Introduction
In patients with gastric cancer, both the stage of
the cancer and the patient’s performance status (PS) are taken into
consideration when selecting the treatment regimen. In elderly
patients, the number of coexisting diseases, the history of the
disease and the extent of the planned surgery may limit the
possibility of general anesthesia and are associated with a high
risk of life-threatening perioperative complications. The
proportion of patients with gastric cancer who did not undergo
surgery increased from 8% prior to 1970 to 29% in 1990, due to
improved pretreatment selection (1). It was estimated that ~10% of patients
with locally advanced gastric cancer are not eligible for surgery
due to their poor general condition or contraindications to general
anesthesia. In such patients, chemotherapy is also usually
contraindicated. A proportion of elderly patients do not agree to
surgery due to their concerns regarding postoperative
complications. For this group of patients, best supportive care
(BSC) is the treatment of choice. BSC may improve the quality of
life, but offers no survival benefits. There is a lack of
alternative treatment regimens for this group of patients.
Palliative radiotherapy provides relief of symptoms in the majority
of patients and marginally prolongs survival (2–7). In
our institution, over the last 10 years neoadjuvant
chemoradiotherapy has been used in patients with operable gastric
cancer (8). This type of therapy
appears to be well tolerated, even by older patients. A high rate
of pathological response and R0 resection, low rate of local
recurrence and high percentage of 2-year survival were observed and
these results prompted us to attempt the use of radical
radiotherapy/chemoradiotherapy in patients with inoperable gastric
cancer. Such treatment may increase the chance of a cure. The aim
of this study was to present our experience with treatment
tolerance and patient outcomes with this regimen.
Materials and methods
Patient population
Patients with biopsy-proven locally advanced
inoperable gastric adenocarcinoma, with no evidence of distant
metastases, were treated with radiotherapy or chemoradiotherapy.
All the patients were required to have a Eastern Cooperative
Oncology Group (ECOG) PS of 0–2, to be aged 20–85 years, have serum
creatinine levels <1.5 mg/dl, serum bilirubin levels <2.0
mg/dl, a granulocyte count >1,500 cells/μl and a platelet count
>100,000 cells/μl.
The pretreatment staging included a complete
physical examination, oesophagogastroscopy with biopsies, chest
X-ray or computed tomography (CT) and CT of the abdomen. Endoscopic
ultrasonography is not yet available in our institution. The
patients were not staged with laparoscopy. This staging was focused
on identifying patients with distant metastases, who were excluded
from this study. The study was performed in accordance with the
Good Clinical Practice guidelines and the Declaration of
Helsinki.
A total of 13 patients were investigated, 3 women
(23.1%) and 10 men (76.9%), with a median age of 74 years (range,
52–83 years). Patients were enrolled in the study from February,
2008 to June, 2013. A total of 6 patients (46.2%) refused surgery
and 7 (53.8%) had contraindications to anesthesia due to
cardiological or respiratory reasons (4 and 3 patients,
respectively). A total of 6 patients (46.1%) had an ECOG PS of 0, 5
(38.5%) had an ECOG PS of 1 and 2 (15.4%) had an ECOG PS of 2. The
tumors were located predominantly in the cardiac region in 9
patients (69.2%), in the body of the stomach in 3 (23.1%) and in
the antral region in 1 patient (7.7%). The pretreatment tumor
stages were as follows: T1-2, 5 patients (38.5%) and T3, 8 patients
(61.5%). There was no nodal involvement (N0) in 8 patients (61.5%)
and N1-3 disease was found in 5 patients (38.5%). The median tumor
volume was 89 cm3 (range, 25–211 cm3).
A total of 4 patients lost >10% of their weight.
A reduced serum albumin level to <3.5 g/dl caused by
malnutrition was observed in 2 patients (15.4%). A total of 38% of
the patients were found to be anemic (hemoglobin concentration
<12 g/dl). The incidence of thrombocytosis, defined as platelet
count >400,000 cells/μl, was 23.1%. Carcinoembryonic antigen
(CEA) and carbohydrate antigen 19-9 (CA 19-9) levels were elevated
above the cutoff level in 30.8 and 46.1% of patients, respectively.
The patient characteristics are listed in Table I.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristics | Patient no. (%)
(n=13) |
|---|
| Age, years |
| Median (range) | 74 (52–83) |
| Gender |
| Male | 10 (76.9) |
| Female | 3 (23.1) |
| Reasons for no
surgery |
| Personal choice | 6 (46.2) |
| Comorbidities | 7 (53.8) |
| ECOG performance
status |
| 0 | 6 (46.1) |
| 1 | 5 (38.5) |
| 2 | 2 (15.4) |
| Tumor location |
| Proximal | 9 (69.2) |
| Middle/distal | 4 (30.8) |
| Histology |
| Adenocarcinoma | 10 (76.9) |
| Signet ring
cell/mucinous adenocarcinoma | 3 (23.1) |
| Tumor stage |
| T1-2 | 5 (38.5) |
| T3 | 8 (61.5) |
| Nodal status |
| N0 | 8 (61.5) |
| N1-3 | 5 (38.5) |
| Maximum tumor
diameter, cm |
| 0.0–5.9 | 2 (15.4) |
| 6.0–10.0 | 5 (38.5) |
| >10.0 | 6 (46.1) |
| Tumor volume,
cm3 |
| 0.0–49.9 | 3 (23.1) |
| 50.0–99.9 | 4 (30.8) |
| >100.0 | 6 (46.1) |
| Body weight loss,
% |
| No loss | 5 (38.4) |
| ≤10 | 4 (30.8) |
| >10 | 4 (30.8) |
| Albumin level prior
to RT/CRT, g/dl |
| Median (range) | 4.0 (3.0–4.8) |
| Hb level prior to
RT/CRT, g/dl |
| Median (range) | 12.3 (8.7–17.5) |
Radiotherapy
The treatment regimen consisted of radiotherapy and
chemotherapy administered for 35 days. The total dose of 45 Gy was
administered in 25 fractions, with 5 fractions per week for 5
weeks. The biologically effective dose (BED), calculated using the
linear-quadratic formalism (9) and
an α/β ratio of 10 for early responding-tissues (tumor), was 53.1
Gy. The tumor volume and location were defined on the basis of CT
scans of the abdomen and upper gastrointestinal endoscopy reports.
The treatment fields encompassed the stomach and regional lymph
nodes (gastric, celiac, gastroduodenal, porta hepatis, splenic,
suprapanceratic, retropanceraticoduodenal and lower oesophageal).
The longitudinal margins of the esophagus or duodenum (5 cm) were
included when the tumor involved the cardia or the gastroduodenal
junction (10). Radiation therapy
was delivered with a high-energy linear accelerator (Clinac 23EX;
Varian Medical Systems, Palo Alto, CA, USA) using 6–20 MV photons.
Three-dimentional conformal treatment planning was used for all the
cases in this study. Radiotherapy was performed using
intensity-modulated radiation therapy (8 patients), four-field
isocentric technique (3 patients) and tomotherapy (1 patient).
Concurrent chemotherapy
The concurrent chemotherapy regimen was based on
5-fluorouracil (5-FU). Chemotherapy was administered at least 1 h
prior to starting irradiation. 5-FU was administered intravenously
as a 10-min bolus injection. Patients routinely received
prophylactic antiemetic support. Bolus infusions of 5-FU (325
mg/m2 of body surface area) were administered
intravenously on days 1–5 and 29–33. Complete blood cell (CBC)
count, liver and renal tests were monitored prior to each course.
CBC counts were evaluated at least once per week. Over half of the
patients were not qualified to receive chemotherapy due to
comorbidities. Five patients (38.5%) received 2 cycles of
concurrent 5-FU during radiotherapy and 1 patient (7.7%) received 2
cycles of epirubicin, oxaliplatin and capecitabine prior to
qualification for radiotherapy.
Toxicity criteria and tumor response
Treatment-related toxicity was classified according
to the Common Terminology Criteria for Adverse Events, version 3.0
(11). Nausea, vomiting, diarrhea,
leukopenia, granulocytopenia, lymphocytopenia and thrombocytopenia
were assessed weekly. The quality of life was assessed using the
European Organization for Research and Treatment of Cancer Quality
of Life Questionnaire-Core 30. Tumor response was assessed based on
CT scans.
Statistical analysis
The survival function was computed using the
Kaplan-Meier method. The overall survival (OS) was calculated from
the start of the radiation therapy. Comparisons of survival curves
were performed using the Cox’s F-test and P<0.05 was considered
to indicate a statistically significant difference. The Chi-square
or Fisher’s exact tests were used to assess the association between
clinical factors and clinical response rates after therapy. All the
statistical computations were performed using Statistica software,
version 10 (StatSoft, Inc., Tulsa, OK, USA).
Follow-up
Each patient was assessed every 3 months after
treatment for 5 years or until death from any cause. The follow-up
evaluations included physical examination, oesophagogastroscopy,
chest X-ray, transabdominal ultrasonography or CT scans of the
abdomen, CBC count, CEA and CA 19-9 levels, liver and renal
function tests.
Results
Tolerance and response to treatment
All 13 patients were assessed for tolerance to
treatment and survival and 12 patients were assessed for clinical
complete response. Of the 13 patients who started treatment, 12
(92.3%) completed radiotherapy. In 1 case (7.7%), radiotherapy was
discontinued after 27 Gy due to worsening of a coexisting condition
and the patient soon succumbed to the disease despite intensive
hospital treatment. In 2 patients (15.4%), treatment was
interrupted for a few days, due to hematological adverse events in
one and due to worsening of a coexisting condition in the other
patient. All 5 patients who were qualified for concurrent
chemotherapy received 2 cycles of 5-FU as planned.
The incidence of the treatment-related adverse
effects is shown in Table II. A
total of 12 patients (92.3%) experienced a maximum of grade 3 or 4
lymphocytopenia. The median of decrease in the concentration of
hemoglobin was −0.7 g/dl (range, −3.9–1.1 g/dl). Other
hematological toxicities were infrequent. Only 1 patient developed
grade 3 nausea/vomiting. There were no cases of grade 4
gastrointestinal toxicities. Tumor regression was assessed in the
12 patients (92.3%) who completed the radiotherapy. Of these 12
patients, 6 (50%) exhibited local response to treatment, with 5/12
(41.7%) displaying clinical complete response and 1/12 (8.3%)
displaying partial response. Local progression and stable disease
were observed in 4 (33.3%) and 2 (16.7%) patients, respectively
(data not shown).
 | Table IITreatment-related adverse events. |
Table II
Treatment-related adverse events.
| Grades of adverse
events | Patient no. (%)
(n=13) |
|---|
| Leukopenia |
| 0 | 8 (61.5) |
| 1, 2 | 4 (30.8) |
| 3, 4 | 1 (7.7) |
| Granulocytopenia |
| 0 | 11 (84.6) |
| 1, 2 | 2 (15.4) |
| 3, 4 | 0 |
| Lymphocytopenia |
| 0 | 0 |
| 1, 2 | 1 (7.7) |
| 3, 4 | 12 (92.3) |
| Thrombocytopenia |
| 0 | 10 (76.9) |
| 1, 2 | 3 (23.1) |
| 3, 4 | 0 |
| Nausea |
| 0 | 8 (61.5) |
| 1, 2 | 4 (30.8) |
| 3, 4 | 1 (7.7) |
| Vomiting |
| 0 | 10 (76.9) |
| 1, 2 | 2 (15.4) |
| 3, 4 | 1 (7.7) |
| Diarrhoea |
| 0 | 11 (84.6) |
| 1, 2 | 2 (15.4) |
| 3, 4 | 0 |
Survival and pattern of failure
The survival analysis was based on all 13 patients
in the group studied. The median follow-up for surviving patients
was 30.1 months (range, 7.3–65.4 months). No patients were lost to
follow-up. At the time of the analysis, 7 (53.9%) of 13 patients
were alive, 4 (30.8%) without signs of disease and 3 (23.1%) with
local progression of the tumor. Among the 6 (46.1%) deceased
patients, 2 (15.4%) succumbed to tumor progression, 2 (15.4%)
succumbed to distant metastases, 1 (7.7%) succumbed to tumor
progression and distant metastases and 1 (7.7%) died during
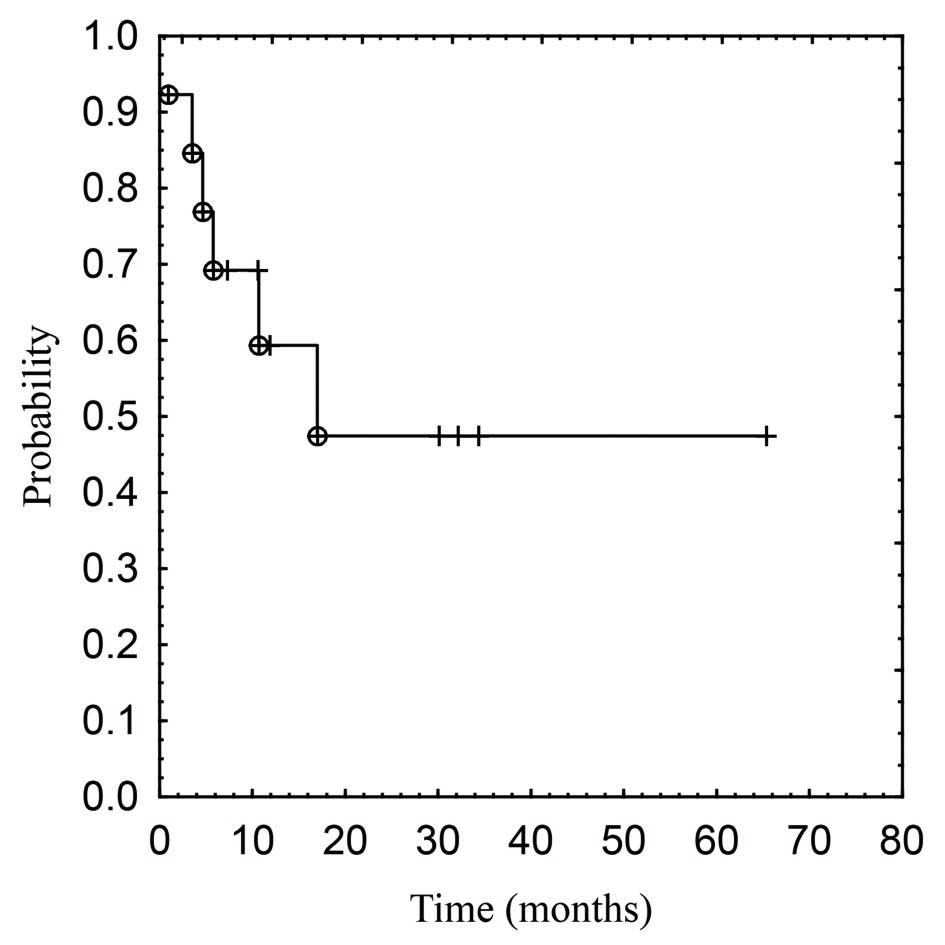
radiotherapy due to worsening of a coexisting disease. The 1- and
3-year OS rates and median survival were 59 and 48% and 17.1
months, respectively. The survival curve is depicted in Fig. 1.
Prognostic factors
Among the different clinical factors affecting the
OS rate, lower tumor stage (T1-2 vs. T3, P<0.031), lymph node
metastasis (present vs. absent, P<0.021) and complete or partial
tumor regression following therapy (yes vs. no, P<0.019) were
statistically significant (data not shown). The following clinical
characteristics were not found to be prognostic: gender, age, PS,
histology, tumor location, tumor volume, type of treatment
(radiotherapy vs. chemoradiotherapy), pretreatment hemoglobin
concentration, CEA and CA 19-9 levels.
Discussion
The definition of ‘inoperable’ gastric cancer should
be discussed. A significant proportion of the patients may not be
suitable for radical treatment due to their poor general condition,
presence of distant metastases or extensive infiltration of the
surrounding organs. Our study focused on patients who were not
surgical candidates due to comorbidities, or who did not agree to
surgery and we only evaluated the outcomes of 13 patients. We
intend to close the present trial due to the low recruitment rate,
which has been <3 patients per year. We are not able to recruit
40 patients as initially planned, the main reason being what in our
opinion is a lack of faith in the medical community as to the
likelihood of effective treatment of patients with medically
inoperable gastric cancer and the subsequent resignation of
directing such patients to cancer centers. Although a proportion of
these patients do not qualify for any treatment due to their poor
general condition, some of these patients should receive palliative
treatment, whereas others may benefit from radical radiotherapy or
chemoradiotherapy. Despite our full awareness of the limited value
of our research due to our small patient sample, we came to the
decision to publish our results due to the unexpected outcomes that
may pave the way for further multicenter studies.
In the past, due to the lack of curative treatment
modalities, palliative radiotherapy and/or chemotherapy and BSC
were widely employed and the median survival of untreated patients
was <2 months (4). BSC improves
median survival by 1–2 months, whereas radiotherapy and
chemoradiotherapy were mainly used as palliative treatment. An
analysis of the previously published studies demonstrated that the
median OS rate ranged between 3.4 and 5.3 months (2–7) and
the 1-year OS varied between 0 and 15% (3,4). The
main goal of palliative radiotherapy is the reduction of symptoms
such as bleeding, stenosis and pain, with satisfactory results in
the majority of the patients. There is currently no data in the
literature comparing the feasibility and efficacy of radical
radiotherapy or chemoradiotherapy for inoperable gastric cancer. In
our study, the median actuarial OS was 17.1 months. The survival
rate at 6, 12 and 36 months was 69, 59 and 48%, respectively. In a
retrospective analysis of 66 patients who received definitive
chemoradiotherapy, the median OS was 14.5 months (12). A third of those patients did not
undergo surgery, due to the presence of comorbidities or through
personal choice. Unfortunately, the authors did not provide an
analysis of the survival rate amongst that group of patients. The
results presented indicate that the median OS and 3-year survival
rate are similar to those reported by Saikawa et al
(13), who evaluated preoperative
chemoradiotherapy for unresectable or incurable gastric cancer.
Better results were observed in trials assessing neoadjuvant
chemoradiotherapy followed by surgery for operable gastric cancer
(8,14–18).
High-dose chemotherapy may be a suitable palliative treatment only
for patients exhibiting a good PS. Multidrug chemotherapy used in
patients with unresectable or metastatic gastric cancer appears to
be intolerably toxic for patients with medically inoperable gastric
cancer. In our group, over half of the patients were disqualified
from 5-FU alone administered at low doses as a radiosensitiser.
However, our results demonstrated that even radiotherapy alone
exerted a beneficial effect on treatment outcome.
Radiation therapy may be considered an effective
treatment for gastric cancer. In our study, a complete or partial
tumor regression following radiotherapy was observed in half of the
patients. Suzuki et al (12) reported that 35% of the patients
achieved a clinical complete response. Unfortunately, in our study,
no parameters were identified which could be used to predict the
degree of clinical tumor response. In the other study, the patients
who achieved clinical complete response exhibited a longer OS
(12). None of our patients
underwent surgery and, therefore, the percentage of pathological
response cannot be determined. Neoadjuvant treatment was found to
induce a high rate of pathological response, ranging between 6 and
36% (14–18). As expected, in our study, tumor
stage and lymph node status affected the OS.
The tolerance to treatment was satisfactory, with a
toxic death rate of 7.7% (1/13). Despite intensive hospital
treatment, the PS of the patient deteriorated and he succumbed to
the disease 2 weeks after the discontinuation of radiotherapy
(after 27 Gy). We cannot unambiguously determine the cause of
death. The patient was the oldest among the group and had a
pretreatment PS of 2. The severity of the side effects was the
highest among all the patients: grade 3 nausea/vomiting, grade 2
diarrhoea and grade 3 lymphocytopenia. This case highlights the
need for careful qualification of patients aged >80 years with a
PS of 2. In previous palliative studies there was no reported
treatment-related mortality (2,3).
However, Saikawa et al (12) reported 1 case (3%) of lethal events
following radical chemoradiotherapy. Myelosuppresion was the most
commonly observed toxicity. Almost all the patients developed grade
3 or 4 lymphocytopenia. Co-trimoxazole was used as a prophylaxis
against Pneumocystis carinii pneumonia when lymphocytopenia
was <500 cells/μl. There were no observed grade 3 or 4
granulocytopenia or thrombocytopenia. In palliative studies, the
incidence of grade 3 or 4 hematological toxicities was low
(2,3). In our study, other observed treatment
toxicities included mild nausea/vomiting and diarrhoea. Other
authors reported grade 3 gastrointestinal toxicity ranging between
3 and 14% (2,3). The toxicity in our study was
comparable to that reported regarding neoadjuvant chemoradiotherapy
(8,13). Modern individual treatment planning
based on CT scans and multi-field conformal techniques allow the
reduction of the risk of localization errors and enable sparing of
normal tissues (kidneys, spinal cord, liver and bowel), while
maintaining a relatively high local control rate. The present study
demonstrated that the prescribed schedule of radiotherapy and
chemoradiotherapy was relatively well tolerated and the
complication rate was considered acceptable; however, it is our
opinion it may be better administered under hospitalization in
elderly patients. In conclusion, inoperable gastric cancer is
currently considered to be an incurable disease. Our results
demonstrated that such an approach may be inaccurate and requires
revision. Radical radiotherapy, alone or in combination with
chemotherapy, is a safe and well-tolerated treatment modality for
patients with primarily inoperable gastric cancer and may prolong
survival.
Acknowledgements
This study was supported by The National Science
Centre of Poland (grant no. NN 403 238 140).
References
|
1
|
Kelsen DP, Van de Velde CJH and Minsky BD:
Gastric cancer: clinical management. Principles and Practice of
Gastrointestinal Oncology. Kelsen DP, Daly JM, Kern SE, Levin B,
Tepper JE and Van Cutsem E: 2nd edition. Wolters Kluwer Health,
Lippincott Williams & Wilkins; Philadelphia: pp. 285–316.
2008
|
|
2
|
Kim MM, Rana V, Janjan NA, et al: Clinical
benefit of palliative radiation therapy in advanced gastric cancer.
Acta Oncol. 47:421–427. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tey J, Back MF, Shakespeare TP, et al: The
role of palliative radiation therapy in symptomatic locally
advanced gastric cancer. Int J Radiat Oncol Biol Phys. 67:385–388.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chaw CL, Niblock PG, Chaw CS, et al: The
role of palliative radiotherapy for haemostasis in unresectable
gastric cancer: a single-institution experience.
Ecancermedicalscience. 8:3842014.PubMed/NCBI
|
|
5
|
Hashimoto K, Mayahara H, Takashima A, et
al: Palliative radiation therapy for hemorrhage of unresectable
gastric cancer: a single institute experience. J Cancer Res Clin
Oncol. 135:1117–1123. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee JA, Lim do H, Park W, et al: Radiation
therapy for gastric cancer bleeding. Tumori. 95:726–730.
2009.PubMed/NCBI
|
|
7
|
Asakura H, Hashimoto T, Harada H, et al:
Palliative radiotherapy for bleeding from advanced gastric cancer:
is a schedule of 30 Gy in 10 fractions adequate? J Cancer Res Clin
Oncol. 137:125–130. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wydmanski J, Suwinski R, Poltorak S, et
al: The tolerance and efficacy of preoperative chemoradiotherapy
followed by gastrectomy in operable gastric cancer, a phase II
study. Radiother Oncol. 82:132–136. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fowler JF: The linear-quadratic formula
and progress in fractionated radiotherapy. Br J Radiol. 62:679–694.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wydmanski J and Mohanti BK: An appraisal
of radiation therapy techniques for adjuvant and neoadjuvant
therapy in gastric cancer. J Radiother Pract. 7:67–75. 2008.
View Article : Google Scholar
|
|
11
|
National Cancer Institute. Common
terminology criteria for adverse events v3.0 (CTCAE). http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf#search=“ctcae version 3.0”"ref-label" rowspan="1" colspan="1">
12
|
Suzuki A, Xiao L, Taketa T, et al:
Localized gastric cancer treated with chemoradation without
surgery: UTMD Anderson Cancer Center experience. Oncology.
82:347–351. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saikawa Y, Kubota T, Kumagai K, et al:
Phase II study of chemoradiotherapy with S-1 and low-dose cisplatin
for inoperable advanced gastric cancer. Int J Radiat Oncol Biol
Phys. 71:173–179. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ajani JA, Mansfield PF, Janjan N, et al:
Multi-institutional trial of preoperative chemoradiotherapy in
patients with potentially resectable gastric carcinoma. J Clin
Oncol. 22:2774–2780. 2004. View Article : Google Scholar
|
|
15
|
Ajani JA, Mansfield PF, Crane CH, et al:
Paclitaxel-based chemoradiotherapy in localized gastric carcinoma:
degree of pathologic response and not clinical parameters dictated
patient outcome. J Clin Oncol. 23:1237–1244. 2005. View Article : Google Scholar
|
|
16
|
Allal AS, Zwahlen D, Brundler MA, et al:
Neoadjuvant radiochemotherapy for locally advanced gastric cancer:
long-term results of a phase I trial. Int J Radiat Oncol Biol Phys.
63:1286–1289. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Balandraud P, Moutardier V, Giovannini M,
et al: Locally advanced adenocarcinomas of the gastric cardia:
results of pre-operative chemoradiotherapy. Gastroenterol Clin
Biol. 28:651–657. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lowy AM, Feig BW, Janjan N, et al: A pilot
study of preoperative chemoradiotherapy for resectable gastric
cancer. Ann Surg Oncol. 8:519–524. 2001. View Article : Google Scholar : PubMed/NCBI
|