras-related
associated with diabetes |
| VWCE | 3.790 | 2.35 | von Willebrand
factor C and EGF domains |
|
ATP6V0D2c | 3.778 | 0.69 | ATPase,
H+ transporting, lysosomal 38kDa, V0 subunit d2 |
| CDH15 | 3.750 | 0.64 | cadherin 15,
M-cadherin (myotubule) |
| HES2 | 3.723 | 0.54 | hairy and enhancer
of split 2 (Drosophila) |
|
IL-32c | 3.711 | 8.91 | interleukin 32 |
| CRELD1 | 3.707 | 2.92 | cysteine-rich with
EGF-like domains 1 |
| PPP1R3E | 3.702 | 0.39 | protein phosphatase
1, regulatory (inhibitor) subunit 3E |
| CLDN14 | 3.560 | 0.20 | claudin 14 |
| ARHGAP8 | 3.547 | 0.23 | Rho GTPase
activating protein 8 |
|
MGC33926 | 3.508 | 5.58 | hypothetical
protein MGC33926 |
|
LOC390937 | 3.497 | 0.34 | similar to ETS
domain transcription factor ERF |
| FUT5 | 3.486 | 0.41 | fucosyltransferase
5 (α (1,3) fucosyltransferase) |
| CLEC4F | 3.459 | 0.47 | C-type lectin
domain family 4, member F |
|
LOC644893 | 3.363 | 0.21 | hypothetical
protein LOC644893 |
|
C11orf34 | 3.359 | 0.83 | chromosome 11 open
reading frame 34 |
| EGR4 | 3.353 | 0.13 | early growth
response 4 |
|
FLJ42258 | 3.324 | 0.56 | FLJ42258
protein |
| CFB | 3.320 | 5.25 | complement factor
B |
| GPR78 | 3.302 | 0.92 | G protein-coupled
receptor 78 |
| MUC3B | 3.300 | 0.49 | mucin 3B, cell
surface associated |
| CRYM | 3.298 | 1.48 | crystallin, μ |
| CYYR1 | 3.294 | 0.14 |
cysteine/tyrosine-rich 1 |
|
LOC196394 | 3.286 | 7.17 | hypothetical
protein LOC196394 |
|
LOC644725 | 3.262 | 0.30 | similar to
γ-tubulin complex component 3 (GCP-3) (Spindle pole body protein
Spc98 homolog) (hSpc98) (hGCP3) (h104p) |
| FGF7 | 3.219 | 0.17 | fibroblast growth
factor 7 (keratinocyte growth factor) |
|
PNLIPRP3 | 3.178 | 1.21 | pancreatic
lipase-related protein 3 |
|
C1orf101 | 3.170 | 0.13 | chromosome 1 open
reading frame 101 |
| ALS2CR7 | 3.164 | 0.49 | amyotrophic lateral
sclerosis 2 (juvenile) chromosome region, candidate 7 |
| IGLL1 | 3.130 | 1.12 | immunoglobulin
λ-like polypeptide 1 |
| GDF15 | 3.112 | 22.10 | growth
differentiation factor 15 |
|
FLJ26850 | 3.082 | 0.23 | FLJ26850
protein |
| PTP4A3 | 3.037 | 9.05 | protein tyrosine
phosphatase type IVA, member 3 |
| TAS2R39 | 3.035 | 0.34 | taste receptor,
type 2, member 39 |
| SGK2 | 3.015 | 0.28 |
serum/glucocorticoid regulated kinase
2 |
| CRNN | 3.005 | 0.20 | cornulin |
|
MnSODc | 1.599 | 13.77 | manganese
superoxide dismutase |
| GAPDH | 0.962 | 100.00 |
glyceraldehyde-3-phosphate
dehydrogenase |
One problem in the measurement of mRNA using qPCR
was determining which was useful as a control RNA. Thus far, a
reference gene, such as GAPDH, has generally been used in
studies. There are no previous data to show that the expression of
such reference genes is stable at acidic pH, particularly in human
cancer nests. The amount of 18S rRNA was constant in mesothelioma
cells at acidic and alkaline pH (data not shown). The amount of 18S
rRNA in total RNA isolated from human cancer specimens was
measured, with the results demonstrating that the content of 18S
rRNA was constant in all the cancer specimens (Table III). The amount of 18S rRNA was
slightly higher in normal areas, but the difference was <2-fold.
These data indicated that 18S rRNA was suitable for use as control
RNA. The Ct value shown in Table
III was similar to that observed in cells cultured in
vitro (data not shown), suggesting that the ribosome content
per cell is constant even when the activity of protein synthesis
varies. As one cell was reported to have ~4×106
ribosomes (18), the approximate
copy number of mRNA can be calculated using this number. The mRNA
level of GAPDH estimated using 18S rRNA as a control RNA
decreased slightly at acidic pH in mesothelioma cells (Table II).
 | Table IIIAmount of 18S rRNA in the human
specimens from patients with colon, stomach, liver and renal
cancer. |
Table III
Amount of 18S rRNA in the human
specimens from patients with colon, stomach, liver and renal
cancer.
| | Ct of 18S rRNA
(mean ± SD) |
|---|
| |
|
|---|
| Tissues | Samples, n | Normal area | Cancer area |
|---|
| Colon | 11 | 11.07±0.64 | 11.71±0.58 |
| Stomach | 10 | 11.03±0.55 | 11.49±1.01 |
| Renal | 10 | 10.97±0.69 | 11.60±0.40 |
| Liver | 10 | 10.63±0.54 | 11.63±0.66 |
| Total | 41 | 10.93±0.61 | 11.61±0.67 |
Expression levels of selected genes in
human cancers
Specimens from patients with lung, colon, stomach,
liver and renal cancer in the Chiba Cancer Center Tissue Bank were
available for the study. The homogenates of specimens from patients
with lung cancer were not used due to a huge amount of skeletal
material, so the measurement of gene expression was not assessed.
Therefore, the expression of 8 selected genes was examined in the
specimens from patients with colon, stomach, liver and renal
cancer.
The specimens from the colon, stomach and renal
cancer tissues showed increased MnSOD, IL-32 and
TNFRSF9 transcripts compared to those from the non-tumorous
regions of the same patients (Fig.
1). Increased expression of AREG was found in colon and
renal cancer specimens (Fig. 1).
Notably, an elevated expression of ATP6V0D2 was found in
stomach cancer specimens, whereas the expression was reduced in the
specimens from patients with colon and renal cancer (Fig. 1). The expression of ErbB3
was shown to be higher in colon, stomach and liver cancer specimens
compared to the normal tissues, but a higher expression was
observed in less than half of the renal cancer samples (Fig. 1). An increased expression of
LOC553158 was found in the specimens from the colon and
stomach cancer nests, but the expression decreased in the liver and
renal cancer specimens (Fig. 1).
The expression of DMGDH was upregulated in the specimens
from the colon cancer tissues, and the upregulated expression was
observed in about half of the samples from the patients with
stomach, liver and renal cancer (Fig.
1).
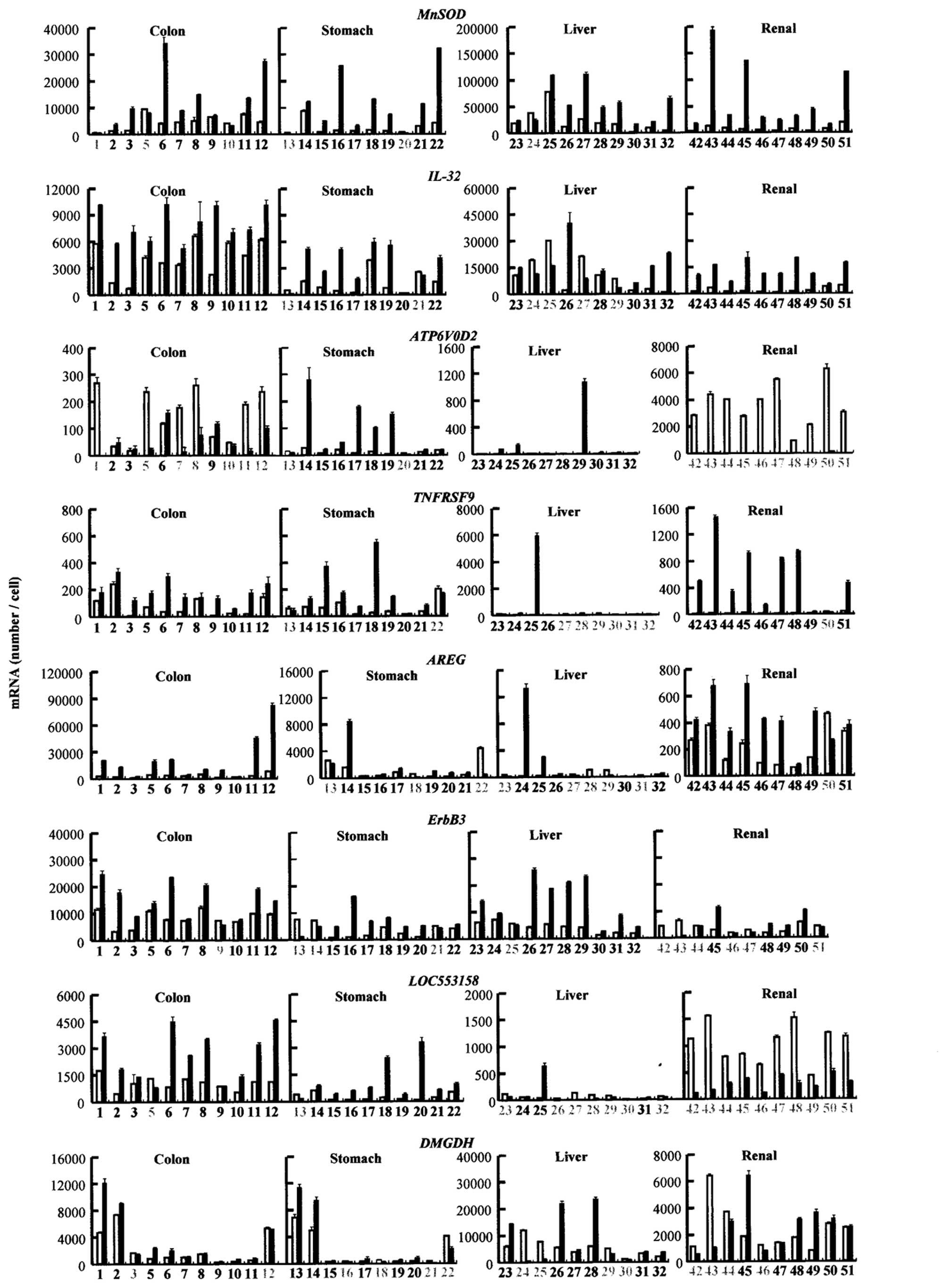 | Figure 1Gene expression in cancer tissues.
RNA was extracted from human tumor (closed bars) and the
corresponding non-tumorous tissues (open bars). The mRNA levels
(MnSOD, IL-32, ATP6V0D2 and TNFRSF9;
AREG, ErbB3, LOC553158 and DMGDH) were
measured as described in the Materials and methods. The averages
and standard deviation values were obtained from three experiments.
The numbers in the horizontal axes correlate to the patient
numbers. Grey numbers are the patients in which the gene expression
decreased in the cancer tissues. MnSOD, manganese superoxide
dismutase; IL-32, interleukin 32; ATP6V0D2, lysosomal
H+ transporting ATPase, V0 subunit d2; TNFRSF9,
tumor necrosis factor receptor superfamily, member 9; AREG,
amphiregulin, schwannoma-derived growth factor;
ErbB3,v-erb-b2 erythroblastic leukemia viral oncogene
homolog 3; LOC553158, PRR5-ARHGAP8; DMGDH,
dimethylglycine dehydrogenase. |
Discussion
For >30 years, it has been well known that cancer
nests are acidified. However, thus far, few in vitro studies
using acidic medium to develop cancer markers and medicines for
cancer therapies have been performed. Our previous studies
suggested that in vitro screening of compounds with
anti-proliferation activity in an acidic medium was useful for
developing anti-cancer drugs (11). A >2-fold increase in expression
was found in ~700 genes in mesothelioma cells as the medium was
acidified (16). Mesothelioma is
one cancer that is hard to treat and remains asymptomatic even at a
late stage.
In the present study, the expression of 8 genes with
acidosis-induced expression in mesothelioma cells were examined in
human specimens from various cancers and corresponding normal
tissues. The expression varied in different tissues and showed a
large variation among patients (Fig.
1). There may be a possibility that the genes that are specific
to acidosis are expressed in a normal tissue area close to cancer
nests as such an area may be acidified even if it contains no
cancer cells. However, it is difficult to measure the pH of normal
tissues prior to surgery as it can change during surgery due to the
limited supply of blood. Furthermore, the pH may vary in different
areas of cancer nests. In particular, the areas far from blood
vessels are strongly acidified as suggested previously (2). Even though the data showed a wide
variation, the present study produced several noteworthy
results.
IL-32, TNFRSF9, AREG, ErbB3,
LOC553158 and DMGDH were expressed at a higher level
than that of the normal areas in almost all the colon cancer
patients. MnSOD, IL-32, ATP6V0D2,
TNFRSF9 and LOC553158 were expressed at a higher
level compared to the normal areas in almost all the patients with
stomach cancer. Therefore, these genes may be candidate therapeutic
or diagnostic marker targets for these cancers, and a combination
use of these genes may be particularly useful for future treatment.
In the liver cancer area, MnSOD and ErbB3 were
expressed at a higher level, but the expression of other genes was
different in various patients. The reason for these differences in
expression change remains unclear. Liver cancer nests may only be
slightly acidified due to the highly organized blood vessel network
in the liver.
IL-32 is a notable cytokine. This cytokine has been
indicated to have a role in immune responses (21). The present data indicates that
IL-32 is an interleukin that is specific to acidic conditions. As
the mRNA level of IL-32 was high in mesothelioma cells
cultured at acidic pH (2.6×105 copies/cell, calculated
from the data shown in Table II)
and the numerous cancer nests measured in the present study
(Fig. 1), this interleukin may be
a predominant candidate for cancer diagnosis as indicated recently
(22). TNFRSF9 has been suggested
to play significant roles in immune responses (23). Our previous study demonstrated that
the expression of TNFRSF9 is induced in mesothelioma cells
cultured in acidic media (16) and
numerous cancer specimens (Fig.
1). Immune cells have to infiltrate into cancer nests or
inflammatory loci to rehabilitate damaged tissues. Since cancer and
inflammatory areas are often acidified, IL-32 and TNFRSF9 may
function under acidic conditions in various cells besides the
immune cells.
The ErbB/HER family, HER1 (epidermal growth factor
receptor), HER2 (ErbB2), HER3 (ErbB3), and HER4 (ErbB4), has been
indicated to have a central role in a wide variety of growth
factor-dependent cell responses (24). This family has been shown to
mediate differentiation in neuroblastoma (25), and a high expression of
ErbB3 was found in neuroblastic tumors (26). High expression of ErbB3 was
also found in various cancers, and ErbB3 has been identified
as an attractive therapeutic target (27). Taken together with the present
data, it can be argued that the gene product of ErbB3
protects against cell death under acidic conditions. AREG
was found to be expressed at high levels in colon and renal
cancers, suggesting a role in carcinogenesis (28,29).
To the best of our knowledge, this is the first study to report the
expression of LOC553158 itself in cancer cells, but the
upregulation of ARHGAP8 has been reported in cervical cancer
(30).
DMGDH is a mitochondrial enzyme that has a role in
choline catabolism [NCBI data base (31)]. No data concerning the role of
DMGDH in carcinogenesis has been reported until the present study,
and furthermore, no data to show the activation of the
mitochondrial function in cancer cells has been reported. The
present data indicate that choline catabolism may be activated in
cancer areas or that DMGDH may mediate an unidentified metabolic
process under acidic conditions besides choline catabolism.
The expression pattern of ATP6V0D2 in renal
tissues was unique. High expression of this gene was detected in
normal areas, whereas almost no expression was observed in the
cancer areas of all the patients. Protons are extruded to urine
(32), and therefore, urine is
often acidified. A high expression of ATP6V0D2 has been
previously reported in normal renal tissues (33). Therefore, it is quite possible that
this gene is expressed in normal renal tissues to protect cells
against external acidosis. The function to extrude protons may be
diminished during carcinogenesis, resulting in the attenuation of
this gene expression.
The genes with elevated expression levels in cancer
specimens as compared to the surrounding normal tissues may be good
candidates as novel targets and markers for cancer therapy.
Particularly, a combination therapy may be more useful for the
diagnosis of carcinogenesis and chemotherapeutics against cancer.
The expression of 8 genes with high expression in cells cultured at
an acidic pH were examined and it was found that the gene
expression was elevated in human cancer tissues in the present
study. Further studies of other acidosis-dependent gene expressions
to promote the development of novel cancer markers and/or
chemotherapeutic targets are warranted in future studies.
Acknowledgements
The authors would like to express their appreciation
to Chiba Cancer Center Tissue Bank (Japan) for providing the human
specimens. We thank Dr Xin Wang for her contribution to this
section of the study.
References
|
1
|
Vaupel P, Kallinowski F and Okunieff P:
Blood flow, oxygen and nutrient supply, and metabolic
microenvironment of human tumors. Cancer Res. 49:6449–6465.
1989.PubMed/NCBI
|
|
2
|
Dellian M, Helmlinger G, Yuan F and Jain
RK: Fluorescence ratio imaging of interstitial pH in solid tumours:
effect of glucose on spatial and temporal gradients. Br J Cancer.
74:1206–1215. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vukovic V and Tannock IF: Influence of low
pH on cytotoxicity of paclitaxel, mitoxantrone and topotecan. Br J
Cancer. 75:1167–1172. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sauvant C, Nowak M, Wirth C, et al:
Acidosis induces multi-drug resistance in rat prostate cancer cells
(AT1) in vitro and in vivo by increasing the activity of the
p-glycoprotein via activation of p38. Int J Cancer. 123:2532–2542.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zucali PA and Giaccone G: Biology and
management of malignant pleural mesothelioma. Eur J Cancer.
42:2706–2714. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Catalano A, Rodilossi S, Rippo MR, Caprari
P and Procopio A: Induction of stem cell factor/c-Kit/Slug signal
transduction in multidrug-resistant malignant mesothelioma cells. J
Biol Chem. 279:46706–46714. 2004. View Article : Google Scholar
|
|
8
|
Fukamachi T, Chiba Y, Wang X, et al: Tumor
specific low pH environments enhance the cytotoxicity of lovastatin
and cantharidin. Cancer Lett. 297:182–189. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nielsen SF, Nordestgaard BG and Bojesen
SE: Statin use and reduced cancer-related mortality. N Engl J Med.
367:1792–1802. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brewer TM, Masuda H and Liu DD: Statin use
in primary inflammatory breast cancer: a cohort study. Br J Cancer.
109:318–324. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fukamachi T, Wang X, Mochizuki Y, et al:
Acidic environments enhance the inhibitory effect of statins on
proliferation of synovial cells. Int Immunopharmacol. 17:148–153.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fukamachi T, Saito H, Kakegawa T and
Kobayashi H: Different proteins are phosphorylated under acidic
environments in Jurkat cells. Immunol Lett. 82:155–158. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hirata S, Fukamachi T, Sakano H, et al:
Extracellular acidic environments induce phosphorylation of ZAP-70
in Jurkat T cells. Immunol Lett. 115:105–109. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lao Q, Fukamachi T, Saito H, Kuge O,
Nishijima M and Kobayashi H: Requirement of an IkappaB-beta COOH
terminal region protein for acidic-adaptation in CHO cells. J Cell
Physiol. 207:238–243. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fukamachi T, Lao Q, Okamura S, Saito H and
Kobayashi H: CTIB (C-Terminus protein of IkappaB-beta): a novel
factor required for acidic adaptation. Adv Exp Med Biol.
584:219–228. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fukamachi T, Ikeda S, Wang X, et al: Gene
expressions for signal transduction under acidic conditions. Genes
(Basel). 4:65–85. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tang X, Lucas JE, Chen JL, et al:
Functional interaction between responses to lactic acidosis and
hypoxia regulates genomic transcriptional outputs. Cancer Res.
72:491–502. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Darnel J, Lodish H and Baltimore D:
Molecular Cell Biology. Scientific American Books, Inc; New York,
NY, USA: 1986
|
|
19
|
Malafa M, Margenthaler J, Webb B, Neitzel
L and Christophersen M: MnSOD expression is increased in metastatic
gastric cancer. J Surg Res. 88:130–134. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meng X, Wu J, Pan C, et al: Genetic and
epigenetic down-regulation of microRNA-212 promotes colorectal
tumor metastasis via dysregulation of MnSOD. Gastroenterology.
145:426–436. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Joosten LA, Heinhuis B, Netea MG and
Dinarello CA: Novel insights into the biology of interleukin-32.
Cell Mol Life Sci. 70:3883–3892. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ishigami S, Arigami T, Uchikado Y, et al:
IL-32 expression is an independent prognostic marker for gastric
cancer. Med Oncol. 30:4722013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao S, Zhang H, Xing Y and Natkunam Y:
CD137 ligand is expressed in primary and secondary lymphoid
follicles and in B-cell lymphomas: diagnostic and therapeutic
implications. Am J Surg Pathol. 37:250–258. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Linggi B and Carpenter G: ErbB receptors:
new insights on mechanisms and biology. Trends Cell Biol.
16:649–656. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Izycka-Swieszewska E, Wozniak A, Drozynska
E, et al: Expression and significance of HER family receptors in
neuroblastic tumors. Clin Exp Metastasis. 28:271–282. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wilzén A, Krona C, Sveinbjörnsson B, et
al: ERBB3 is a marker of a ganglioneuroblastoma/ganglioneuroma-like
expression profile in neuroblastic tumours. Mol Cancer.
12:702013.PubMed/NCBI
|
|
27
|
Sithanandam G and Anderson LM: The ERBB3
receptor in cancer and cancer gene therapy. Cancer Gene Ther.
15:413–448. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guzman MJ, Shao J and Sheng H:
Pro-neoplastic effects of amphiregulin in colorectal
carcinogenesis. J Gastrointest Cancer. 44:211–221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yotsumoto F, Yagi H, Suzuki SO, et al:
Validation of HB-EGF and amphiregulin as targets for human cancer
therapy. Biochem Biophys Res Commun. 365:555–561. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Song JY, Lee JK, Lee NW, et al: Microarray
analysis of normal cervix, carcinoma in situ, and invasive cervical
cancer: identification of candidate genes in pathogenesis of
invasion in cervical cancer. Int J Gynecol Cancer. 18:1051–1059.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Binzak BA, Wevers RA, Moolenaar SH, et al:
Cloning of dimethylglycine dehydrogenase and a new human inborn
error of metabolism, dimethylglycine dehydrogenase deficiency. Am J
Hum Genet. 68:839–847. 2001. View
Article : Google Scholar
|
|
32
|
Zeidel ML, Silva P and Seifter JL:
Intracellular pH regulation and proton transport by rabbit renal
medullary collecting duct cells. Role of plasma membrane proton
adenosine triphosphatase. J Clin Invest. 77:113–120. 1986.
View Article : Google Scholar
|
|
33
|
Smith AN, Borthwick KJ and Karet FE:
Molecular cloning and characterization of novel tissue-specific
isoforms of the human vacuolar H+-ATPase C, G and d
subunits, and their evaluation in autosomal recessive distal renal
tubular acidosis. Gene. 297:169–177. 2002. View Article : Google Scholar : PubMed/NCBI
|















