Introduction
The association between pregnancy and thyroid
carcinoma has been debated upon for over half a century; however,
no definitive conclusion has been reached. Differentiated thyroid
cancer ranks second in incidence among tumors diagnosed during
pregnancy, affecting 14/100,000 individuals (1). Over the last few decades, a number of
scholars indicated that reproductive factors played a significant
role in thyroid cancer. Certain investigators considered that the
risk of thyroid carcinoma increased with an increasing number of
births (2). Moreover, thyroid
carcinoma during pregnancy was found to be more aggressive and its
prognosis was worse following radical resection, whereas thyroid
carcinoma during pregnancy was a predictor of recurrence, which was
considered to be associated with hormone receptors and hormone
level fluctuations (3). However,
other scholars reported that the development and progression of
thyroid carcinoma were not significantly associated with pregnency.
Negri et al (4) conducted a
meta-analysis in 1999 investigating the correlation between
reproductive factors and thyroid carcinoma. That study indicated a
weak correlation, whereas the reproductive factors in young women
were significantly associated with thyroid carcinoma. Decades
later, following the publication of numerous large-scale clinical
trials, this remains a controversial subject. Therefore, we
performed this meta-analysis to investigate the association between
reproductive factors and thyroid carcinoma.
Materials and methods
Study selection
We followed the guidelines of Meta-analysis of
Observational Studies in Epidemiology (5) to conduct a meta-analysis in order to
determine the association between thyroid carcinoma and pregnancy.
The search terms used were pregnancy, reproduction and thyroid
neoplasms. Three main databases, namely PubMed, OVID and the
Cochrane Library, were searched from their inception to April 1st,
2013. The search strategies were medical subject headings combined
with key words. To ensure a comprehensive search, we also searched
the reference lists of the included studies and previously
published reviews. The authors were contacted when data were
ambiguous or missing. Subsequently, the abstracts were screened and
the full-text articles were accessed. No language restriction was
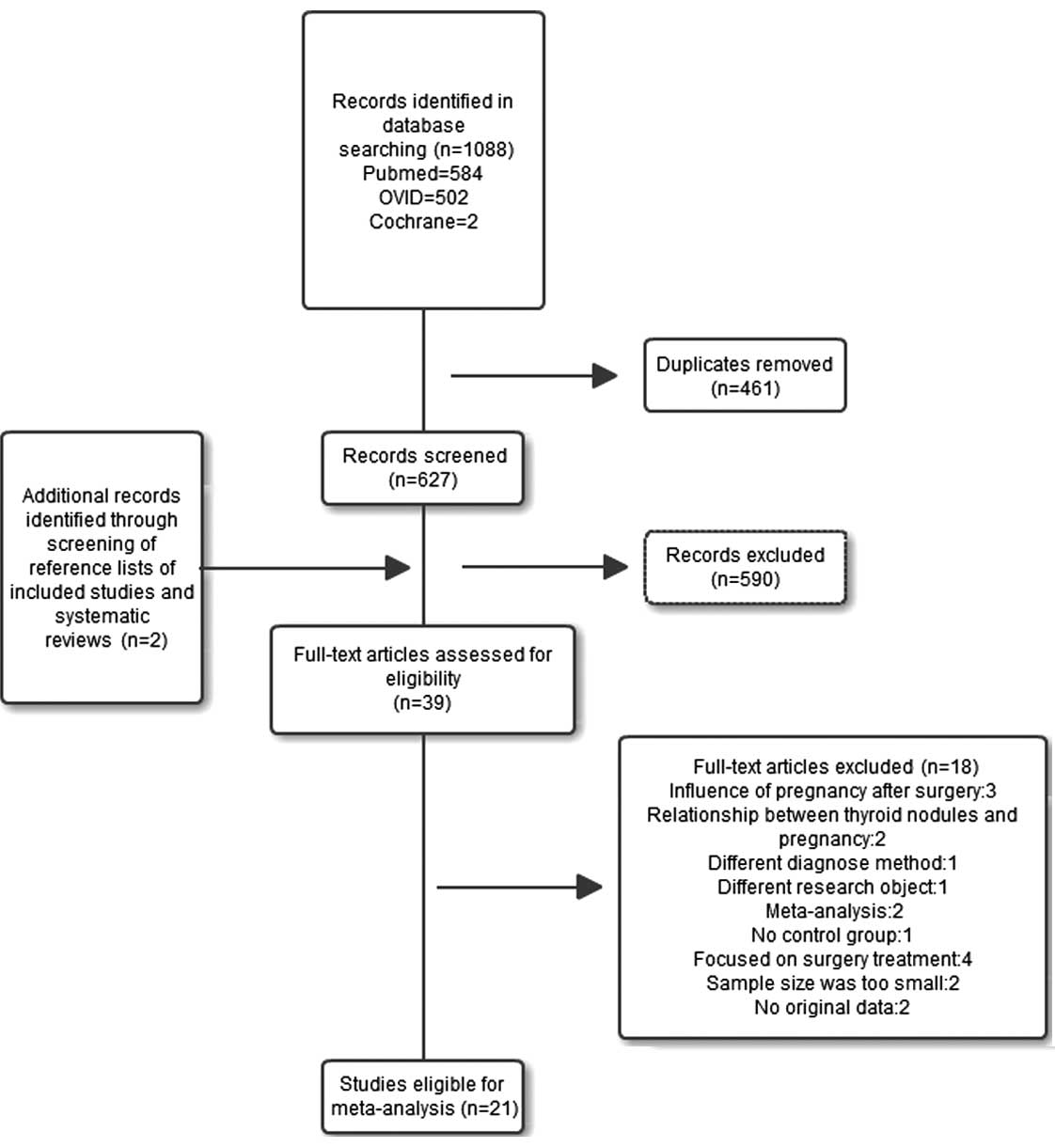
applied. The study selection process is summarized in Fig. 1.
Inclusion criteria
The original studies were prospective or
retrospective random case-control trials; except for the
investigated factors, there was no other difference between the
experimental and control groups. All the diagnoses were
pathologically confirmed, pregnancy was diagnosed by ultrasound and
the follow-up period in all the cases was > 6 months.
Exclusion criteria
We excluded reviews, editorials, letters, case
series, case reports and conference proceedings; studies other that
random case-control trials; studies with different diagnostic
standards and inappropriate outcome measurements; and studies that
provided no original data or only unadjusted analysis. Studies with
limited follow-up period were also eliminated.
Data extraction and quality
assessment
Two investigators extracted data from the eligible
studies. The data included first author, year of publication, study
design (case-control or prospective cohort), compared populations,
inclusion and exclusion criteria, total sample size, number of
patients in the thyroid carcinoma and control groups and number of
pregnant individuals in each group. We evaluated and filtered all
the eligible clinical studies, grading each study according to the
Newcastle-Ottawa scale (NOS) (6),
with 0–4 points reflecting low quality and 5–9 high quality. Any
disagreements or discrepancies were resolved by consensus.
Statistical analysis
We classified the selected studies, extracted data
and performed a meta-analysis using RevMan 5.1 software (http://tech.cochrane.org/revman/download), which was
provided by the Cochrane Library.
Results
Search results and basic
characteristics of eligible studies
Of the 627 retrieved articles, 39 abstracts were
selected for full-text screening, including 1 Chinese study written
in English. The inclusion criteria were met by 21 of the 39 studies
(Fig. 1). According to the
exclusion criteria, 18 studies were eliminated (3 studies reported
the effect of pregnancy after surgery, 2 focused on the association
between thyroid nodules and pregnancy, 1 used fine needle
aspiration as the diagnostic method, 1 had a different research
object, 2 were meta-analyses, 1 had no control group, 4 focused on
surgical treatment, 2 had insufficient samples and 2 did not
provide original data). The 21 selected studies included 406,329
cases in total (Table I).
 | Table I.Basic characteristics of eligible
studies. |
Table I.
Basic characteristics of eligible
studies.
| Authors | Year of
publication | Study design | Population | Case s | Control s | NOS | (Refs.) |
|---|
| Akslen et
al | 1992 | Prospective
cohort | Norway | 124 | 62,966 | 8 | (7) |
| Brindel et
al | 2008 | Case-control | France | 201 | 324 | 7 | (2) |
| Galanti et
al | 1996 | Case-control | Norway-Sweden | 191 | 341 | 7 | (8) |
| Hallquist et
al | 1994 | Case-control | Sweden | 180 | 360 | 6 | (9) |
| Horn-Ross et
al | 2011 | Case-control | USA | 233 | 117,413 | 7 | (10) |
| Kolonel et
al | 1990 | Case-control | USA | 140 | 328 | 7 | (11) |
| Levi et
al | 1993 | Case-control | Switzerland | 91 | 306 | 6 | (12) |
| Mack et
al | 1999 | Case-control | USA | 292 | 292 | 5 | (13) |
| McTiernan et
al | 1984 | Case-control | USA | 185 | 359 | 6 | (14) |
| Memon et
al | 2002 | Case-control | Kuwait | 238 | 238 | 8 | (15) |
| Moosa and
Mazzaferri | 1997 | Case-control | USA | 61 | 528 | 6 | (16) |
| Preston-Martin et
al | 1987 | Case-control | USA | 292 | 292 | 5 | (17) |
| Preston-Martin et
al | 1993 | Case-control | China | 207 | 207 | 5 | (18) |
| Rossing et
al | 2000 | Case-control | USA | 410 | 574 | 6 | (19) |
| Schonfeld et
al | 2011 | Prospective
cohort | USA | 312 | 187,553 | 8 | (20) |
| Takezaki et
al | 1996 | Case-control | Japan | 94 | 26,666 | 5 | (21) |
| Truong et
al | 2005 | Case-control | France | 293 | 354 | 9 | (22) |
| Vannucchi et
al | 2010 | Case-control | England | 15 | 61 | 7 | (3) |
| Wingren et
al | 1993 | Case-control | Sweden | 132 | 203 | 7 | (23) |
| Yasmeen et
al | 2005 | Case-control | USA | 595 | 2,270 | 7 | (24) |
| Zivaljevic et
al | 2003 | Case-control | Serbia | 204 | 204 | 6 | (25) |
Association between thyroid carcinoma
and reproductive factors Association between thyroid carcinoma and
pregnancy history
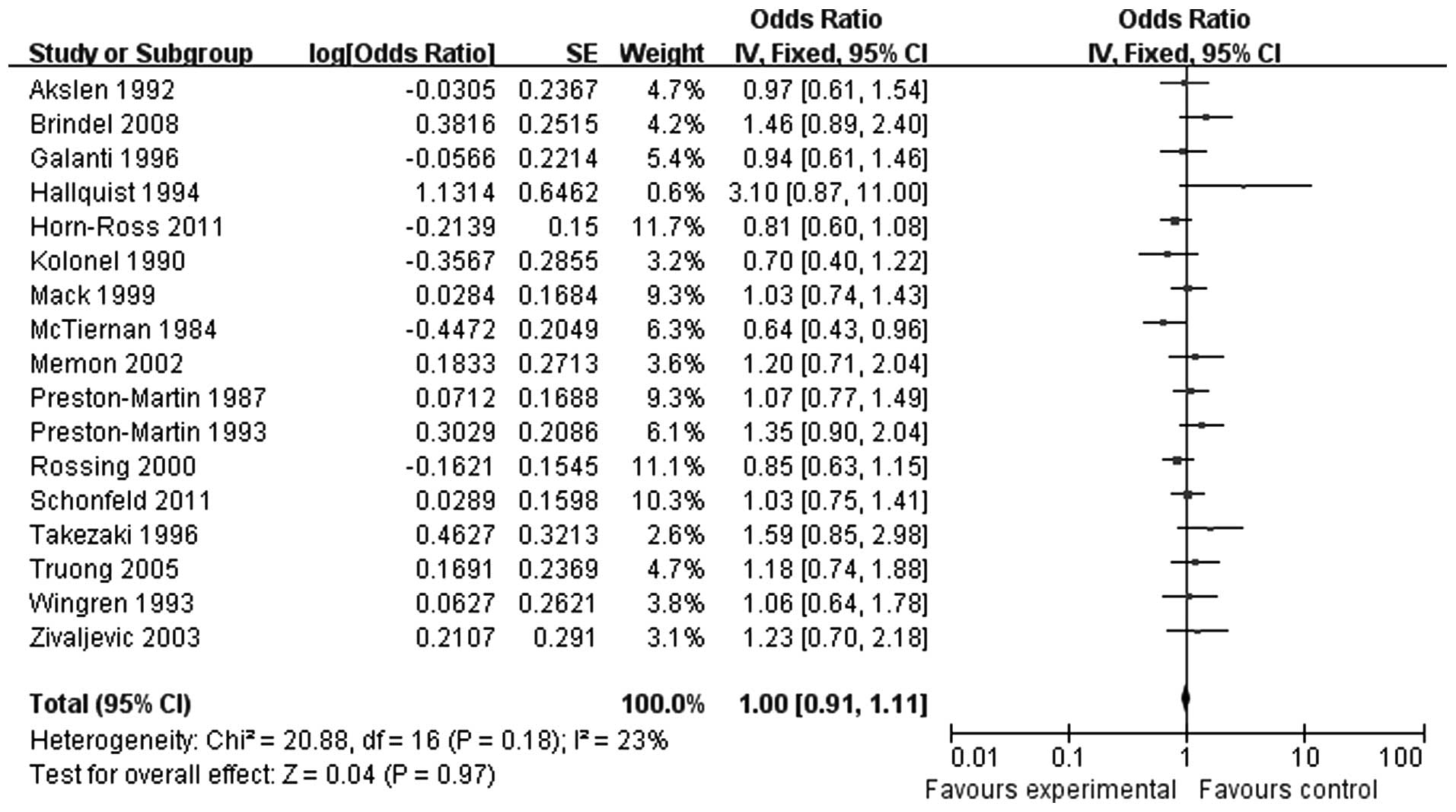
A total of 17 studies, including 402,402 cases,
investigated the association between pregnancy and thyroid cancer.
Of those 17 studies, 2 were prospective studies of high quality. A
proportion of the studies did not include detailed information,
such as the number of pregnancies or the age at pregnancy. As there
was no heterogeneity (P=0.18), the fixed-effects model was
selected. A combined analysis of the 17 studies [odds ratio
(OR)=1.00, 95% confidence interval (CI): 0.91-1.11] revealed that
pregnancy history is not significantly associated with the risk of
thyroid carcinoma (Fig. 2).
Association between thyroid carcinoma
and ≥ 3 pregnancies
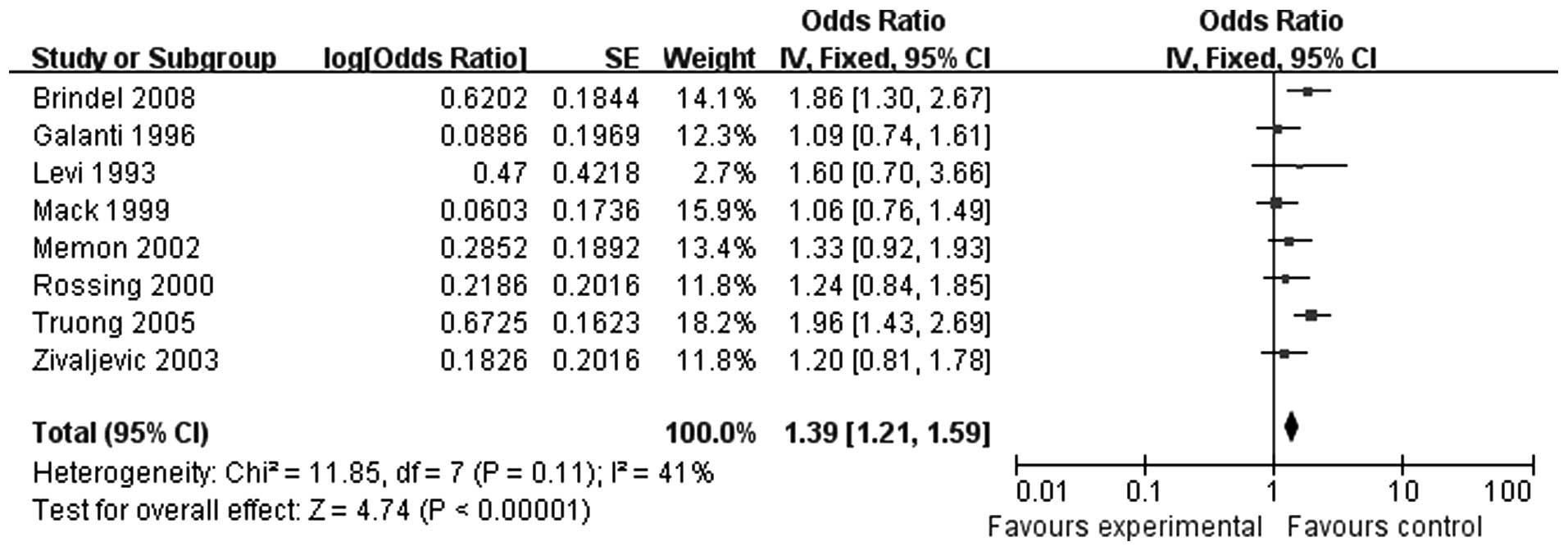
Following a precision screening, 8 studies were
included. Of those 8 studies, 2 indicated that ≥ 3 pregnancies was
a risk factor for thyroid cancer, while the remaining 6 studies
reported opposing results. There were 4,553 cases in total, without
obvious heterogeneity (P=0.11); therefore, the fixed-effects model
was selected. In a combined analysis of all 8 studies (OR=1.39, 95%
CI: 1.21-1.59), the results demonstrated that women with ≥ 3
pregnancies exhibited an increased risk for thyroid carcinoma
(Fig. 3).
Association between thyroid carcinoma
and an interval of ≤ 5 years since the last pregnancy
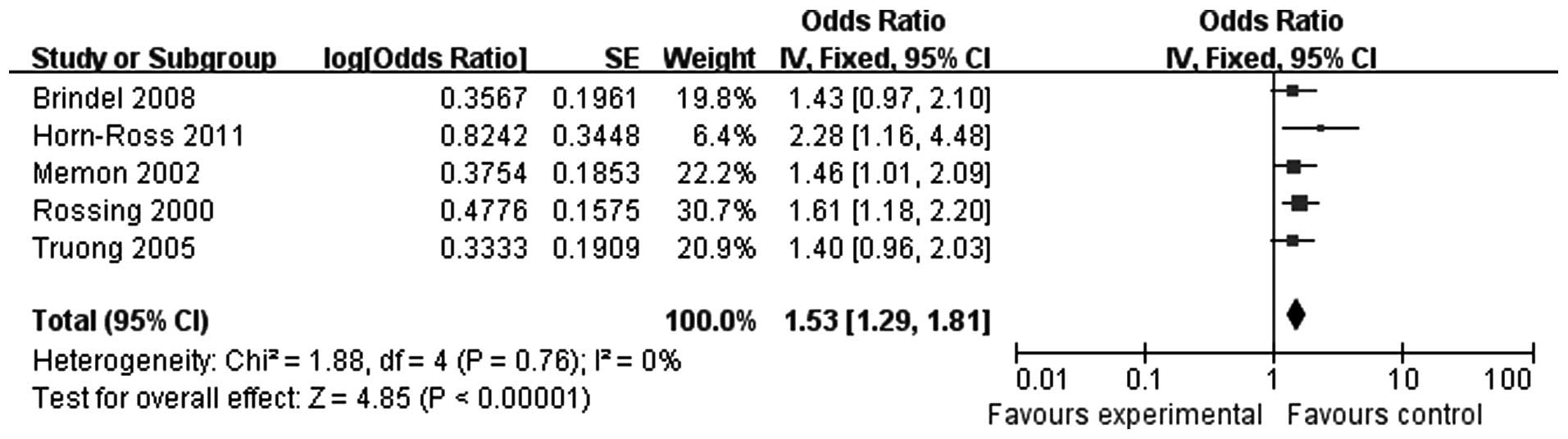
Five studies were included, with a total of 120,278
cases. There was no heterogeneity (P=0.76), so the fixed-effects
model was selected. In a combined analysis of all 5 studies
(OR=1.53, 95% CI: 1.29-1.81), we observed that, compared to the
control group, more women in the thyroid carcinoma group had become
pregnant within 5 years since their last pregnancy (Fig. 4). Therefore, an interval of ≤5
years since the last pregnancy is associated with a higher risk for
thyroid carcinoma.
Effect of pregnancy on lymph node and
distant metastasis Thyroid carcinoma during pregnancy and distant
metastasis
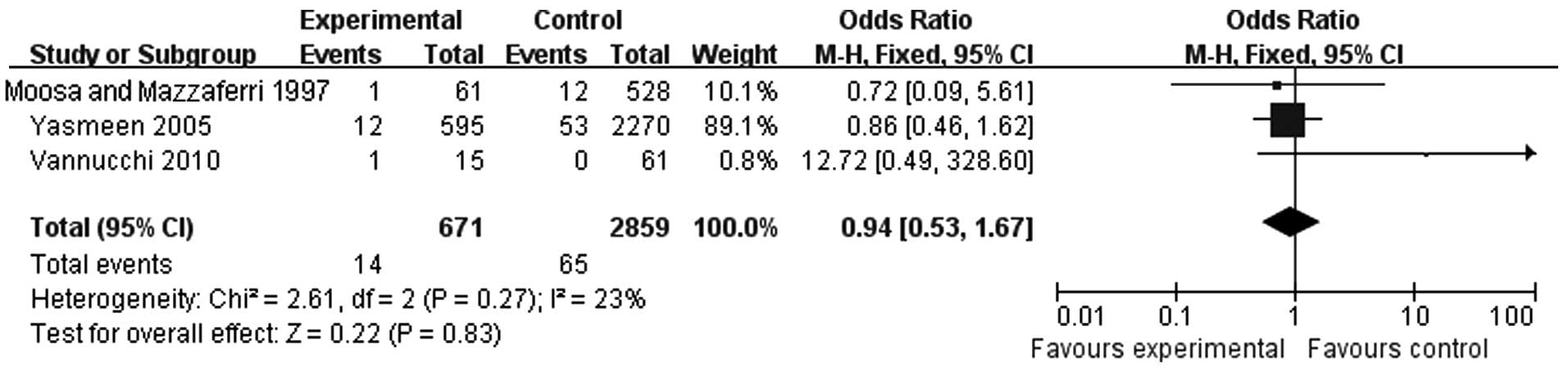
A total of 3 studies were included in the analysis
of the association between thyroid carcinoma during pregnancy and
distant metastasis. There were 671 cases in the experimental and
2,859 cases in the control group. There was no heterogeneity
(P=0.27) and the fixed-effects model was used (OR=0.94, 95% CI:
0.53-1.67) (Fig. 5). The results
indicated that pregnancy did not increase the distant metastasis
rate.
Thyroid carcinoma during pregnancy and
lymphatic metastasis
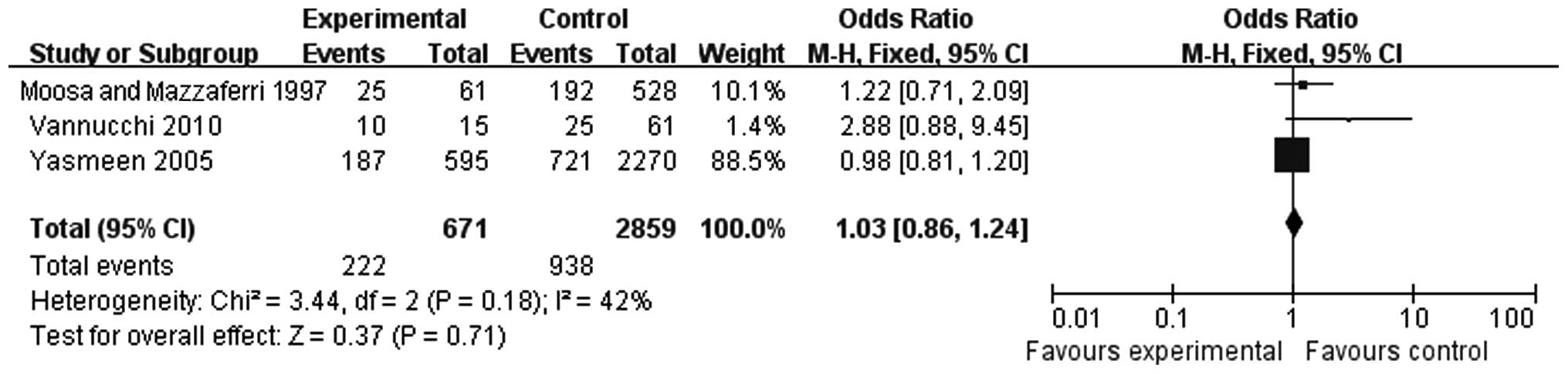
A total of 3 studies were included in the analysis
of the association between pregnancy and lymphatic metastasis of
thyroid carcinoma (Fig. 6). There
were 671 cases in the experimental and 2,859 cases in the control
group. There was no significant heterogeneity (P=0.18) and the
fixed-effects model was used (OR=1.03, 95% CI: 0.86-1.24). The
results indicated that pregnancy did not increase the rate of
lymphatic metastasis.
Publication bias and sensitivity
analysis
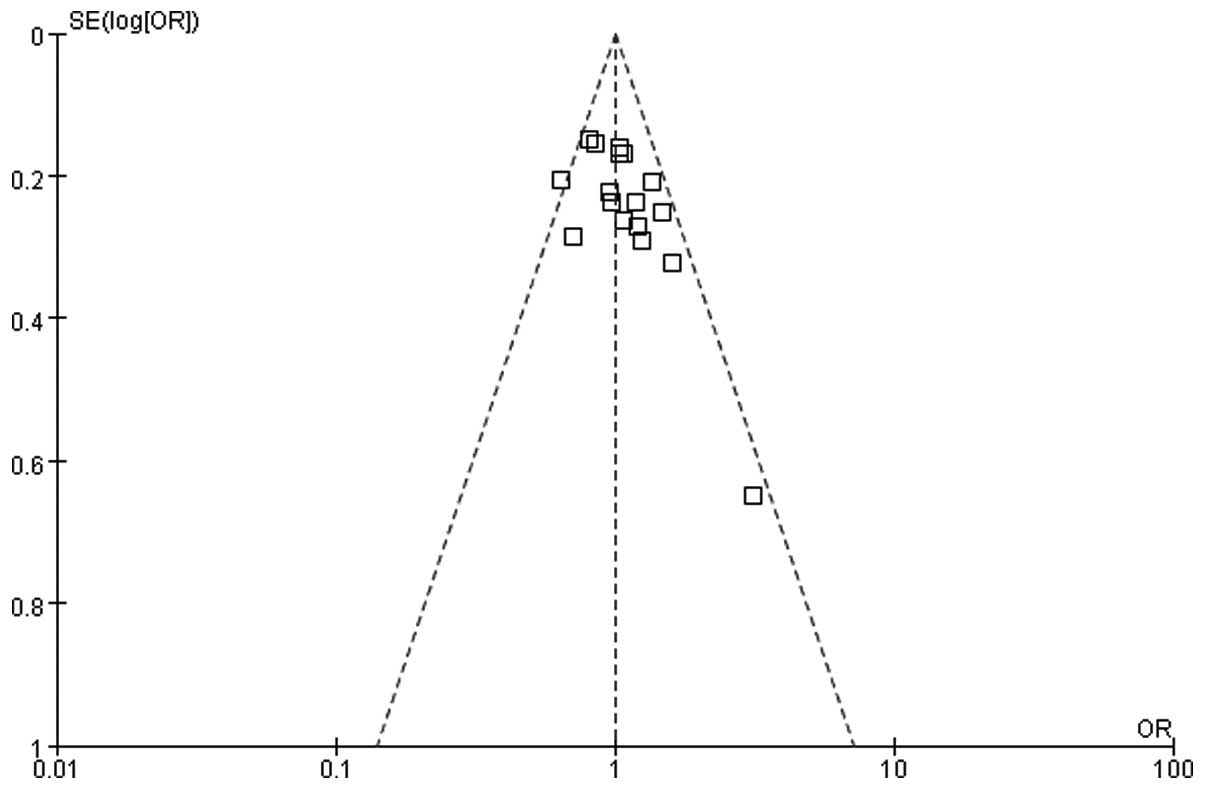
We used a funnel graph to represent publication bias
and 17 studies that investigated the association between thyroid
carcinoma and pregnancy history did not exhibit publication bias
(Fig. 7). There was no significant
heterogeneity in the analysis of thyroid carcinoma during pregnancy
and lymphatic metastasis, but the heterogeneity was significant, so
the sensitivity was analyzed. The effect of each study on the
combined estimate was assessed by removing one study at a time,
which did not affect the significance of the combined estimate or
heterogeneity. It appeared that the heterogeneity originated from
the study by Vannucchi et al (3), which included a relatively limited
number of cases.
Discussion
Our study suggested a significant association
between pregnancy and thyroid carcinoma. The majority of the
subjects in the included studies were diagnosed with differentiated
thyroid carcinoma. Several investigators have focused on the
mechanism underlying this correlation and a likely explanation is
hormone level fluctuation. The trophoblast cells secrete human
chorionic gonadotropin (hCG) and progesterone during pregnancy, as
well as estrogen during late pregnancy, to regulate the delivery
process. As hCG is considered to have a similar structure with
thyroid-stimulating hormone (TSH), it may combine with the
thyroid-stimulating hormone receptor (TSHR). When high
concentrations of hCG are secreted into the bloodstream, it may
combine with TSHR more than TSH and promote thyroid cell
proliferation (26). Estrogen has
been proven to promote tumor growth significantly and this effect
is achieved by combining with the estrogen receptor (ER). Kumar
et al (27) reported that
estrogen acts like an ER agonist, which may significantly promote
the growth of differentiated thyroid carcinoma. Moreover, estrogen
may inhibit apoptosis of thyroid carcinoma cells by increasing the
expression of a series of proteins, such as Bcl-2. The role of
progesterone in thyroid carcinoma remains controversial. In our
meta-analysis, we observed a significant association between a
history of multiple pregnancies and thyroid carcinoma. In addition,
an interval of ≤5 years since the last pregnancy significantly
increased the risk of thyroid carcinoma. These results indicate
that hormone level fluctuation increases the risk of thyroid
carcinoma.
The data in our study were independently extracted
by two investigators. There was no obvious publication bias and no
language restriction. The included studies involved a total of
406,329 cases and were of clinical significance. The regions and
ethnicity of the studies reflected international diversity,
including countries such as China, USA, Britain and Norway.
Although our meta-analysis was not the first to investigate the
association between pregnancy and thyroid carcinoma, it was an
important supplementary study to earlier research. We evaluated all
the included studies by the NOS and then identified the studies
with significant bias.
There were certain limitations to our study and
heterogeneity is a major issue that must be taken into
consideration. There was no significant heterogeneity in each
analysis, while the analysis of the association between thyroid
carcinoma during pregnancy and lymphatic metastasis revealed
significant heterogeneity. All the included studies were of high
quality. The funnel plot revealed no publication bias, but the
evaluating ability of the funnel plot declines when there are fewer
studies included. Sensitivity was then analyzed. The heterogeneity
appeared to originate from the study of Vannucchi et al
(3), which included a relatively
limited number of cases. In addition, other factors may affect the
incidence or invasive potential of thyroid cancer, such as age,
tumor size and the degree of differentiation, but we were unable to
assess these factors due to the lack of detailed data.
Although this study has several limitations, as
mentioned above, it demonstrated a close association between
pregnancy and thyroid carcinoma in certain aspects. In this study,
the risk for thyroid carcinoma was not found to be increased during
pregnancy, but the limited number of studies and included cases
were not sufficient to reach definitive conclusions. Further large
prospective clinical trials are required, focusing on thyroid
carcinoma during pregnancy, to supplement and update our data and
also perform a subgroup analysis according to age, history and
pathological types, to draw a more definitive conclusion.
In summary, pregnancy was identified as a risk
factor of thyroid carcinoma and multiparous women should be closely
followed up to detect the disease early. Thyroid carcinoma during
pregnancy does not increase the probability of lymphatic or distant
metastasis. Treatment should be individualized and the significance
of follow-up must be emphasized.
Acknowledgements
This study was supported by the Shanghai Municipal
Health Bureau (grant no. 2012029).
References
|
1
|
Smith LH, Danielsen B, Allen ME and Cress
R: Cancer associated with obstetric delivery: results of linkage
with the California cancer registry. Am J Obstet Gynecol.
189:1128–1135. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brindel P, Doyon F, Rachedi F, et al:
Menstrual and reproductive factors in the risk of differentiated
thyroid carcinoma in native women in French Polynesia: a
population-based case-control study. Am J Epidemiol. 167:219–229.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vannucchi G, Perrino M, Rossi S, et al:
Clinical and molecular features of differentiated thyroid cancer
diagnosed during pregnancy. Eur J Endocrinol. 162:145–151. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Negri E, Dal Maso L, Ron E, et al: A
pooled analysis of case-control studies of thyroid cancer. II.
Menstrual and reproductive factors. Cancer Causes Control.
10:143–155. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stroup DF, Berlin JA, Morton SC, et al:
Meta-analysis of observational studies in epidemiology: a proposal
for reporting. Meta-analysis Of Observational Studies in
Epidemiology (MOOSE) group. JAMA. 283:2008–2012. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ownby RL, Crocco E, Acevedo A, John V and
Loewenstein D: Depression and risk for Alzheimer disease:
systematic review, meta-analysis, and metaregression analysis. Arch
Gen Psychiatry. 63:530–538. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Akslen LA, Nilssen S and Kvale G:
Reproductive factors and risk of thyroid cancer. A prospective
study of 63,090 women from Norway. Br J Cancer. 65:772–774. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Galanti MR, Hansson L, Lund E, et al:
Reproductive history and cigarette smoking as risk factors for
thyroid cancer in women: a population-based case-control study.
Cancer Epidemiol Biomarkers Prev. 5:425–431. 1996.PubMed/NCBI
|
|
9
|
Hallquist A, Hardell L, Degerman A and
Boquist L: Thyroid cancer: reproductive factors, previous diseases,
drug intake, family history and diet. A case-control study. Eur J
Cancer Prev. 3:481–488. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Horn-Ross PL, Canchola AJ, Ma H, Reynolds
P and Bernstein L: Hormonal factors and the risk of papillary
thyroid cancer in the California Teachers Study cohort. Cancer
Epidemiol Biomarkers Prev. 20:1751–1759. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kolonel LN, Hankin JH, Wilkens LR,
Fukunaga FH and Hinds MW: An epidemiologic study of thyroid cancer
in Hawaii. Cancer Causes Control. 1:223–234. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Levi F, Franceschi S, Gulie C, Negri E and
La Vecchia C: Female thyroid cancer: the role of reproductive and
hormonal factors in Switzerland. Oncology. 50:309–315. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mack WJ, Preston-Martin S, Bernstein L,
Qian D and Xiang M: Reproductive and hormonal risk factors for
thyroid cancer in Los Angeles County females. Cancer Epidemiol
Biomarkers Prev. 8:991–997. 1999.PubMed/NCBI
|
|
14
|
McTiernan AM, Weiss NS and Daling JR:
Incidence of thyroid cancer in women in relation to reproductive
and hormonal factors. Am J Epidemiol. 120:423–435. 1984.PubMed/NCBI
|
|
15
|
Memon A, Darif M, Al-Saleh K and Suresh A:
Epidemiology of reproductive and hormonal factors in thyroid cancer
evidence from a case-control study in the Middle East. Int J
Cancer. 97:82–89. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Moosa M and Mazzaferri EL: Outcome of
differentiated thyroid cancer diagnosed in pregnant women. J Clin
Endocrinol Metab. 82:2862–2866. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Preston-Martin S, Bernstein L, Pike MC,
Maldonado AA and Henderson BE: Thyroid cancer among young women
related to prior thyroid disease and pregnancy history. Br J
Cancer. 55:191–195. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Preston-Martin S, Jin F, Duda MJ and Mack
WJ: A case-control study of thyroid cancer in women under age 55 in
Shanghai (People's Republic of China). Cancer Causes Control.
4:431–440. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rossing MA, Voigt LF, Wicklund KG and
Daling JR: Reproductive factors and risk of papillary thyroid
cancer in women. Am J Epidemiol. 151:765–772. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schonfeld SJ, Ron E, Kitahara CM, et al:
Hormonal and reproductive factors and risk of postmenopausal
thyroid cancer in the NIH-AARP Diet and Health Study. Cancer
Epidemiol. 35:e85–e90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Takezaki T, Hirose K, Inoue M, et al: Risk
factors of thyroid cancer among women in Tokai, Japan. J Epidemiol.
6:140–147. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Truong T, Orsi L, Dubourdieu D, Rougier Y,
Hemon D and Guenel P: Role of goiter and of menstrual and
reproductive factors in thyroid cancer: a population-based
case-control study in New Caledonia (South Pacific), a very high
incidence area. Am J Epidemiol. 161:1056–1065. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wingren G, Hatschek T and Axelson O:
Determinants of papillary cancer of the thyroid. Am J Epidemiol.
138:482–491. 1993.PubMed/NCBI
|
|
24
|
Yasmeen S, Cress R, Romano PS, et al:
Thyroid cancer in pregnancy. Int J Gynaecol Obstet. 91:15–20. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zivaljevic V, Vlajinac H, Jankovic R, et
al: Case-control study of female thyroid cancer - menstrual,
reproductive and hormonal factors. Eur J Cancer Prev. 12:63–66.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yoshimura M, Nishikawa M, Yoshikawa N, et
al: Mechanism of thyroid stimulation by human chorionic
gonadotropin in sera of normal pregnant women. Acta Endocrinol
(Copenh). 124:173–178. 1991.PubMed/NCBI
|
|
27
|
Kumar A, Klinge CM and Goldstein RE:
Estradiol-induced proliferation of papillary and follicular thyroid
cancer cells is mediated by estrogen receptors α and β. Int J
Oncol. 36:1067–1080. 2010.PubMed/NCBI
|