Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most
common type of non-Hodgkin's lymphoma (1). DLBCL is responsive to chemotherapy and
patients with relapsed or refractory disease may be treated with
salvage chemotherapy followed by autologous stem cell
transplantation (ASCT). In a previous study, the 3-year overall
survival rate was 49% for relapsed lymphoma without central nervous
system (CNS) involvement (2).
Patients with disease progression into the CNS exhibit poor
survival, despite aggressive interventions, with a median survival
of 2–5 months (3–5). The reported incidence of CNS involvement
varies from 2.8 to 25%, depending on the population under
investigation and the diagnostic tests used (5–7). The CNS
lymphoma may involve the brain parenchyma and/or the leptomeninges.
CNS may be the only disease site or may be associated with other
sites of disease at relapse. There is currently no consensus
regarding the optimal strategy to prevent CNS dissemination.
Traditionally, the prophylactic measures include intrathecal (IT)
injection of methotrexate, cytarabine and steroid, mainly in
high-risk patients, i.e., those with marrow, testicular, orbital
and nasal sinus involvement.
The addition of rituximab to cyclophosphamide,
doxorubicin, vincristine and prednisolone (CHOP) chemotherapy has
improved the remission rate and the overall survival in DLBCL
patients. Considering the effective eradication of systemic disease
in responsive patients, we were prompted to investigate whether
rituximab was also able to reduce CNS events. This is a major
practical question, since CNS prophylactic measures may require
improvement if CHOP with the addition of rituximab (R-CHOP) is
found to be inadequate. The aim of this study was to perform a
historical analysis of the patients treated with CHOP and R-CHOP to
evaluate whether the CNS disease rate was reduced with the addition
of rituximab and to identify the risk factors associated with CNS
involvement.
Patients and methods
Patient selection and treatment
Patients aged ≥18 years, diagnosed with DLBCL and
treated with CHOP or R-CHOP chemotherapy with curative intent in
the Tuen Mun Hospital (Hong Kong, China) between January, 1996 and
December, 2012, were recruited in this study. The exclusion
criteria included human immunodeficiency virus (HIV) positivity and
CNS disease at initial diagnosis.
For early-stage patients (stage I or II) with
non-bulky disease, 4 courses of CHOP (cyclophosphamide 750
mg/m2 on day 1, doxorubicin 50 mg/m2 on day
1, vincristine 1.4 mg/m2 on day 1 and prednisolone 40
mg/m2 on days 1–5) or R-CHOP (addition of rituximab 375
mg/m2 on day 1) were administered. Following
chemotherapy, regional radiation therapy (RT) was delivered to the
involved area. For patients with advanced stages (III or IV) or
early-stage bulky disease, 6–8 courses of CHOP or R-CHOP were
administered. The chemotherapy or chemoimmunotherapy were
administered every 3 weeks. Patients with bulky disease also
received RT. Bulky disease was defined as a mediastinal mass with a
maximal width exceeding one-third of the maximal diameter on a
standing posteroanterior chest X-ray, or any mass >10 cm in
diameter. IT prophylaxis with 8 doses of methotrexate (12 mg) was
administered to high-risk patients, such as those with bone marrow,
testicular, nasal sinus, kidney or breast involvement, unless
patients declined prophylactic treatment.
The evaluation of CNS involvement was performed as
clinically indicated and included computed tomography (CT) or
magnetic resonance imaging (MRI) and lumbar puncture with
cerebrospinal fluid (CSF) analysis by cytological examination. CSF
analysis was also performed in high-risk patients, such as those
with bone marrow, nasal sinus or testicular involvement. CNS
involvement was defined as the presence of lymphoma cells in the
CSF or the presence of typical lesion(s) on CT or MRI. The rate of
CNS disease progression was compared between the CHOP and the
R-CHOP groups.
Endpoints
Baseline parameters such as age, disease stage,
marrow disease, bulky disease, B symptoms, number and sites of
extranodal disease, international prognostic index (IPI) score and
lactate dehydrogenase (LDH) level were collected. The prognostic
factors associated with subsequent CNS disease were investigated.
The primary endpoint was the time-to-CNS disease, which was
calculated from the date of pathological diagnosis of lymphoma to
the date of diagnosis of CNS disease. Patients not developing CNS
lymphoma were censored at the last date of follow-up.
Statistical analysis
Time-to-CNS disease and survival were compared
between the two groups using the Kaplan-Meier method and the
log-rank test. Baseline parameters associated with CNS disease were
compared using the chi-square test and the Fisher's exact test was
used for categorical variables. To evaluate the risk factors for
CNS events, a univariate analysis was initially performed using
time-to-CNS disease as the endpoint. Subsequently, the cox
proportional hazards model was applied in the multivariate analysis
to include factors with P<0.05 in the univariate analysis and
assess the effect of these factors on the risk of CNS events.
Overall survival was calculated from the date of DLBCL diagnosis
until the date of death or censored at the date of the last
follow-up. All the P-values were two-sided and P<0.05 was
considered to indicate statistically significant differences. Data
were analyzed using SPSS software, version 11 (SPSS Inc., Chicago,
IL, USA).
Results
Baseline characteristics
A total of 111 patients with DLBCL were identified
and 1 patient who was HIV-positive was excluded from the study. The
final cohort included 110 patients, with 45 (41%) receiving CHOP
and 65 (59%) receiving R-CHOP. The baseline parameters were
comparable between the two groups. Over 90% of the patients in this
study received planned doses of chemotherapy. The median patient
age was 55 years (range, 20–77 years) for the CHOP group and 56
years (range, 18–86 years) for the R-CHOP group. The majority of
the patients had advanced stage III or IV disease (69% in the CHOP
group and 74% in the R-CHOP group). A total of 20% of all the
patients exhibited marrow involvement and 21% exhibited involvement
of >1 extranodal sites. The extranodal sites included bone
marrow, breast, kidney, liver, ovary, nasopharynx and lungs
(Table I).
 | Table I.Baseline characteristics of the two
treatment groups. |
Table I.
Baseline characteristics of the two
treatment groups.
| Characteristics | CHOPa, no. (%) (n=45) | R-CHOPb, no. (%) (n=65) | P-value |
|---|
| Age, years |
|
|
|
|
<60 | 31 (69.0) | 41 (63.0) | 0.55 |
| ≥60 | 14 (31.0) | 24 (37.0) | 0.55 |
| Gender |
|
|
|
| Male | 21 (47.0) | 27 (42.0) | 0.69 |
|
Female | 24 (53.0) | 38 (58.0) | 0.69 |
| LDH >2×ULN | 17 (39.0) | 23 (35.0) | 0.84 |
| Stage |
|
|
|
| III or
IV | 31 (69.0) | 48 (74.0) | 0.67 |
| IV | 17 (38.0) | 31 (47.0) | 0.33 |
| B symptoms | 23 (51.0) | 39 (60.0) | 0.43 |
| Bulky disease | 2 (4.4) | 8 (12.0) | 0.19 |
| ECOG performance
status >1 | 3 (6.6) | 7 (11.0) | 0.52 |
| IPI 3–5 | 15 (33.0) | 12 (48.0) | 0.17 |
| Extranodal siteS
>1 | 6 (13.0) | 17 (26.0) | 0.15 |
| Bone marrow
involvement | 10 (22.0) | 12 (18.0) | 0.63 |
| Breast
involvement | 2 (4.5) | 1 (1.5) | 0.56 |
| Nasopharyngeal
involvement | 1 (2.2) | 4 (6.1) | 0.65 |
| Kidney
involvement | 1 (2.2) | 2 (3.1) | 1.00 |
| Pulmonary
involvement | 4 (8.9) | 10 (15.3) | 0.39 |
| Hepatic
involvement | 1 (2.2) | 2 (3.1) | 1.00 |
| Ovarian
involvement | 1 (2.2) | 2 (3.1) | 1.00 |
| CNS prophylaxis
with IT chemotherapy | 5 (11.1) | 9 (13.8) | 0.77 |
The compliance of patients to IT prophylaxis was
low, with a total of 5 (11.1%) and 9 (13.8%) patients in the CHOP
and R-CHOP groups, respectively, receiving IT prophylaxis.
Characteristics of CNS diseases
The median follow-up time for patients treated with
CHOP and R-CHOP was 58 months (range, 1.1–207 months) and 32 months
(range, 1.2–131 months), respectively. In agreement with previously
published studies, patients treated with R-CHOP exhibited a higher
complete remission rate compared with those treated with CHOP (69
vs. 40%, respectively; P=0.003) and a higher 5-year overall
survival (70 vs. 49%, respectively; P=0.01) (data not shown).
The median time from diagnosis to CNS disease was
6.7 months (range, 1.3–23.8 months). A total of 4 patients
developed CNS lymphoma following initial complete remission (1
patient received CHOP chemotherapy and relapsed 8 months after
initial remission; the remaining 3 patients received R-CHOP therapy
and relapsed 5–14 months after their initial remission).
A total of 12 patients (10.9%) subsequently
developed CNS involvement; 3 patients received IT prophylaxis and 4
patients exhibited involvement of >1 extranodal site. The
incidence of CNS events was 15.5% (7/45) in patients receiving CHOP
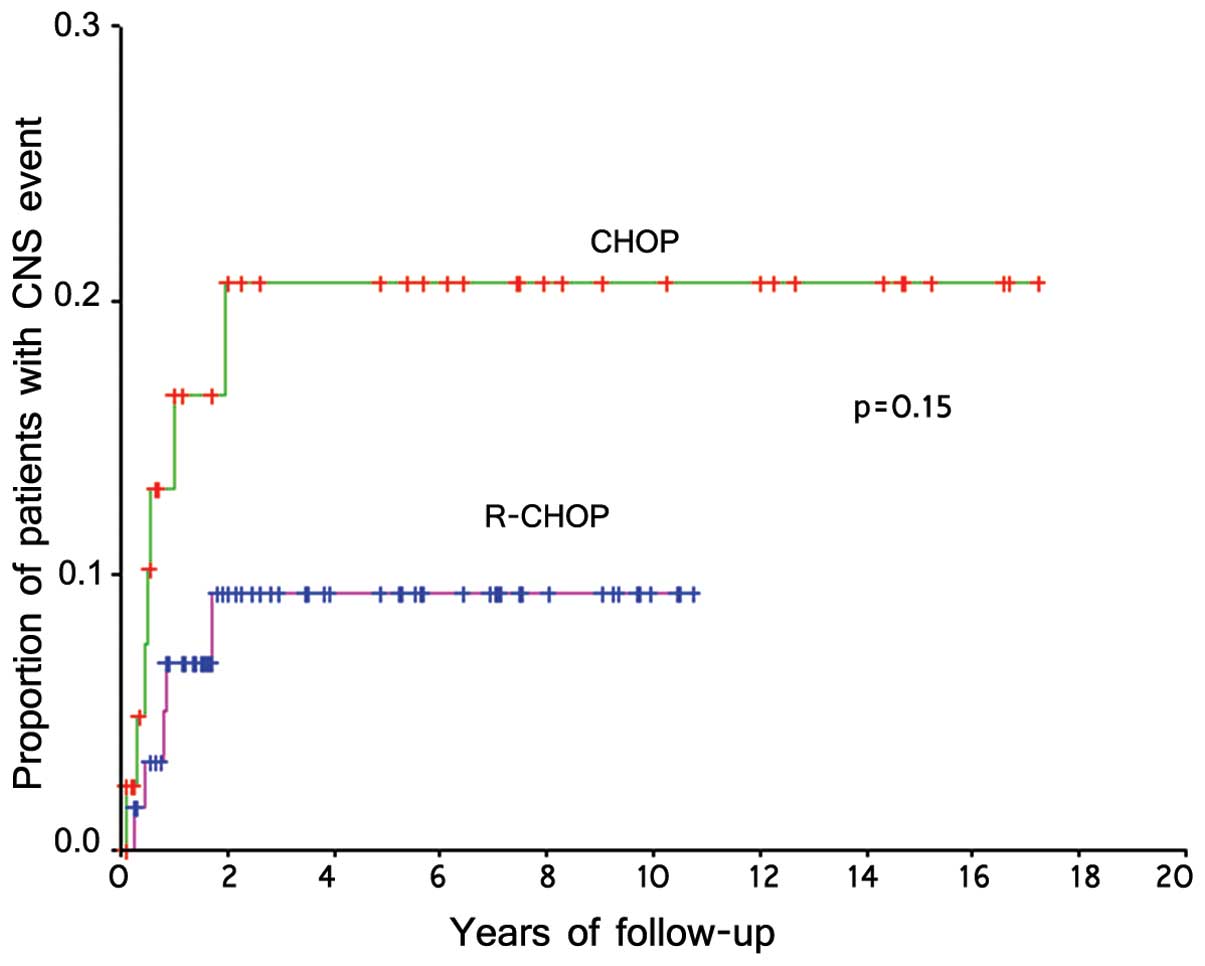
and 7.6% (5/65) in those receiving R-CHOP. The projected risk of
CNS events at 3 years was 9% in the R-CHOP group compared with 18%
in the CHOP group (P=0.15) (Fig. 1).
Parenchymal relapse appeared to be more common among patients
receiving R-CHOP (R-CHOP vs. CHOP, 80 vs. 29%, respectively)
(Table II).
 | Table II.Characteristics of patients with
central nervous system (CNS) relapse. |
Table II.
Characteristics of patients with
central nervous system (CNS) relapse.
|
Characteristics | CHOPa, NO. (%) (n=7) | R-CHOPb, no. (%) (n=5) |
|---|
| Site of CNS
relapse |
|
|
|
Parenchymal | 2 (29.0) | 4 (80.0) |
|
Leptomeningeal | 5 (71.0) | 1 (20.0) |
| Diagnosis of CNS
relapse |
|
|
|
Imaging | 2 (29.0) | 4 (80.0) |
|
CSF | 3 (42.0) | 1 (20.0) |
|
Both | 2 (29.0) | 0 (0.0) |
| Relapse within the
first year from diagnosis | 5 (71.0) | 4 (80.0) |
| Treatment of CNS
relapse |
|
|
|
High-dose methotrexate | 5 (71.0) | 3 (60.0) |
| IT
chemotherapy | 6 (86.0) | 4 (80.0) |
|
Whole-brain irradiation | 2 (29.0) | 3 (60.0) |
|
Supportive therapy | 1 (14.0) | 1 (20.0) |
| Outcome |
|
|
|
Lymphoma-related death | 6 (86.0) | 4 (80.0) |
| Alive
in second remission | 1 (14.0) | 1 (20.0) |
Treatment of CNS disease
A total of 8 patients suffering from CNS disease
were re-treated with high-dose methotrexate and it methotrexate and
cytarabine; 5 of these patients also underwent whole-brain RT; 2
patients with systemic and CNS disease were administered
dexamethasone, cytarabine and cisplatin chemotherapy, IT
methotrexate and cytarabine; and 2 patients received supportive
therapy due to their advanced age and refractory lymphoma. However,
the survival of all patients with CNS disease was poor, with a
median survival time of 5.8 months.
Risk factors for CNS involvement
On univariate analysis, the following factors were
significantly associated with subsequent secondary CNS disease:
Stage IV disease [hazard ratio (HR)=8.79, 95% confidence interval
(CI): 1.91–40.34, P=0.005], bone marrow involvement (HR=5.24, 95%
CI: 1.69–16.31), P=0.004) and elevated LDH level >2 times the
upper limit of normal (HR=3.01, 95% CI: 0.97–9.61, P=0.05)
(Table III). on multivariate
analysis using the Cox proportional model, stage IV disease
remained an independent predictor for CNS disease (HR=7.75, 95% CI:
1.67–35.92, P=0.009) (data not shown).
 | Table III.Risk factors for central nervous
system relapse in univariate analysis. |
Table III.
Risk factors for central nervous
system relapse in univariate analysis.
| FactorS | Hazard ratio (95%
CI) | P-value |
|---|
| Age >60
years | 0.39
(0.09–1.78) | 0.23 |
| Male gender | 2.01
(0.63–6.33) | 0.23 |
| LDH >1x ULN | 1.21
(0.26–5.51) | 0.81 |
| LDH >2x ULN | 3.01
(0.97–9.61) | 0.05 |
| Bone marrow
involvement | 5.24
(1.69–16.31) | 0.004 |
| Stage IV
disease | 8.79
(1.91–40.34) | 0.005 |
| Stage III and IV
disease | 5.27
(0.68–40.9) | 0.11 |
| Extranodal sites
>1 | 2.39
(0.71–8.03) | 0.15 |
| IPI >2 | 1.12
(0.35–3.54) | 0.84 |
| B symptoms | 1.69
(0.51–5.61) | 0.39 |
| Bulky disease | 2.43
(0.53–11.15) | 0.25 |
| IT prophylaxis | 2.69
(0.74–10.0) | 0.14 |
| Rituximab | 0.43
(0.14–1.38) | 0.15 |
Discussion
The addition of rituximab to CHOP has been shown to
improve the remission rate and the overall and event-free survival
of patients with DLBCL (8–11). Based on the eradication of systemic
disease achieved by rituximab, we investigated whether it was able
to reduce the incidence of CNS involvement since its introduction
into our practice. The overall CNS event rate for DLBCL was 10.9%
in our study. The majority of CNS events developed within 1 year of
the diagnosis of lymphoma. The CNS disease rate was 15.5% (7/45) in
the CHOP group vs. 7.6% (5/65) in the R-CHOP group. The projected
3-year CNS disease rate was 18% in the CHOP group vs. 9% in the
R-CHOP group (P=0.15). Therefore, the addition of rituximab to
chemotherapy did not significantly reduce the risk of CNS events in
our study.
The risk of CNS events was higher in our cohort
compared with that in previous studies. The CNS disease rate was
previously reported to be 2.2–10.4% (3,5,12–15). This
difference may be attributed to our inclusion of more patients with
advanced-stage (III or IV) disease (~70%). Moreover, lumbar
puncture with CSF analysis was not routinely performed at diagnosis
to allow for the detection and exclusion of patients with occult
CNS disease. The early CNS relapse and higher proportion of
isolated CNS relapses observed in our study may also reflect the
presence of subclinical CNS disease at diagnosis.
Stage IV lymphoma was identified as a significant
predictor of CNS disease in our cohort. Elevated LDH levels,
extranodal site involvement and advanced stage of lymphoma are the
most commonly reported risk factors. Our findings are consistent
with those of previous studies (12–17).
Rituximab penetrates poorly across the blood-brain
barrier. A pharmacokinetic study demonstrated that, following an
intravenous (i.v.) dose of rituximab, its levels in the CSF were
only 0.1% of their corresponding levels in the serum (18). Therefore, it is unlikely that
rituximab is able to directly access the lymphoma cells in the CNS.
We observed a relatively higher proportion of parenchymal disease
in the R-CHOP group compared with that in the CHOP group (80 vs.
29%, respectively). The pharmacokinetic constraints of rituximab
may limit its ability to prevent lymphoma progression in the
parenchymal compartment. We also observed that isolated CNS disease
was more common in the R-CHOP group in our cohort. This may be due
to the fact that R-CHOP is more effective in preventing systemic
relapse of lymphoma.
The optimal treatment for patients with disease
progression into the CNS has not yet been standardized (19,20). The
majority of our patients were treated with high-dose methotrexate,
with or without whole-brain RT. It was previously demonstrated that
treatment with high-dose methotrexate was effective and improved
survival (19). Whole-brain RT is
effective for initial control of CNS lymphoma; however, it is
associated with increased risk of delayed treatment-related
neurotoxicity, particularly in elderly patients (21). There is also a potential role for
high-dose chemotherapy followed by ASCT. Bromberg et al
reported an overall survival benefit in patients undergoing ASCT
and long-term survival is more likely in these patients (22).
Our study was limited by its retrospective nature
and the small sample size. Not all patients underwent lumbar
puncture and CSF analysis at the beginning of therapy; therefore, a
proportion of patients with subclinical CNS disease at diagnosis
may have been missed. There was also a difference in follow-up time
between the two groups.
The efficacy of IT prophylaxis could not be properly
assessed in our study due to the low compliance. Previous published
studies on the effect of rituximab on CNS events also reported a
similar low rate of IT prophylaxis, even in high-risk patients
(4,13,14). It
was found the effect of rituximab on the risk of CNS disease could
be assessed regardless of whether IT prophylaxis had been
administered (4). Although certain
studies support the efficacy of IT chemotherapy, several others
have questioned its ability to prevent CNS recurrence (14,23–26).
Although IT chemotherapy has been effective in preventing or
treating leptomeningeal disease, its efficacy in preventing
parenchymal disease has been questioned due to the low penetration
into the brain parenchyma and the uneven distribution within the
neuroaxis. Lumbar administration of IT methotrexate also results in
marked differences in peak levels throughout the subarachnoid
space. Subtherapeutic levels are common due to differences in CSF
movement, choroidal uptake and drug clearance (27). IT prophylaxis is associated with
several rare but severe neurological complications, such as
seizures, encephalopathy and spinal cord lesions manifesting as
tetraplegia, paraplegia and cauda equina syndrome (28). These complications may defer patients
receiving IT chemotherapy.
Patients with CNS relapse have a poor prognosis. The
incidence of CNS events may be reduced by increasing the
sensitivity of diagnosis of CNS disease and applying more effective
prophylactic therapeutic regimens. The application of more
sensitive tests may facilitate the diagnosis of occult CNS disease.
Flow cytometry of CSF may prove useful for the detection of
leptomeningeal involvement and it is more sensitive compared with
conventional cytological analysis of CSF (29). Brain MRI or CT scan is indicated for
the detection of parenchymal involvement, particularly in high-risk
patients, such as those with stage IV DLBCL. More intensive upfront
CNS-directed prophylaxis may be used for high-risk patients.
High-dose i.v. methotrexate or cytosine arabinoside may cross the
blood-brain barrier and have been used to treat established CNS
disease. The incorporation of high-dose i.v. methotrexate into the
rituximab combination may be a rational prophylactic approach for
high-risk patients. A retrospective study with a median of 3 cycles
of i.v. methotrexate 3.5 g/m2 administered to a
high-risk group of DLBCL patients reported a significant reduction
of CNS recurrence, with a recurrence rate of only 3% in the
high-risk group at a median follow-up of 33 months (30).
Another multicenter retrospective study of patients
at high-risk for CNS relapse demonstrated that the addition of
high-dose i.v. methotrexate and/or cytarabine was associated with a
lower incidence of CNS relapse compared with IT chemotherapy alone
(31). The 3-year actuarial rates of
CNS relapse in the groups receiving IT methotrexate, IT
methotrexate with 2 cycles of high-dose i.v. methotrexate, and
chemotherapy regimens containing high-dose i.v. methotrexate and
cytarabine, were 18.4, 6.9 and 2.3%, respectively (P=0.009). The
most frequent toxicity of i.v. methotrexate was renal impairment,
which was grade 1 in the majority of the cases.
A prospective study of patients with DLBCL or grade
III follicular lymphoma reported that 6 courses of R-CHEOP
(rituximab, cyclophosphamide, doxorubicin, etoposide, vincristine
and prednisolone) followed by high-dose cytarabine (3
g/m2 twice daily for 2 days) and a course of high-dose
methotrexate (3 g/m2 i.v. as a 24-h infusion) achieved a
CNS relapse rate of 4.5% (32). There
is growing evidence that high-dose antimetabolite therapy may
provide effective CNS prophylaxis in patients with DLBCL.
The optimal time of systemic high-dose chemotherapy
has not yet been defined. CNS relapse is most common within the
first 12 months from the completion of primary therapy (14,25–26,33).
This may suggest the presence of occult CNS disease at diagnosis
and early use of high-dose i.v. methotrexate is recommended.
In conclusion, although the addition of rituximab to
chemotherapy may improve the remission rate and overall survival of
patients with DLBCL, it did not appear to decrease the risk of CNS
events in our study. There is a need for a better prophylactic
strategy by increasing the sensitivity of diagnosis of CNS disease
and more effective prophylactic therapeutic regimens, such as
high-dose i.v. methotrexate, in order to reduce CNS events,
particularly in high-risk patients.
References
|
1
|
Martelli M, Ferreri AJ, Agostinelli C, Di
Rocco A, Pfreundschuh M and Pileri SA: Diffuse large B-cell
lymphoma. Crit Rev Oncol Hematol. 87:146–171. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gisselbrecht C, Glass B, Mounier N, et al:
Salvage regimens with autologous transplantation for relapsed large
B-cell lymphoma in the rituximab era. J Clin Oncol. 28:4184–4190.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Feugier P, Virion JM, Tilly H, Haioun C,
Marit G, Macro M, Bordessoule D, Recher C, Blanc M, Molina T,
Lederlin P and Coiffier B: Incidence and risk factors for central
nervous system occurrence in elderly patients with diffuse
large-B-cell lymphoma: influence of rituximab. Ann Oncol.
15:129–133. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boehme V, Schmitz N, Zeynalova S, Loeffler
M and Pfreundschuh M: CNS events in elderly patients with
aggressive lymphoma treated with modern chemotherapy (CHOP-14) with
or without rituximab: an analysis of patients treated in the
RICOVER-60 trial of the German high-grade non-Hodgkin lymphoma
study group (DSHNHL). Blood. 113:3896–3902. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bernstein SH, Unger JM, Leblanc M,
Friedberg J, Miller TP and Fisher RI: Natural history of CNS
relapse in patients with aggressive non-Hodgkin's lymphoma: a
20-year follow-up analysis of SWOG 8516-the southwest oncology
group. J Clin Oncol. 27:114–119. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bierman P and Giglio P: Diagnosis and
treatment of central nervous system involvement in non-Hodgkin's
lymphoma. Hematol Oncol Clin North Am. 19:597–609. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hegde U, Filie A, Little RF, Janik JE,
Grant N, Steinberg SM, Dunleavy K, Jaffe ES, Abati A,
Stetler-Stevenson M and Wilson WH: High incidence of occult
leptomeningeal disease detected by flow cytometry in newly
diagnosed aggressive B-cell lymphomas at risk for central nervous
system involvement: the role of flow cytometry vs. cytology. Blood.
105:496–502. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Coiffier B, Lepage E, Briere J, et al:
CHOP chemotherapy plus rituximab compared with CHOP alone in
elderly patients with diffuse large-B-cell lymphoma. N Engl J Med.
346:235–242. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Coiffier B, Thieblemont C, Van Den Neste
E, et al: Long-term outcome of patients in the LNH-98.5 trial, the
first randomized study comparing rituximab-CHOP to standard CHOP
chemotherapy in DLBCL patients: a study by the Groupe d'Etudes des
Lymphomes de l'Adulte. Blood. 116:2040–2045. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pfreundschuh M, Trumper L, Osterborg A, et
al: MabThera International Trial Group: CHOP-like chemotherapy plus
rituximab vs. CHOP-like chemotherapy alone in young patients with
good prognosis diffuse large-B-cell lymphoma: a randomised
controlled trial by the MabThera International Trial (MInT) Group.
Lancet Oncol. 7:379–391. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pfreundschuh M, Schubert J, Ziepert M, et
al: German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL):
Six versus eight cycles of bi-weekly CHOP-14 with or without
rituximab in elderly patients with aggressive CD20+
B-cell lymphomas: a randomized controlled trial (RICOVER-60).
Lancet Oncol. 9:105–116. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boehme V, Zeynalova S, Kloess M, Loeffler
M, Kaiser U, Pfreundschuh M and Schmitz N: German High-Grade
Non-Hodgkin's Lymphoma Study Group (DSHNHL): Incidence and risk
factors of central nervous system recurrence in aggressive lymphoma
- a survey of 1693 patients treated in protocols of the German
High-Grade Non-Hodgkin's Lymphoma Study Group (DSHNHL). Ann Oncol.
18:149–157. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shimazu Y, Notohara K and Ueda Y: Diffuse
large B-cell lymphoma with central nervous system relapse:
prognosis and risk factors according to retrospective analysis from
a single-center experience. Int J Hematol. 89:577–583. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Villa D, Connors JM, Shenkier TN, Gascoyne
RD, Sehn LH and Savage KJ: Incidence and risk factors for central
nervous system relapse in patients with diffuse large B-cell
lymphoma: the impact of the addition of rituximab to CHOP
chemotherapy. Ann Oncol. 21:1046–1052. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kridel R and Dietrich PY: Prevention of
CNS relapse in diffuse large B-cell lymphoma. Lancet Oncol.
12:1258–1266. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jahnke K, Thiel E, Martus P, Schwartz S
and Korfel A: Retrospective study of prognostic factors in
non-Hodgkin lymphoma secondarily involving the central nervous
system. Ann Hematol. 85:45–50. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tomita N, Yokoyama M, Yamamoto W, et al:
Central nervous system event in patients with diffuse large B-cell
lymphoma in rituximab era. Cancer Sci. 103:245–251. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rubenstein JL, Combs D, Rosenberg J, Levy
A, McDermott M, Damon L, Ignoffo R, Aldape K, Shen A, Lee D,
Grillo-Lopez A and Shuman MA: Rituximab therapy for CNS lymphomas:
targeting the leptomeningeal compartment. Blood. 101:466–468. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Doolittle ND, Abrey LE, Shenkier TN, et
al: Brain parenchyma involvement as isolated central nervous system
relapse of systemic non-Hodgkin lymphoma: an international primary
CNS lymphoma collaborative group report. Blood. 111:1085–1093.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim SJ, Oh SY, Kim JS, Kim H, Lee GW, Won
JH, Shin HJ, Yang DH, Choi CW, Park J, Kim WS and Suh C: Secondary
central nervous system (CNS) involvement in patients with diffuse
large B-cell lymphoma: a therapeutic dilemma. Ann Hematol.
90:539–546. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gavrilovic IT, Hormigo A, Yahalom J,
DeAngelis LM and Abrey LE: Long-term follow-up of high-dose
methotrexate-based therapy with and without whole brain irradiation
for newly diagnosed primary CNS lymphoma. J Clin Oncol.
24:4570–4574. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bromberg JE, Doorduijn JK, Illerhaus G,
Jahnke K, Korfel A, Fischer L, Fritsch K, Kuittinen O, Issa S, van
Montfort C and van den Bent MJ: Central nervous system recurrence
of systemic lymphoma in the era of stem cell transplantation - an
international primary central nervous system lymphoma study group
project. Haematologica. 98:808–813. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chua SL, Seymour JF, Streater J, Wolf MM,
Januszewicz EH and Prince HM: Intrathecal chemotherapy alone is
inadequate central nervous system prophylaxis in patients with
intermediate-grade non-Hodgkin's lymphoma. Leuk Lymphoma.
43:1783–1788. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Arkenau HT, Chong G, Cunningham D, Watkins
D, Agarwal R, Sirohi B, Trumper M, Norman A, Wotherspoon A and
Horwich A: The role of intrathecal chemotherapy prophylaxis in
patients with diffuse large B-cell lymphoma. Ann Oncol. 18:541–545.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tai WM, Chung J, Tang PL, Koo YX, Hou X,
Tay KW, Quek R, Tao M and Lim ST: Central nervous system (CNS)
relapse in diffuse large B cell lymphoma (DLBCL): pre- and
post-rituximab. Ann Hematol. 90:809–818. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schmitz N, Zeynalova S, Glass B, Kaiser U,
Cavallin-Stahl E, Wolf M, Haenel M, Loeffler M, Truemper L and
Pfreundschuh M: CNS disease in younger patients with aggressive
B-cell lymphoma: an analysis of patients treated on the Mabthera
international trial and trials of the German high-grade non-Hodgkin
lymphoma study group. Ann Oncol. 23:1267–1273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shapiro WR, Young DF and Mehta BM:
Methotrexate: distribution in cerebrospinal fluid after
intravenous, ventricular and lumbar injections. N Engl J Med.
293:161–166. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kwong YL, Yeung DY and Chan JC:
Intrathecal chemotherapy for hematologic malignancies: drugs and
toxicities. Ann Hematol. 88:193–201. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hegde U, Filie A, Little RF, Janik JE,
Grant N and Steinberg SM: High incidence of occult leptomeningeal
disease detected by flow cytometry in newly diagnosed aggressive
B-cell lymphomas at risk for CNS involvement: the role of flow
cytometry vs. cytology. Blood. 105:496–502. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Abramson JS, Hellmann M, Barnes JA,
Hammerman P, Toomey C, Takvorian T, Muzikansky A and Hochberg EP:
Intravenous methotrexate as central nervous system (CNS)
prophylaxis is associated with a low risk of CNS recurrence in
high-risk patients with diffuse large B-cell lymphoma. Cancer.
116:4283–4290. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cheah CY, Herbert KE, O'Rourke K, et al: A
multicentre retrospective comparison of central nervous system
prophylaxis strategies among patients with high-risk diffuse large
B-cell lymphoma. Br J Cancer. 111:1072–1079. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Holte H, Leppa S, Bjorkholm M, et al:
Dose-densified chemoimmunotherapy followed by systemic central
nervous system prophylaxis for younger high-risk diffuse large
B-cell/follicular grade 3 lymphoma patients: results of a phase II
Nordic lymphoma group study. Ann Oncol. 24:1385–1392. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yamamoto W, Tomita N, Watanabe R, Hattori
Y, Nakajima Y, Hyo R, Hashimoto C, Motomura S and Ishigatsubo Y:
Central nervous system involvement in diffuse large B-cell
lymphoma. Eur J Haematol. 85:6–10. 2010.PubMed/NCBI
|