Introduction
Over the last few years, minimally invasive
laparoscopic surgery has become widespread. The indications for
such surgery have been extended to various conditions, including
additional bowel resection for stage I rectal cancer, radical
resection of stage II or III rectal cancer and palliative surgery
in patients with stage IV rectal cancer (1–6). With
conventional laparotomy (CL) for rectal cancer, it is difficult to
visualize areas such as the pelvic floor, the ventral part of the
bladder and the posterior to apical regions of the prostate and
almost blind manipulation is required. By contrast, endoscopic
magnification and viewing a monitor allows procedures to be
performed more safely, although the difficulty of rectal cancer
surgery increases with the depth of the lesion in the pelvis,
particularly lower rectal cancer located at the pelvic floor
(4). In Japan, pure
laparoscopy-assisted colorectal surgery (LACS) is frequently
performed using 5–6 ports, including a camera port and a small
incision of 35–45 mm for anastomosis. However, 4 forceps are
required for several procedures in pure LACS; therefore, at least 2
surgeons skilled in LACS must participate in the operation, while
the long operating time puts significant pressure on the
anesthesiologists and the availability of operating theaters. In
addition, LACS requires additional education and technical
guidance, as well as costly equipment and surgical materials; thus,
performing pure LACS at medium-sized hospitals with 400–500 beds is
associated with various problems (4–8). The
majority of the studies comparing pure LACS with CL demonstrated
that the former method is associated with a lower incidence of
wound infections and a shorter hospital stay, while achieving a
comparable or superior survival, with a more acceptable cosmetic
outcome (7–10). However, hand-assisted laparoscopic
surgery (HALS) and hybrid HALS (HH), which are based on
manipulation under direct vision, are more popular compared to pure
LACS in Europe and the United States. HH has the following
advantages: i) It allows safe palpation and grasping with the left
hand according to the techniques learned for laparotomy, which
allows smooth handling of large, heavy tumors and also allows the
surgeon to rapidly and easily pull the resected intestines away
from the pelvic floor under direct vision, as in laparotomy; ii)
Since HH is based on laparotomy, the operating time is relatively
short; and iii) it does not require a long time to learn the skills
necessary to perform HH (8,9,11–17). In Japan, CL accounts for ~50% of
rectal cancer surgery and pure LACS accounts for ~30–40%, with HALS
and small incision surgery constituting the remaining 10–20%
(18). The majority of the studies
comparing HALS and pure LACS have demonstrated that the operating
time is shorter with HALS and the rate of conversion to open
laparotomy is low; HALS may thus be considered as a surgical
technique between CL and pure LACS (8,9,19–23). In
Japan, HALS was widely used as an adjunctive method for a brief
period until pure LACS was introduced in 2000; however, the use of
HALS has noticeably decreased since the standardization of pure
LACS. Therefore, although certain single-center studies on HALS
have been performed overseas, no such report has been published in
Japan (9,24,25).
Accordingly, the objective of this study was to compare the
clinical outcome of HALS and CL in patients with rectal cancer
treated at a single center in Japan.
Patients and methods
Patients
A total of 850 patients underwent curative resection
of primary colorectal cancer at Tokai University Hachioji Hospital
(Tokyo, Japan) between April, 2002 and December, 2012. HALS was
employed to treat colorectal cancer from July, 2007 onwards and was
used in at least 350 patients. A total of 54 patients who underwent
conventional CL prior to the introduction of HALS were selected
from the 850 patients as stage-matched historical controls, whereas
the HALS group comprised 57 patients. The mean/median age was
65.4/65.0 years (range, 55–81 years) in the HALS group and
67.0/68.5 years (range, 35–92 years) in the CL group (P=0.095)
(Table IA). Of the 57 patients in the
hals group, 43 (75.4%) were male and 14 (24.6%) were female; of the
54 CL patients, 35 (64.8%) were male and 19 (35.2%) were female,
with no significant differences between the two groups (P=0.221)
(Table IB). As regards tumor
location, the tumor was located at the rectosigmoid region in 20
patients (35.1%) from the HALS group and 20 (37.0%) from the CL
group (P=0.831); in the upper rectum in 21 patients (36.8%) from
the HALS group and 14 (25.9%) from the CL group (P=0.216); and in
the lower rectum in 16 patients (28.1%) from the HALS group and 20
(37.0%) from the CL group (P=0.313). There were no significant
differences between the two groups (Table
IC). A total of 11 patients (19.3%) in the HALS group and 12
(22.2%) in the CL group underwent anterior resection (P=0.704); 39
patients (68.4%) in the HALS group and 33 (61.1%) in the CL group
underwent low anterior resection (P=0.420); and 7 patients (12.3%)
in the HALS group and 9 (16.7%) in the CL group underwent Miles'
operation (P=0.511). There were no significant differences between
the two groups (Table. IIA).
 | Table I.Comparison of patient age, gender and
tumor location between the HALS (n=57) and CL (n=54) groups. |
Table I.
Comparison of patient age, gender and
tumor location between the HALS (n=57) and CL (n=54) groups.
| A, Comparison of
patient age |
|
|
|
|
| Age (years) | HALS | CL | P-valuea |
|
| Average | 65.4 | 67.0 | 0.095 |
| Median (range) | 65.0 (55–81) | 68.5 (35–92) |
|
|
| B, Comparison of
patient gender |
|
|
|
|
| Gender | HALS, no. (%) | CL, no. (%) | P-valueb |
|
| Male | 43 (75.4) | 35 (64.8) | 0.221 |
| Female | 14 (24.6) | 19 (35.2) |
|
|
| C, Comparison of
tumor location |
|
|
|
|
| Location | HALS, no. (%) | CL, no. (%) |
P-valueb |
|
| RS | 20 (35.1) | 20 (37.0) | 0.831 |
| Ra | 21 (36.8) | 14 (25.9) | 0.216 |
| Rb | 16 (28.1) | 20 (37.0) | 0.313 |
 | Table II.Comparison of operative method and
tumor stage between the HALS (n=57) and CL (n=54) groups. |
Table II.
Comparison of operative method and
tumor stage between the HALS (n=57) and CL (n=54) groups.
| A, Comparison of
operative method |
|
|
|
|
| Operative
method | HALS, no. (%) | CL, no. (%) |
P-valuea |
|
| Anterior
resection | 11 (19.3) | 12 (22.2) | 0.704 |
| Low anterior
resection | 39 (68.4) | 33 (61.1) | 0.420 |
| Miles'
operation | 7 (12.3) | 9 (16.7) | 0.511 |
|
| B, Comparison of
tumor stage |
|
|
|
|
| Tumor stage | HALS, no. (%) | CL, no. (%) |
P-valuea |
|
| I | 17 (29.8) | 10 (18.5) | 0.165 |
| II | 14 (24.6) | 20 (37.0) | 0.154 |
| III | 26 (45.6) | 24 (44.4) | 0.901 |
The CL group included 54 patients who underwent
radical resection (stage I, 10 patients; stage II, 20 patients; and
stage III, 24 patients) prior to August, 2007 and received the same
postoperative adjuvant chemotherapy and follow-up as the those in
the HALS group (Table IIB). These
historical controls were matched for stage with the 57 patients who
underwent HALS (stage I, 17 patients; stage II, 14 patients; and
stage III, 26 patients) from 2007 onwards. For all patients in both
groups, the operative indications were as follows: A performance
status of 0–2, no severe cardiopulmonary complications, no lateral
lymph node metastasis or invasion of multiple organs and tumor not
filling the pelvic cavity prior to surgery (4,26,27).
The present study was approved by the Institutional
Review Board of Tokai University Hachioji Hospital and all the
patients provided written informed consent.
Treatment
CL involved a midline laparotomy with an incision of
30 cm or longer, whereas HALS was performed with 3 ports (rectum,
5/12/5 mm) and a vertical incision of ~45–55 mm in the umbilical
region (4,26,27). In
accordance with the Japanese General Rules for Classification of
Colorectal Carcinoma, a D2,3 resection was performed and at least
12 lymph nodes were harvested in all the patients from both groups
(28–30). No postoperative adjuvant chemotherapy
was administered to stage I patients; oral anticancer agents were
administered to stage II patients (400 mg/m2
tegafur/uracil and 3 g of polysaccharide K 5 days/week for at least
6 months); and modified 5-fluorouracil/leucovorin (5-FU/LV) or
modified FOLFIRI (5-FU/LV + irinotecan: 85 mg/m2 of
irinotecan twice a month and 350 mg/m2 of 5-FU plus 150
mg/m2 LV on 5 consecutive days/month) was administered
to stage III patients for at least 6 months (31–36).
Survival
To identify metastasis/recurrence, we performed
ultrasound scan/computerized tomography (US/CT) and measured tumor
markers 3–4 times/year. If metastasis/recurrence was identified by
both US and CT, this was defined as metastasis/recurrence in the
present study (31–36). For patientsateach stage (I, II and
III) from the two groups, the 3-year relapse-free survival (3Y-RFS)
and 3-year overall survival (3Y-OS) were calculated. The mean and
median values of blood loss, operating time and postoperative
hospital stay, as well as the rate of conversion to open laparotomy
(only in the HALS group) were also calculated. Furthermore, we
compared postoperative complications, such as wound infection,
ileus, anastomotic leakage and re-operation, between the two
groups.
Statistical analysis
The Kaplan-Meier method was employed to estimate
3Y-RFS and 3Y-OS, while the log-rank test and hazard ratio (HR)
[(95% confidence interval (CI)] were used for comparisons between
the two groups. For other analyses, the χ2 test and
Mann-Whitney U test were used. P<0.05 was considered to indicate
a statistically significant difference. Analyses were performed
with SPSS 21.0 statistical software (IBM Corporation, Armonk, NY,
USA).
Results
Comparison of survival between the two
groups
The 3Y-RFS of patients with stage I, II and III
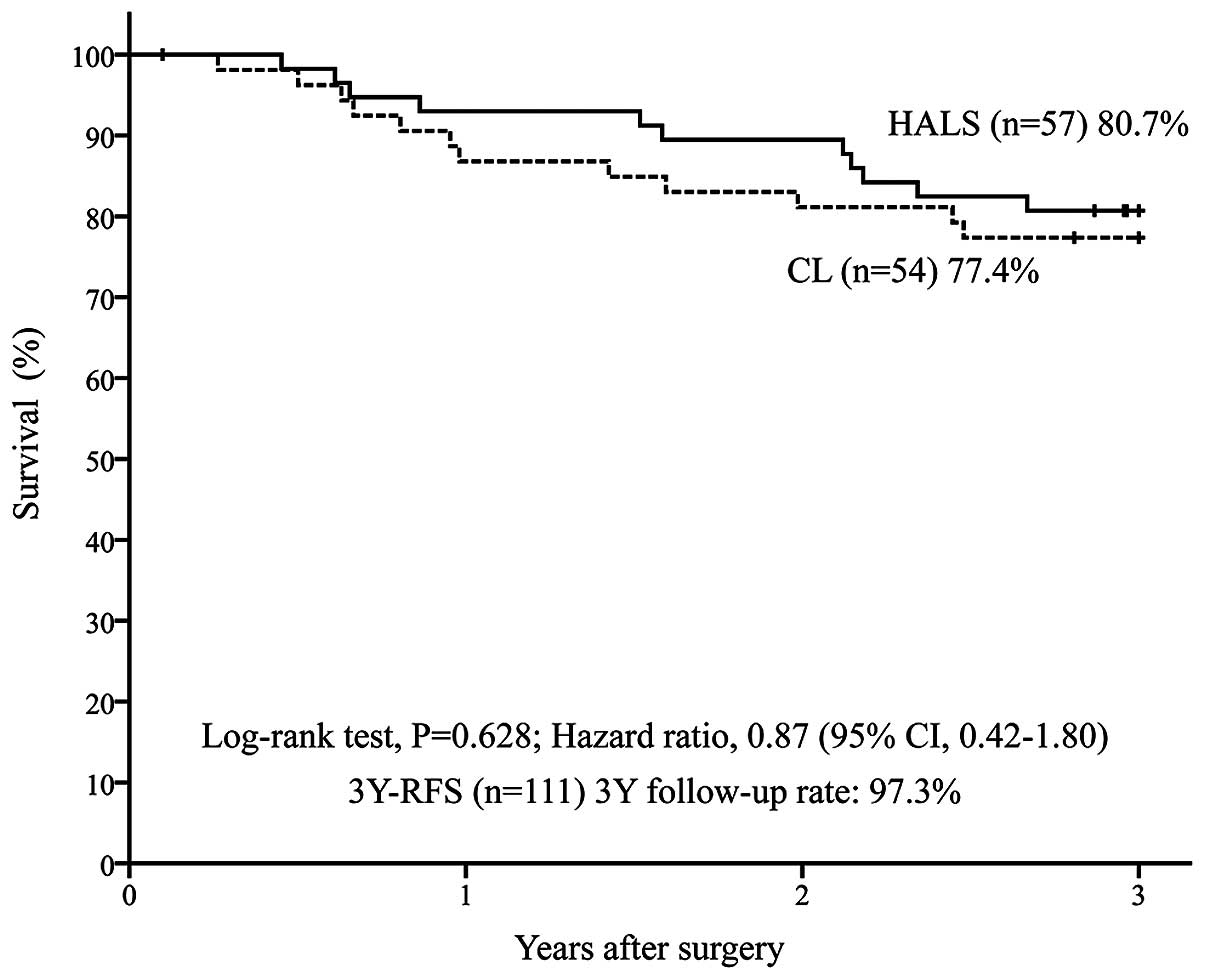
disease (n=111) was 80.7% in the HALS group (n=57) and 77.4% in the
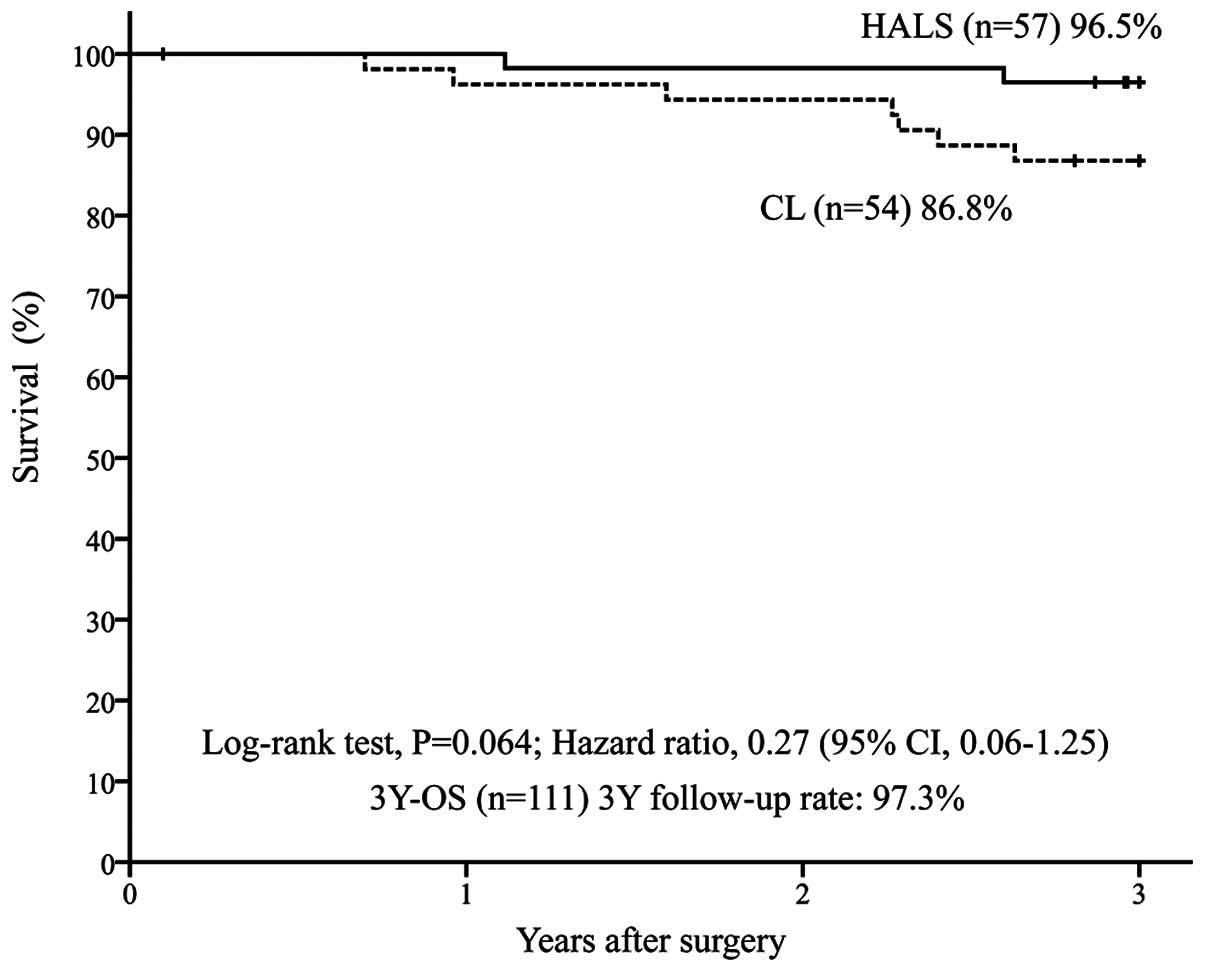
CL group (n=54) [P=0.628; HR=0.87 (95% CI: 0.42–1.80)] (Fig. 1). In addition, the 3Y-OS was 96.5% in
the HALS group (n=57) vs. 86.8% in the CL group (n=54) [P=0.064;
HR=0.27 (95% CI: 0.06–1.25)] (Fig.
2). The 3-year follow-up rate was 98.3% in the HALS group and
96.3% in the CL group (mean, 97.3%).
Intraoperative factors and hospital
stay
The mean/median intraoperative blood loss was
344.0/247.0 (range, 15–1969) ML in the HALS group (n=57) and
807.5/555.5 (121–4293) ML in the CL group (n=54) (P<0.001)
(Table III). The mean/median
operating time was 3 h 51 min/3 h 34 min (1 h 57 min-7 h 49 min) in
the HALS group (n=57) and 3 h 52 min/3 h 38 min (2 h 06 min-7 h 12
min) in the CL group (n=54) (P=0.454) (Table III). The mean/median postoperative
hospital stay was 19.8/17.0 (8–55) days in the HALS group (n=57)
and 25.5/18.5 (12–97) days in the CL group (n=54) (P=0.039)
(Table III).
 | Table III.Intraoperative factors and hospital
stay in the HALS and CL groups. |
Table III.
Intraoperative factors and hospital
stay in the HALS and CL groups.
| Variables | HALS (n=57) | CL (n=54) |
P-valuea |
|---|
| Blood
lossb |
|
| <0.001 |
|
Mean | 344.0 ml | 807.5 ml |
|
| Median
(range) | 247.0 (15–1,969)
ml | 555.5 (121–4,293)
ml |
|
| Operating time |
|
|
|
|
Mean | 3 h 51 min | 3 h 52 min | 0.454 |
| Median
(range) | 3 h 34 min (1 h 57
min–7 h 49 min) | 3 h 38 min (2 h 06
min–7 h 12 min) |
|
| Postoperative
hospital stay |
|
|
|
|
Mean | 19.8 days | 25.5 days | 0.039 |
| Median
(range) | 17.0 (8–55)
days | 18.5 (12–97)
days |
|
Postoperative complications
In the HALS group (n=57), the postoperative
complications included wound infection in 4 patients (7.0%), ileus
in 4 patients (7.0%), anastomotic leakage in 3 patients (5.3%),
urinary tract injury in 1 patient (1.8%) and re-operation in 2
patients (3.5%). There were no cases of conversion to open
laparotomy (0.0%) (Table IV). In the
CL group (n=54), these complications occurred in 9 (16.7%), 2
(3.7%), 3 (5.6%), 4 (7.4%) and 3 patients (5.6%), respectively;
there were no significant differences in the incidence of
complications between the two groups (Table IV).
 | Table IV.Postoperative complications in the
HALS and CL groups. |
Table IV.
Postoperative complications in the
HALS and CL groups.
| Complications | HALS, no (%)
(n=57) | CL, no (%)
(n=54) |
P-valuea |
|---|
| Wound
infection | 4 (7.0) | 9 (16.7) | 0.114 |
| Ileus | 4 (7.0) | 2 (3.7) | 0.440 |
| Anastomotic
leakage | 3 (5.3) | 3 (5.6) | 0.946 |
| Urinary tract
injury | 1 (1.8) | 4 (7.4) | 0.151 |
| Re-operation | 2 (3.5) | 3 (5.6) | 0.603 |
| Others | 3 (5.3) | 5 (9.3) | 0.416 |
| Conversion to
CL | 0 (0.0) |
|
|
Discussion
In Japan, ~30–40% of rectal cancer surgeries are
performed by pure LACS, CL accounts for ~50%, while HALS and small
incision surgery are used for the remaining 10–20% (18). Since pure LACS has rapidly been
adopted over the last few years, several studies comparing pure
LACS with CL or HALS have been reported (8,9,19–23).
However, there is a major problem with the majority of the studies.
Usually, single-center comparison of surgical procedures employs
the CL group as a control; however, it is difficult to avoid bias
of background factors in studies of pure LACS or HALS, as these
procedures tend to be used for low-risk patients with a relatively
good general condition, who are able to tolerate the oblique
position with the head down, or patients with early-stage disease.
In addition, it may be difficult to achieve unification of
second-line treatment, including postoperative chemotherapy and
radiotherapy, as well as treatment following recurrence (4–10).
Moreover, if national clinical databases or guidelines are used as
controls, the study becomes a stage-stratified comparison of
outcomes with the national standards, which is not appropriate for
comparing surgical procedures. In our study, the CL group was
selected from patients who underwent surgery prior to the
introduction of HALS and we ensured that they were matched for
stage and received the same postoperative adjuvant chemotherapy as
the HALS group. All operations in both groups were performed by
Mukai et al (4,27), so the management of stage I, II and
III rectal cancer was standardized and at least 6 months of
treatment was completed by >80% of the patients in the two
groups (data not shown). In addition, <20% of the patients were
censored from our database, including those with unknown
details/dropout, which conforms to the Japanese Society for Cancer
of the Colon and Rectum Guidelines 2010 for the Treatment of
Colorectal Cancer and the database used in this study had a total
censored rate of 11.9% for the HALS group vs. 1.8% for the CL group
(P=0.001, data not shown). Furthermore, all the patients were
followed up for ≥3 years, with a 3-year follow-up rate of 98.3% for
the HALS group and 96.3% for the CL group. We are planning to
perform a final analysis in the next 2 years.
Studies comparing pure LACS with CL have identified
problems with the former, including a longer operating time and
increased cost, although the hospital stay is shorter and analgesic
use is decreased (7–10). There are also other problems with
performing pure LACS at medium-sized hospitals with 400–500 beds,
including the need for skilled surgeons, the training requirements,
the pressure on the anesthesiologists due to longer operations,
longer occupation of operating theaters and greater consumption of
materials. The comparison of pure LACS with HALS has also
identified problems, such as the slower learning curve for pure
LACS, additional time required and differences in the conversion
rate to open surgery (8,9,19–23). It has been reported that HALS is
associated with a markedly lower conversion rate compared to pure
LACS, with the rate being 0.0% (0/57 patients) in our study. This
result indicates that preoperative diagnosis and our indications
for HALS were strict and appropriate (2,4,7). In addition, there was less blood loss
and a shorter hospital stay with HALS compared to CL, suggesting
that HALS may be performed safely based on strict indications by
employing the magnified view obtained with laparoscopy.
In conclusion, HALS is a safe and reliable procedure
that utilizes the same left-hand manipulation as CL and allows
palpation/touch as it is positioned between pure LACS and CL. Since
HALS may be performed relatively easily at a low cost, we consider
it to be an excellent therapeutic option that deserves
re-evaluation, particularly in Japan, due to the decreasing
availability of surgeons and anesthesiologists.
Acknowledgements
This study was supported by a grant from the
Hand-Assisted Laparoscopic Surgery Research Group (grant no.
2012-5007; Tokai University Hachioji Hospital, Hachioji, Tokyo,
Japan) and the Research and Study Program of Tokai University
Educational System General Research Organization (grant no.
2012-03; Tokai University Hospital, Isehara, Kanagawa, Japan).
Glossary
Abbreviations
Abbreviations:
|
HALS
|
hand-assisted laparoscopic surgery
|
|
CL
|
conventional laparotomy
|
|
LACS
|
laparoscopy-assisted colorectal
surgery
|
References
|
1
|
Franklin ME Jr, Rosenthal D, Abrego-Medina
D, Dorman JP, Glass JL, Norem R and Diaz A: Prospective comparison
of open vs. laparoscopic colon surgery for carcinoma. Five-year
results. Dis Colon Rectum. 39 (Suppl 10):S35–S46. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yano H, Ohnishi T, Kanoh T and Monden T:
Hand-assisted laparoscopic low anterior resection for rectal
carcinoma. J Laparoendosc Adv Surg Tech A. 15:611–614. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mukai M, Tanaka A, Tajima T, Fukasawa M,
Yamagiwa T, Okada K, Sato K, Tobita K, Oida Y and Makuuchi H:
Two-port hand-assisted laparoscopic surgery for the 2-stage
treatment of a complete bowel obstruction by left colon cancer: A
case report. Oncol Rep. 19:875–879. 2008.PubMed/NCBI
|
|
4
|
Mukai M, Kishima K, Tajima T, Hoshikawa T,
Yazawa N, Fukumitsu H, Okada K, Ogoshi K and Makuuchi H: Efficacy
of hybrid 2-port hand-assisted laparoscopic surgery (Mukai's
operation) for patients with primary colorectal cancer. Oncol Rep.
22:893–899. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koh DC, Law CW, Kristian I, Cheong WK and
Tsang CB: Hand-assisted laparoscopic abdomino-perineal resection
utilizing the planned end colostomy site. Tech Coloproctol.
14:201–206. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guerrieri M, Campagnacci R, De Sanctis A,
Lezoche G, Massucco P, Summa M, Gesuita R, Capussotti L, Spinoglio
G and Lezoche E: Laparoscopic versus open colectomy for TNM stage
III colon cancer: Results of a prospective multicenter study in
Italy. Surg Today. 42:1071–1077. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chung CC, Ng DC, Tsang WW, Tang WL, Yau
KK, Cheung HY, Wong JC and Li MK: Hand-assisted laparoscopic versus
open right colectomy: A randomized controlled trial. Ann Surg.
246:728–733. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yin WY, Wei CK, Tseng KC, Lin SP, Lin CH,
Chang CM and Hsu TW: Open colectomy versus laparoscopic-assisted
colectomy supported by hand-assisted laparoscopic colectomy for
resectable colorectal cancer: A comparative study with minimum
follow-up of three years. Hepatogastroenterology. 56:998–1006.
2009.PubMed/NCBI
|
|
9
|
Pendlimari R, Holubar SD, Pattan-Arun J,
Larson DW, Dozois EJ, Pemberton JH and Cima RR: Hand-assisted
laparoscopic colon and rectal cancer surgery: Feasibility,
short-term, and oncological outcomes. Surgery. 148:378–385. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aimaq R, Akopian G and Kaufman HS:
Surgical site infection rates in laparoscopic versus open
colorectal surgery. Am Surg. 77:1290–1294. 2011.PubMed/NCBI
|
|
11
|
Nakajima K, Lee SW, Cocilovo C, Foglia C,
Sonoda T and Milsom JW: Laparoscopic total colectomy: Hand-assisted
vs. standard technique. Surg Endosc. 18:582–586. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kang JC, Chung MH, Chao PC, Yeh CC, Hsiao
CW, Lee TY and Jao SW: Hand-assisted laparoscopic colectomy vs.
open colectomy: A prospective randomized study. Surg Endosc.
18:577–581. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ringley C, Lee YK, Iqbal A, Bocharev V,
Sasson A, McBride CL, Thompson JS, Vitamvas ML and Oleynikov D:
Comparison of conventional laparoscopic and hand-assisted oncologic
segmental colonic resection. Surg Endosc. 21:2137–2141. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Young-Fadok TM: Colon cancer: Trials,
results, techniques (LAP and HALS), future. J Surg Oncol.
96:651–659. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tjandra JJ, Chan MK and Yeh CH:
Laparoscopic- vs. hand-assisted ultralow anterior resection: A
prospective study. Dis Colon Rectum. 51:26–31. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pattana-arun J, Sahakitrungruang C,
Atithansakul P, Tantiphlachiva K, Khomvilai S and Rojanasakul A:
Multimedia article. Hand-assisted laparoscopic total mesorectal
excision: A stepwise approach. Dis Colon Rectum. 52:17872009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oncel M, Akin T, Gezen FC, Alici A and
Okkabaz N: Left inferior quadrant oblique incision: A new access
for hand-assisted device during laparoscopic low anterior resection
of rectal cancer. J Laparoendosc Adv Surg Tech A. 19:663–666. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kitano S, Yamashita N, Shiraishi N, et al:
11th Nationwide surgery of endoscopic surgery in Japan. J Jpn Soc
Endoscopic Surg. 17:595–611. 2012.(In Japanese).
|
|
19
|
CimA R and Pemberton JH: How a hand-
assist can help in lap colectomy. Contemp Surg. 63:19–23. 2007.
|
|
20
|
Cima RR, Pattana-arun J, Larson DW, Dozois
EJ, Wolff BG and Pemberton JH: Experience with 969 minimal access
colectomies: The role of hand-assisted laparoscopy in expanding
minimally invasive surgery for complex colectomies. J Am Coll Surg.
206:946–952. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sheng QS, Lin JJ, Chen WB, Liu FL, Xu XM,
Lin CZ, Wang JH and Li YD: Hand-assisted laparoscopic versus open
right hemicolectomy: Short-term outcomes in a single institution
from China. Surg Laparosc Endosc Percutan Tech. 22:267–271. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ng LW, Tung LM, Cheung HY, Wong JC, Chung
CC and Li MK: Hand-assisted laparoscopic versus total laparoscopic
right colectomy: A randomized controlled trial. Colorectal Dis.
14:e612–e617. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sim JH, Jung EJ, Ryu CG, Paik JH, Kim G,
Kim SR and Hwang DY: Short-term outcomes of hand-assisted
laparoscopic surgery vs. open surgery on right colon cancer: A
case-controlled study. Ann Coloproctol. 29:72–76. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Meshikhes AW, El Tair M and Al Ghazal T:
Hand-assisted laparoscopic colorectal surgery: Initial experience
of a single surgeon. Saudi J Gastroenterol. 17:16–19. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tajima T, Mukai M, Yamazaki M, Higami S,
Yamamoto S, Hasegawa S, Nomura E, Sadahiro S, Yasuda S and Makuuchi
H: Comparison of hand-assisted laparoscopic surgery and
conventional laparotomy for colorectal cancer: Interim results from
a single institution. Oncol Lett. 8:627–632. 2014.PubMed/NCBI
|
|
26
|
Mukai M, Fukasawa M, Kishima K, Iizuka S,
Fukumitsu H, Yazawa N, Tajima T, Nakamura M and Makuuchi H:
Trans-anal reinforcing sutures after double stapling for lower
rectal cancer: Report of two cases. Oncol Rep. 21:335–339.
2009.PubMed/NCBI
|
|
27
|
Mukai M, Sekido Y, Hoshikawa T, Yazawa N,
Fukumitsu H, Okada K, Tajima T, Nakamura M and Ogoshi K: Two-stage
treatment (Mukai's method) with hybrid 2-port HALS (Mukai's
operation) for complete bowel obstruction by left colon cancer or
rectal cancer. Oncol Rep. 24:25–30. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mukai M, Ito I, Mukoyama S, Tajima T,
Saito Y, Nakasaki H, Sato S and Makuuchi H: Improvement of 10-year
survival by Japanese radical lymph node dissection in patients with
Dukes' B and C colorectal cancer: A 17-year retrospective study.
Oncol Rep. 10:927–934. 2003.PubMed/NCBI
|
|
29
|
Japanese Society for Cancer of the Colon
and Rectum (JSCCR), . General Rules for Clinical and Pathological
Studies on Cancer of the Colon, Rectum and Anus. 7th. revised
version. Kanehara Shuppan; Tokyo, Japan: 2009
|
|
30
|
Japanese Society for Cancer of the Colon
and Rectum, . JSCCR Guidelines 2010 for the Treatment of Colorectal
Cancer. Kanehara & Co., Ltd; Tokyo, Japan: 2010
|
|
31
|
Mukai M, Tajima T, Nakasaki H, Sato S,
Ogoshi K and Makuuchi H: Efficacy of postoperative adjuvant oral
immunochemotherapy in patients with Dukes' B colorectal cancer. Ann
Cancer Res Therap. 11:201–214. 2003. View Article : Google Scholar
|
|
32
|
Mukai M, Tajima T, Nakasaki H, Sato S,
Ogoshi K and Makuuchi H: Efficacy of postoperative adjuvant oral
immunochemotherapy in patients with Dukes' C colorectal cancer. Ann
Cancer Res Therap. 11:215–229. 2003. View Article : Google Scholar
|
|
33
|
Ito I, Mukai M, Ninomiya H, Kishima K,
Tsuchiya K, Tajima T, Oida Y, Nakamura M and Makuuchi H: Comparison
between intravenous and oral postoperative adjuvant
immunochemotherapy in patients with stage II colorectal cancer.
Oncol Rep. 20:1189–1194. 2008.PubMed/NCBI
|
|
34
|
Ito I, Mukai M, Ninomiya H, Kishima K,
Tsuchiya K, Tajima T, Nakamura M and Makuuchi H: Comparison between
intravenous and oral postoperative adjuvant immunochemotherapy in
patients with stage III colorectal cancer. Oncol Rep. 20:1521–1526.
2008.PubMed/NCBI
|
|
35
|
Mukai M, Okada K, Fukumitsu H, Yazawa N,
Hoshikawa T, Tajima T, Hirakawa H, Ogoshi K and Makuuchi H:
Efficacy of 5-FU/LV plus CPT-11 as first-line adjuvant chemotherapy
for stage IIIa colorectal cancer. Oncol Rep. 22:621–629. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mukai M, Kishima K, Uchiumi F, Ishibashi
E, Fukasawa M, Tajima T, Nakamura M and Makuuchi H: Clinical
comparison of QOL and adverse events during postoperative adjuvant
chemotherapy in outpatients with node-positive colorectal cancer or
gastric cancer. Oncol Rep. 21:1061–1066. 2009. View Article : Google Scholar : PubMed/NCBI
|