Introduction
Nasopharyngeal carcinoma (NPC) is widespread in
Southern China and Southeastern Asia, although it is less common in
North America and Western Europe. Among head and neck carcinomas,
NPC is characterized by clinical, pathological, phenotypic and
biological heterogeneity (1). Radical
external radiotherapy (RT) has always been the mainstay of
treatment for all-stage NPC (2).
Currently, although patients with early-stage NPC may be cured by
RT alone, the majority of NPC patients present with stage III or IV
disease and have a poor prognosis (3). Numerous attempts have been made to
improve the outcome of locoregionally advanced NPC (4).
As NPC has been found to be radiosensitive as well
as chemosensitive and responds well to various chemotherapeutic
agents, such as cisplatin, fluorouracil and paclitaxel (5–9), combined
chemotherapy and RT have become the standard treatment strategy for
locoregionally advanced NPC (10,11),
particularly concurrent chemoradiotherapy (CCRT), on the basis of
the INT 0099 trial (12). Randomized
trials of induction chemotherapy followed by RT alone have resulted
in encouraging response rates and improvement in disease-free
survival (DFS), but not overall survival (OS) (13). The development of a sequential
schedule of induction chemotherapy followed by chemoradiotherapy is
a logical strategy to maximize the benefit from the two approaches,
which has been widely used in Southern China. However, the high
incidence of severe toxicity with this approach is the biggest
obstacle to its wider application in the treatment of Asian
patients with advanced NPC. The majority of the trials consistently
demonstrated that CCRT increased acute toxicity by ~30%. Although
most of these toxicities were recovered uneventfully, they were
associated with 1% increased mortality in all Asian trials
(14). New drugs and regimens have
been combined with RT in an attempt to maximize efficacy and
minimize toxicity. S-1 (TS-1; Taiho Pharmaceutical, Co., Ltd.,
Tokyo, Japan) is an orally active combination of tegafur, gimeracil
and oteracil; its efficacy and safety have been investigated in
gastric cancer, non-small-cell lung cancer and head and neck
squamous cell carcinoma (15–18). Therefore, we designed a new strategy
of CCRT with S-1. The objective of the present study was to
determine the efficacy and tolerance of this strategy in
locoregionally advanced NPC.
Patients and methods
Eligibility criteria
The patients were evaluated using the American Joint
Committee on Cancer 2002 staging system. Patients with stage III–IV
(M0) histologically proven NPC were eligible for this trial. The
patients were required to have no prior history of cancer, apart
from carcinoma in situ of the cervix or non-melanoma cancers
of the skin. The inclusion criteria were as follows: Karnofsky
performance status ≥60%; WBC count ≥4,000/mm3; platelet
count ≥100,000/mm3; serum creatinine level ≤1.6 mg/dl;
normal liver function with total bilirubin ≤2.5 mg/dl; and no
evidence of systemic metastasis. This study was performed following
approval from the Institutional Ethics Committee. All the patients
were randomly assigned to the treatment groups and each patient
provided written informed consent prior to treatment.
Pretreatment evaluation
All the patients underwent endoscopy and biopsy to
obtain specimens for pathological diagnosis. Additional
pretreatment evaluation included a complete history and physical
examination; chest X-ray; nasopharyngoscopy; computed tomography
scan of the nasopharynx, neck and thorax; ultrasound of the
abdomen; hematology; biochemistry, including 24-h creatinine
clearance; and urinalysis. Magnetic resonance imaging examination
was not mandatory. Bone scan was performed only when bone
metastasis was suspected.
Trial design
A total of 105 patients were enrolled in this trial.
The randomization code was developed using a computerized random
number generator. The patients were randomly assigned into the
groups receiving CCRT with S-1 (S-1 arm) or weekly cisplatin
(control arm), using blocks of 4 based on 1:1 treatment allocation.
The design was not stratified, as the participant characteristics
were well balanced by the large patient sample in this trial. The
clinicians who assessed the treatment outcomes were blinded to the
patients' group assignments.
Chemotherapy
For the S-1 arm, oral S-1, 400 mg twice per day, 7
days a week, was administered for 4 weeks concurrent with RT. For
the control arm, the patients received RT concurrent with cisplatin
40 mg/m2, administered for 7 weeks. All the patients
received antiemetic prophylaxis of 5-hydroxytryptamine-3 receptor
antagonists and were encouraged to ingest large amounts of water
during chemotherapy infusion. The second chemotherapy cycle was
delayed in case of any persistent leucopenia or severe mucositis
and was promptly resumed after recovery.
Radiotherapy
All the patients were treated in a uniform manner,
with intention-to-treat RT in both study arms. A 6-MV linear
accelerator was used for treatment, using the split-field technique
consisting of two lateral opposed faciocervical fields to the
primary tumor and upper neck, supplemented by a single anterior
field to the lower neck with a central block. The nasopharynx and
the adjacent muscles and bones were treated by a shrinking-field
technique to avoid further irradiation of the spinal cord. An
anterior facial electron field was added for cases with nasal and
ethmoidal tumor extensions. The bulky nodal area was boosted with a
posteroanterior neck field of an electron beam of appropriate
energy. The total planned dose was 66–76 Gy/7–8 weeks to the
primary tumor, 60–66 Gy/6–7 weeks to the positive neck region and
50–55 Gy/5–6 weeks to the negative neck region.
Patient assessment
After completing the combined treatments, the
patients were followed up every 2 months over the first year, every
3 months for the second and third years and every 6 months
thereafter. Patients who developed local or distant recurrence were
subjected to any treatment considered appropriate in the opinion of
the attending physician, including surgery, chemotherapy, or
RT.
Two months after completing all the treatment
schemes, the response to the combined modalities was assessed by
MRI and clinically by flexible nasopharyngoscopy. The response to
combined treatment was evaluated according to the World Health
Organization response criteria. Treatment-related toxicities were
recorded according to the National Cancer Institute Common Toxicity
Criteria (NCI-CTC) classification, version 2.0. Hematological
assessments were performed weekly to determine the worst toxicity
points. For the toxicity analysis, the worst data for each patient
in all the cycles of chemotherapy and RT were used.
Endpoints and analysis
The primary endpoints of this study were
progression-free survival (PFS) and OS at 2 years in both arms.
Distant metastasis DFS was also evaluated. PFS was defined as the
time from randomization to the time of disease progression; and OS
was defined as the time from the first day of treatment to the date
of death from any cause, or the date of the last follow-up visit.
The analyses assumed the intention-to-treat approach. Kaplan-Meier
survival curves were used to analyze time-to-event endpoints.
Toxicity and response were analyzed with χ2 tests. All
the reported significance levels were based on two-sided tests.
Results
Patient characteristics
Between January, 2007 and December, 2010, a total of
105 patients were randomly assigned to the S-1 or control arms; 2
patients (1 from the S-1 and 1 from the control arm) did not
complete the entire course of treatment due to the treatment cost,
but were included in the analysis according to the
intention-to-treat principle. No other patients refused or
discontinued their treatment due to toxicities, coexisting illness,
or other causes. The baseline characteristics, including age,
gender, Karnofsky performance status, pathology, T stage and N
stage, did not significantly differ between the two arms (Table I).
 | Table I.Characteristics of the eligible
patients. |
Table I.
Characteristics of the eligible
patients.
| Characteristics | S-1 arm, no. (%)
(n=55) | Control arm, no. (%)
(n=50) |
|---|
| Age, years |
|
Median | 48 | 46 |
|
Range | 25–68 | 20–69 |
| Gender |
| Male | 36 (65.5) | 30 (60.0) |
|
Female | 19 (34.5) | 20 (40.0) |
| Karnofsky PS |
|
>80 | 31 (56.4) | 31 (62.0) |
| ≤80 | 24 (43.6) | 19 (38.0) |
| Stagea |
| III | 38 (69.1) | 32 (64.0) |
| IV | 17 (30.9) | 18 (36.0) |
|
Pathologyb |
| Type
I | 2 (3.6) | 2 (4.0) |
| Type
II | 40 (72.7) | 39 (78.0) |
| Type
III | 13 (23.7) | 9 (18.0) |
| T stagea |
|
T1-T2 | 22 (40.0) | 22 (44.0) |
|
T3-T4 | 33 (60.0) | 28 (56.0) |
| N stagea |
|
N0-N1 | 28 (50.9) | 25 (50.0) |
|
N2-N3 | 27 (49.1) | 25 (50.0) |
Tumor response
Response was evaluated by MRI at 2 months after
completion of treatment (Table II).
We considered 2 patients in the S-1 arm and 3 in the control arm to
be unevaluable due to lack of treatment, incomplete treatment, or
major protocol violations. In the S-1 arm, the complete response
(CR) rate was 67.3% (37/55) and the partial response (PR) rate
23.6% (13/55), with an overall response rate (ORR) of 90.9%. In the
control arm, CR and PR were 54.0% (28/50) and 26.0% (13/50),
respectively, with an ORR of 80.0%. The two arms did not
significantly differ in ORR (χ2=1.551, P=0.299).
Additionally, as there was residual primary tumor and the neck
nodes usually regress slowly or may become fibrotic after several
months, no planned neck dissection was performed for 6 months.
 | Table II.Response to combined chemotherapy and
radiotherapy. |
Table II.
Response to combined chemotherapy and
radiotherapy.
| Response | S-1 arm, no. (%)
(n=55) | Control arm, no. (%)
(n=50) |
|---|
| Not assessable | 2 (3.0) | |
| Assessable | 53 (47.0) | |
| CR | 37 (67.3) | 28 (54.0) |
| PR | 13 (23.6) | 13 (26.0) |
| No change | 2 (3.6) | 4 (8.0) |
| Progression | 1 (1.8) | 2 (4.0) |
Survival
The median follow-up time was 28.4 months (range,
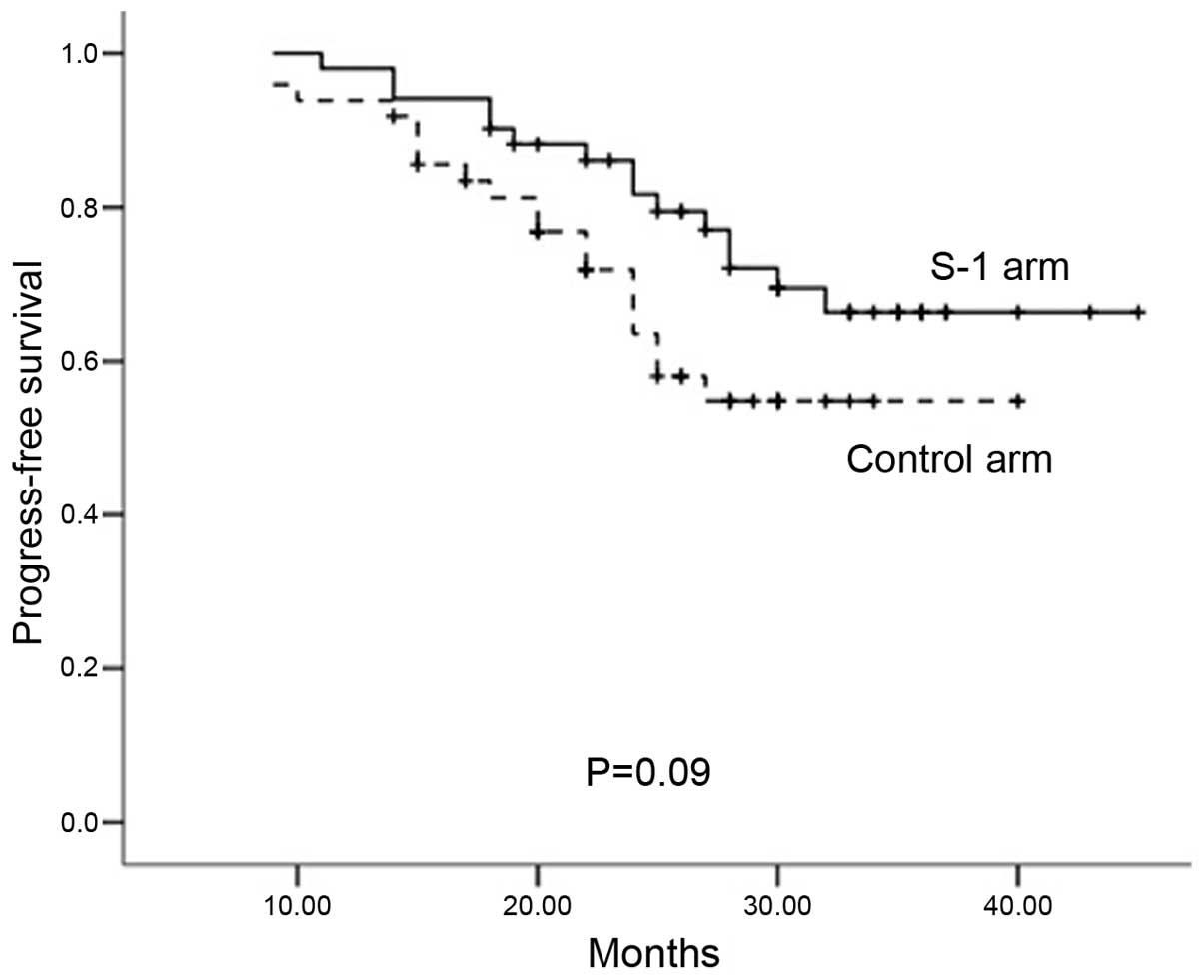
9–50 months). The rates for 2-year PFS (S-1 arm, 81.3%; control
arm, 65.8%; P=0.090; Fig. 1) and
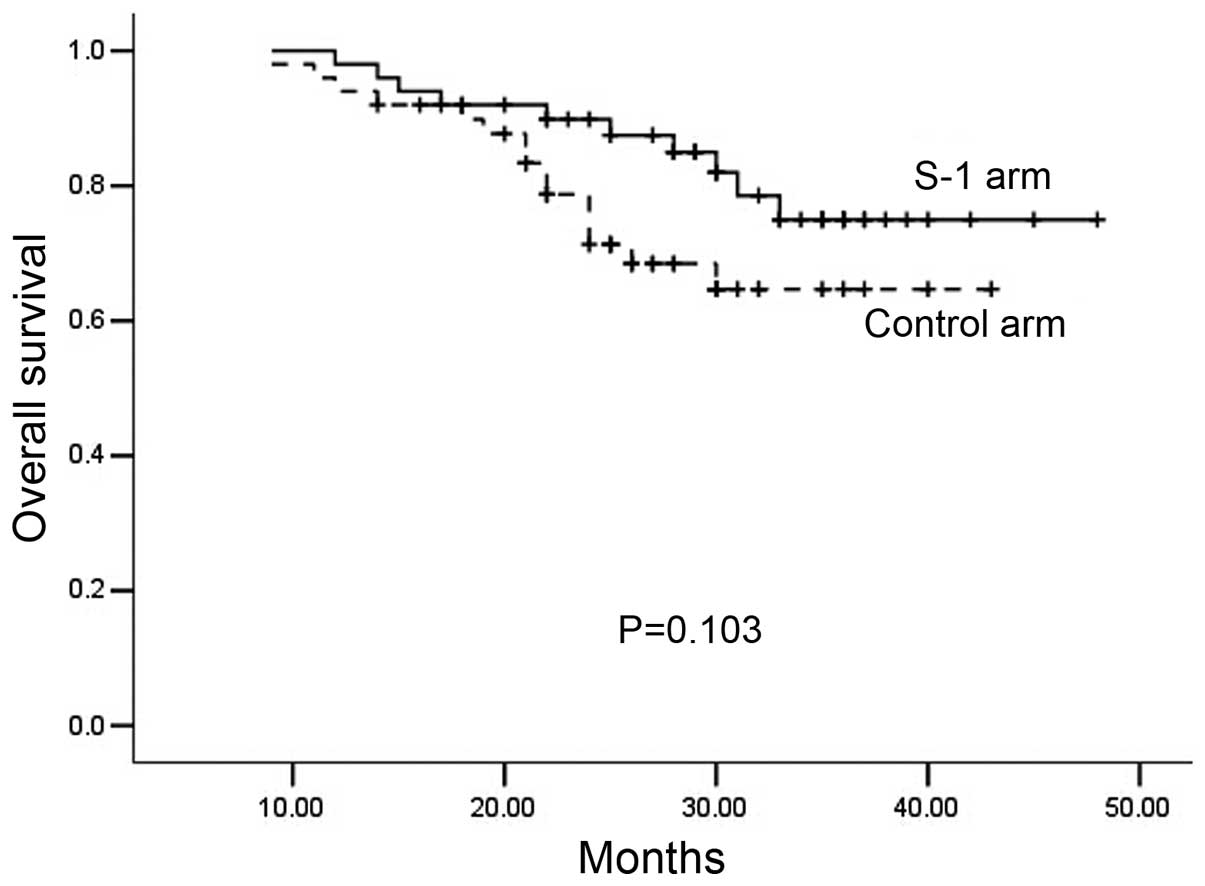
2-year OS (S-1 arm, 86.2%; control arm, 82.5%; P=0.103; Fig. 2) did not differ significantly between
the two arms.
Toxicity and compliance
Grade 3–4 toxicities according to the NCI CTC 2.0
classification are listed in Table
III. No fatal treatment-related toxicities occurred in either
arm. No patient developed grade 3–4 liver or renal function
impairment in either arm. The main toxicities were leukopenia,
mucositis, dermatitis and nausea/vomiting in both arms; the
secondary hematological toxicities were anemia and thrombocytopenia
and the secondary non-hematological toxicities were mouth dryness,
fatigue and otitis externa.
 | Table III.Summary of grade 3–4 adverse events
during treatment according to the National Cancer Institute Common
Toxicity Criteria classification, version 2.0. |
Table III.
Summary of grade 3–4 adverse events
during treatment according to the National Cancer Institute Common
Toxicity Criteria classification, version 2.0.
| Toxicity | S-1 arm, no. (%)
(n=55) | Control arm, no. (%)
(n=50) |
|---|
| Hematological |
|
Leukopenia | 3 (5.5) | 11 (22.0) |
|
Anemia | 1 (6.2) | 7 (14.0) |
|
Thrombocytopenia | 0 (0.0) | 2 (4.0) |
|
Non-hematological |
|
Mucositis | 11 (20.0) | 23 (46.0) |
|
Dermatitis | 8 (14.5) | 18 (36.0) |
|
Nausea/vomiting | 5 (9.1) | 20 (40.0) |
| Mouth
dryness | 5 (9.1) | 7 (14.0) |
|
Fatigue | 2 (3.6) | 7 (14.0) |
| Otitis
externa | 1 (1.8) | 1 (2.0) |
Of note, the main non-hematological grade 3–4
toxicities were significantly less frequent in the S-1 arm compared
to the control arm (mucositis, 20.0 vs. 46.0%, P=0.004; dermatitis,
14.5 vs. 36.0%, P=0.011; and nausea/vomiting, 9.1 vs. 40.0%,
P=0.000). In the S-1 arm, leukopenia (the main hematological
toxicity) was also less frequent compared to the control arm (5.5
vs. 22.0%, P=0.013). Additionally, the incidence rate of grade 1–2
nausea was 23.6% (13/55) in the S-1 arm and 52.0% (26/50) in the
control arm (P=0.03). Clearly, nausea was a significantly less
important issue, in terms of degree and extent, in the S-1 arm
compared to the control arm (P<0.05).
Discussion
Over the last few years, numerous trials have
investigated optimal strategies of combined chemoradiotherapy
(19). Induction chemotherapy appears
to be a logical and attractive method to control subclinical
metastatic foci and may help reduce distant metastasis, thus
improving OS. CCRT has been established as standard treatment for
locoregionally advanced NPC on the basis of the Intergroup Trial
00–99 (12) in 1998, the first
randomized trial to demonstrate a survival benefit for NPC with
combined treatment modalities. However, the suitability of CCRT for
patients in China remains controversial due to its significant
toxicity. Therefore, drug selection and dosage are crucial, as
overly toxic schedules may impair RT delivery. In China, various
chemotherapeutic agents have been combined with RT to establish
less toxic regimens for locoregionally advanced NPC.
Recently, S-1, as a novel oral chemotherapeutic
agent, has been investigated for use in gastric cancer,
non-small-cell lung cancer and head and neck squamous cell
carcinoma. The development of anticancer drugs has favored oral
over intravenous regimens, due to their relative ease of
administration and lower hospital resource demands. Oral
fluoropyrimidines, in particular, appear to possess at least
equivalent efficacy and potentially lower toxicity compared to
intravenous therapies. Using rational drug design, several oral
fluoropyrimidines have been developed, including capecitabine, UFT
(tegafur and uracil), eniluracil plus oral 5-fluorouracil and S-1.
Numerous studies have shown S-1 with CCRT to exhibit significant
antitumor activity and safety in cancers of the rectum, pancreas,
esophagus and oral cavity. Interestingly, S-1 has exhibited higher
efficacy and less toxicity in squamous cell carcinoma of the head
and neck (SCCHN). However, NPC has a natural history distinct from
that of other SCCHNs and, to date, no studies have determined
whether S-1 with RT yields the same benefit in NPC as in other
SCCHNs.
To the best of our knowledge, the present study was
the first to introduce oral S-1 with concurrent RT for
locoregionally advanced NPC. In China, CCRT with weekly cisplatin
is widely popular in clinical practice. However, several patients
experienced severe toxicities when administered CCRT with
cisplatin. With the aim to maximize efficacy and minimize toxicity,
we designed a CCRT + S-1 regimen and then compared this strategy
with standard CCRT + cisplatin for locoregionally advanced NPC. Our
results demonstrated that CR and PR were similar in the S-1 and
control arms (67.3 vs. 54.0%, respectively, P=0.235; and 23.6 vs.
26.0%, respectively, P=0.779), which is consistent with the
literature (20). The 2-year PFS and
OS were also similar in the S-1 and control arms (81.3 vs. 65.8%,
respectively, P=0.090; and 86.2 vs. 82.5%, respectively, P=0.103).
Therefore, our results demonstrated that CCRT + S-1 exhibited
similar efficacy to that of CCRT + cisplatin in this
population.
The aim of the present study was to determine the
optimal strategy for CCRT, with a focus on improved tolerance to
combined modalities. We observed that the main non-hematological
toxicities in the S-1 arm were significantly less frequent compared
to the control arm (mucositis, 20.0 vs. 46.0%, P=0.004; dermatitis,
14.5 vs. 36.0%, P=0.011; and nausea/vomiting, 9.1 vs. 40.0%,
P=0.000) and were also significantly less frequent compared to the
majority of the trials of CCRT in NPC (5,9,21). Our results suggest that S-1 increased
tolerance to the regimen in this study. Chemotherapy as well as RT
may lead to gastrointestinal reactions (i.e., anorexia, nausea,
nausea, constipation and skin or mucosal injury). Therefore, CCRT +
S-1 is associated with a high incidence of non-hematological
toxicities. As oral S-1 exhibits relatively low toxicity, its use
in CCRT lowers the risk of toxicity. Leukopenia was the most common
hematological adverse effect in our study. Grade 3–4 leukopenia was
significantly less frequent in the S-1 compared to the control arm
(5.5 vs. 22.0%, P=0.013). Moreover, cases of severe leukopenia
during induction chemotherapy and the concurrent S-1 phase were all
uncomplicated and manageable. Hematological toxicity may be further
ameliorated with the use of growth factor support and prophylactic
antibiotics. Additionally, as a linkage effect, fewer severe
toxicities encourage patients to complete their treatment course,
thus improving the PFS and OS of patients with locoregionally
advanced NPC.
In conclusion, this novel strategy may be considered
as an alternative approach to treat locoregionally advanced NPC in
a population in whom NPC is particularly common. We found the
combination of CCRT and S-1 to be efficacious, feasible and well
tolerated; therefore, an optimal regimen and schedule should be
established, with more randomized trials on larger patient samples
with longer follow-up. Moreover, as molecular-targeted agents
become increasingly available and refined, their use should also be
investigated in this context.
References
|
1
|
Cheng SH, Jian JJ, Tsai SY, Chan KY, Yen
LK, Chu NM, Tan TD, Tsou MH and Huang AT: Prognostic features and
treatment outcome in locoregionally advanced nasopharyngeal
carcinoma following concurrent chemotherapy and radiotherapy. Int J
Radiat Oncol Biol Phys. 41:755–762. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sultanem K, Shu HK, Xia P, Akazawa C,
Quivey JM, Verhey LJ and Fu KK: Three-dimensional
intensity-modulated radiotherapy in the treatment of nasopharyngeal
carcinoma: the University of California-San Francisco experience.
Int J Radiat Oncol Biol Phys. 48:711–722. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cheng SH, Yen KL, Jian JJ, Tsai SY, Chu
NM, Leu SY, Chan KY, Tan TD, Cheng JC, Hsieh CY and Huang AT:
Examining prognostic factors and patterns of failure in
nasopharyngeal carcinoma following concomitant radiotherapy and
chemotherapy: impact on future clinical trials. Int J Radiat Oncol
Biol Phys. 50:717–726. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fareed MM, AlAmro AS, Bayoumi Y, Tunio MA,
Ismail AS, Akasha R, Mubasher M and Al Asiri M: Intensity-modulated
radiotherapy with simultaneous modulated accelerated boost
technique and chemotherapy in patients with nasopharyngeal
carcinoma. BMC Cancer. 13:3182013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chan AT, Ma BB, Lo YM, Leung SF, Kwan WH,
Hui EP, Mok TS, Kam M, Chan LS, Chiu SK, Yu KH, Cheung KY, Lai K,
Lai M, Mo F, Yeo W, King A, Johnson PJ, Teo PM and Zee B: Phase II
study of neoadjuvant carboplatin and paclitaxel followed by
radiotherapy and concurrent cisplatin in patients with
locoregionally advanced nasopharyngeal carcinoma: therapeutic
monitoring with plasma Epstein-Barr virus DNA. J Clin Oncol.
22:3053–3060. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zheng J, Wang G, Yang GY and Wang D, Luo
X, Chen C, Zhang Z, Li Q, Xu W, Li Z and Wang D: Induction
chemotherapy with nedaplatin with 5-FU followed by
intensity-modulated radiotherapy concurrent with chemotherapy for
locoregionally advanced nasopharyngeal carcinoma. Jpn J Clin Oncol.
40:425–431. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Du C, Ying H, Zhou J, Hu C and Zhang Y:
Experience with combination of docetaxel, cisplatin plus
5-fluorouracil chemotherapy and intensity-modulated radiotherapy
for locoregionally advanced nasopharyngeal carcinoma. Int J Clin
Oncol. 18:464–471. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Niu X, Hu C and Kong L: Experience with
combination of cetuximab plus intensity-modulated radiotherapy with
or without chemotherapy for locoregionally advanced nasopharyngeal
carcinoma. J Cancer Res Clin Oncol. 139:1063–1071. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhong YH, Dai J, Wang XY, Xie CH, Chen G,
Zeng L and Zhou YF: Phase II trial of neoadjuvant docetaxel and
cisplatin followed by intensity-modulated radiotherapy with
concurrent cisplatin in locally advanced nasopharyngeal carcinoma.
Cancer Chemother Pharmacol. 71:1577–1583. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin JC, Jan JS, Hsu CY, Liang WM, Jiang RS
and Wang WY: Phase III study of concurrent chemoradiotherapy versus
radiotherapy alone for advanced nasopharyngeal carcinoma: positive
effect on overall and progression-free survival. J Clin Oncol.
21:631–637. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma J, Mai HQ, Hong MH, Min HQ, Mao ZD, Cui
NJ, Lu TX and Mo HY: Results of a prospective randomized trial
comparing neoadjuvant chemotherapy plus radiotherapy with
radiotherapy alone in patients with locoregionally advanced
nasopharyngeal carcinoma. J Clin Oncol. 19:1350–1357.
2001.PubMed/NCBI
|
|
12
|
Al-Sarraf M, LeBlanc M, Giri PG, Fu KK,
Cooper J, Vuong T, Forastiere AA, Adams G, Sakr WA, Schuller DE and
Ensley JF: Chemoradiotherapy versus radiotherapy in patients with
advanced nasopharyngeal cancer: phase III randomized Intergroup
study 0099. J Clin Oncol. 16:1310–1317. 1998.PubMed/NCBI
|
|
13
|
Hong RL, Ting LL, Ko JY, Hsu MM, Sheen TS,
Lou PJ, Wang CC, Chung NN and Lui LT: Induction chemotherapy with
mitomycin, epirubicin, cisplatin, fluorouracil and leucovorin
followed by radiotherapy in the treatment of locoregionally
advanced nasopharyngeal carcinoma. J Clin Oncol. 19:4305–4313.
2001.PubMed/NCBI
|
|
14
|
Chan AT, Teo PM, Ngan RK, Leung TW, Lau
WH, Zee B, Leung SF, Cheung FY, Yeo W, Yiu HH, Yu KH, Chiu KW, Chan
DT, Mok T, Yuen KT, Mo F, Lai M, Kwan WH, Choi P and Johnson PJ:
Concurrent chemotherapy-radiotherapy compared with radiotherapy
alone in locoregionally advanced nasopharyngeal carcinoma:
progression-free survival analysis of a phase III randomized trial.
J Clin Oncol. 20:2038–2044. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zang DY, Lee BH, Park HC, Song HH, Kim HJ,
Jung JY, Kim JH, Kim HY, Kwon JH, Hwang SW, Park SR, Park CH, Kim
KO, Kim MJ and Jang KM: Phase II study with oxaliplatin and S-1 for
patients with metastatic colorectal cancer. Ann Oncol. 20:892–896.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bae WK, Hwang JE, Shim HJ, Cho SH, Lee KH,
Han HS, Song EK, Yun HJ, Cho IS, Lee JK, Lim SC, Chung WK and Chung
IJ: Multicenter phase II study of weekly docetaxel, cisplatin and
S-1 (TPS) induction chemotherapy for locally advanced squamous cell
cancer of the head and neck. BMC Cancer. 13:1022013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ikeda M, Ioka T, Ito Y, Yonemoto N, Nagase
M, Yamao K, Miyakawa H, Ishii H, Furuse J, Sato K, Sato T and
Okusaka T: A multicenter phase II trial of S-1 with concurrent
radiation therapy for locally advanced pancreatic cancer. Int J
Radiat Oncol Biol Phys. 85:163–169. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shin SJ, Kim NK, Keum KC, Kim HG, Im JS,
Choi HJ, Baik SH, Choen JH, Jeung HC, Rha SY, Roh JK, Chung HC and
Ahn JB: Phase II study of preoperative chemoradiotherapy (CRT) with
irinotecan plus S-1 in locally advanced rectal cancer. Radiother
Oncol. 95:303–307. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hui EP, Ma BB, Leung SF, King AD, Mo F,
Kam MK, Yu BK, Chiu SK, Kwan WH, Ho R, Chan I, Ahuja AT, Zee BC and
Chan AT: Randomized phase II trial of concurrent
cisplatin-radiotherapy with or without neoadjuvant docetaxel and
cisplatin in advanced nasopharyngeal carcinoma. J Clin Oncol.
27:242–249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Geara FB, Glisson BS, Sanguineti G, Tucker
SL, Garden AS, Ang KK, Lippman SM, Clayman GL, Goepfert H, Peters
LJ and Hong WK: Induction chemotherapy followed by radiotherapy
versus radiotherapy alone in patients with advanced nasopharyngeal
carcinoma: results of a matched cohort study. Cancer. 79:1279–1286.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Airoldi M, Gabriele P, Gabriele AM,
Garzaro M, Raimondo L, Pedani F, Beatrice F, Pecorari G and
Giordano C: Induction chemotherapy with carboplatin and taxol
followed by radiotherapy and concurrent weekly carboplatin+taxol in
locally advanced nasopharyngeal carcinoma. Cancer Chemother
Pharmacol. 67:1027–1034. 2011. View Article : Google Scholar : PubMed/NCBI
|