Introduction
After Morton et al reported the usefulness of
intraoperative lymphatic mapping for melanoma in the early 1990s
(1), the sentinel node (SN) concept
has been widely accepted for the treatment of various types of
cancer, including breast, gastric and head and neck cancer
(1–5).
Sentinel node navigation surgery (SNNS) is currently a standard
procedure for early-stage melanoma and breast cancer. SNNS for
gastric cancer, however, is currrently in the research phase,
having been investigated at only a few institutes to date (6,7).
Furthermore, two large prospective multicenter trials in Japan have
reported conflicting results regarding the clinical application of
SN biopsies for gastric cancer (8,9),
suggesting that it is difficult to precisely detect SNs in gastric
cancer compared with melanoma or breast cancer.
A hindrance in establishing SNNS as a standard
procedure in gastric cancer is the complexity of the lymphatic flow
and the numerous regional lymph nodes in the stomach. The number of
SNs in gastric cancer is higher compared with that in melanoma or
breast cancer; for example, the mean number of SNs in melanoma or
breast cancer is 1–3 (10), whereas
the mean number in gastric cancer is 4–7 (7). Moreover, when several SNs are detected,
it may be difficult to determine which SN should be examined during
surgery.
In the present study, we aimed to determine which
SNs should be preferentially examined during gastric cancer surgery
in order to detect metastatic SNs.
Patients and methods
Patients
In total, 824 SNs from 113 patients with clinically
determined T1–2 gastric cancer with no apparent lymph node
metastases were included in this study. We attempted to detect SNs
in these patients through the use of radioisotope (RI) and dye
methods during the period between November, 2002 and August, 2011.
There were a total of 35 metastasis-positive and 789
metastasis-negative SNs.
SN identification
SNs were identified by a combination of the RI and
dye methods and classified as hot nodes (HNs) and/or green nodes
(GNs) as follows: In the RI method, 0.5 ml of 99mTc-tin
colloid solution was injected into each of four sites surrounding
the tumor on the day prior to surgery and HNs were defined as lymph
nodes with a radioactivity of ≥10 counts per 10 sec. In the dye
method, 1 ml of 1.25% indocyanine green solution was injected into
each of four sites surrounding the tumor and GNs were defined
macroscopically.
Five minutes following dye injection, we attempted
to determine the SN stations (SSs) where SNs were distributed. We
then dissected the remaining lymph node stations, which was
required for the preoperatively planned dissection. The SNs were
examined on a back table in the operating room.
Tracer accumulation and radioactivity
count of the HNs (RI count)
We focused on the accumulation of tracers expressed
by HNs and GNs, the RI count and the size of the SNs. To establish
a cut-off value for the RI count, we evaluated the diagnostic
characteristics from the receiver operating characteristic (ROC)
curve of the RI count. We also determined the number of SNs that
must be examined to detect metastatic SNs when HNs and GNs with
high RI counts are preferentially examined.
The SN biopsies and SNNS procedures reported in this
study were reviewed and approved by the Institutional Review Board
of the National Defense Medical College (Saitama, Japan) and
written informed consent was obtained from all the patients prior
to conducting the procedures for SN identification.
Statistical analysis
All the data were analyzed using Dr. SPSS II
software for Windows (SPSS Japan Inc., Tokyo, Japan). Data are
expressed as means ± standard deviations and median (range). The
Mann-Whitney U test and Chi-square test were used for comparisons
between the metastatic and non-metastatic groups. P-values of
<0.05 were considered to indicate statistically significant
differences.
Results
Demographic data
Of the 113 patients with cT1–2 gastric cancer, 4
(3.5%) were diagnosed as ≥pT3, whereas 23 (20%) exhibited lymph
node metastases (Table I).
 | Table I.Demographic data. |
Table I.
Demographic data.
| Characteristics | Patient no.
(n=113) |
|---|
| Age, years |
|
| Mean ±
SD | 64±11 |
| Gender |
|
| Male | 77 |
|
Female | 36 |
| Histology |
|
|
Differentiated | 68 |
|
Undifferentiated | 45 |
| Depth |
|
|
Mucosa | 52 |
|
Submucosa | 43 |
|
Muscularis propria | 14 |
|
Subserosa | 3 |
|
Serosa | 1 |
| LN
metastasisa |
|
| N0 | 90 |
| N1 | 15 |
| N2 | 8 |
| Tumor size, cm |
| Mean ±
SD | 3.2±1.6 |
Number of SNs and surgical
procedures
A total of 824 lymph nodes were examined. The median
number (range) of SNs, HNs and GNs was 6 (1–22), 4 (0–22) and 4
(0–17), respectively (Table II). The
surgical procedures included SNNS with a negative SN biopsy
(partial gastrectomy, 9 cases; and sleeve gastrectomy, 31 cases;
Table II).
 | Table II.Number of SNs and surgical
procedure. |
Table II.
Number of SNs and surgical
procedure.
| Variables | Values |
|---|
| Total cases, no. | 113 |
| Total SNs, no. | 824 |
| SN no., median
(range) | 6 (1–22) |
| HNs | 4 (0–22) |
| GNs | 4 (0–17) |
| HNs and
GNs | 2 (0–14) |
| SS no. (median,
range) | 2 (1–4) |
| Surgical procedure,
no. |
|
| Partial
gastrectomy | 9 |
| Sleeve
gastrectomy | 31 |
|
Pylorus-preserving
gastrectomy | 22 |
| Distal
gastrectomy | 35 |
| Proximal
gastrectomy | 10 |
| Total
gastrectomy | 6 |
Accumulation of tracers and SN
size
We compared the accumulation of tracers and size of
lymph nodes between the metastasis-positive and metastasis-negative
SNs. The ratio of HNs in metastasis-positive SNs was significantly
higher compared with that in negative SNs (91 vs. 67%,
respectively; P<0.01). The ratio of GNs was also significantly
higher in metastasis-positive compared with that in negative SNs
(97 vs. 76%, respectively; P<0.01). The most significant
difference between the two groups was observed in the ratio of the
combination of HNs and GNs (89 vs. 43%, respectively; P<0.01).
The RI count of the metastatic SNs was significantly higher
compared with that of the negative SNs [median (range): 361
(0–10,670) vs. 53 (0–9,931), respectively; P<0.01]. There was no
significant difference in SN size between the two groups [median
(range): 4.0 (1.7–15.0) vs. 4.0 (0.5–20.0) mm, respectively;
Table III).
 | Table III.Accumulation of tracers and size of
SNs. |
Table III.
Accumulation of tracers and size of
SNs.
| SN metastasis | Positive (n=35) | Negative (n=789) | P-value |
|---|
| Hot nodes, no.
(%) | 32 (91) | 528 (67) | <0.01 |
| Green nodes | 34 (97) | 601 (76) | <0.01 |
| Hot and green
nodes | 31 (89) | 341 (43) | <0.01 |
| RI counta, median (range) | 361 (0–10,670) | 53 (0–9,931) | <0.01 |
| SN size (mm), median
(range) | 4.0 (1.7–15.0) | 4.0 (0.5–20.0) | 0.40 |
ROC curve and diagnostic
characteristics of the RI count
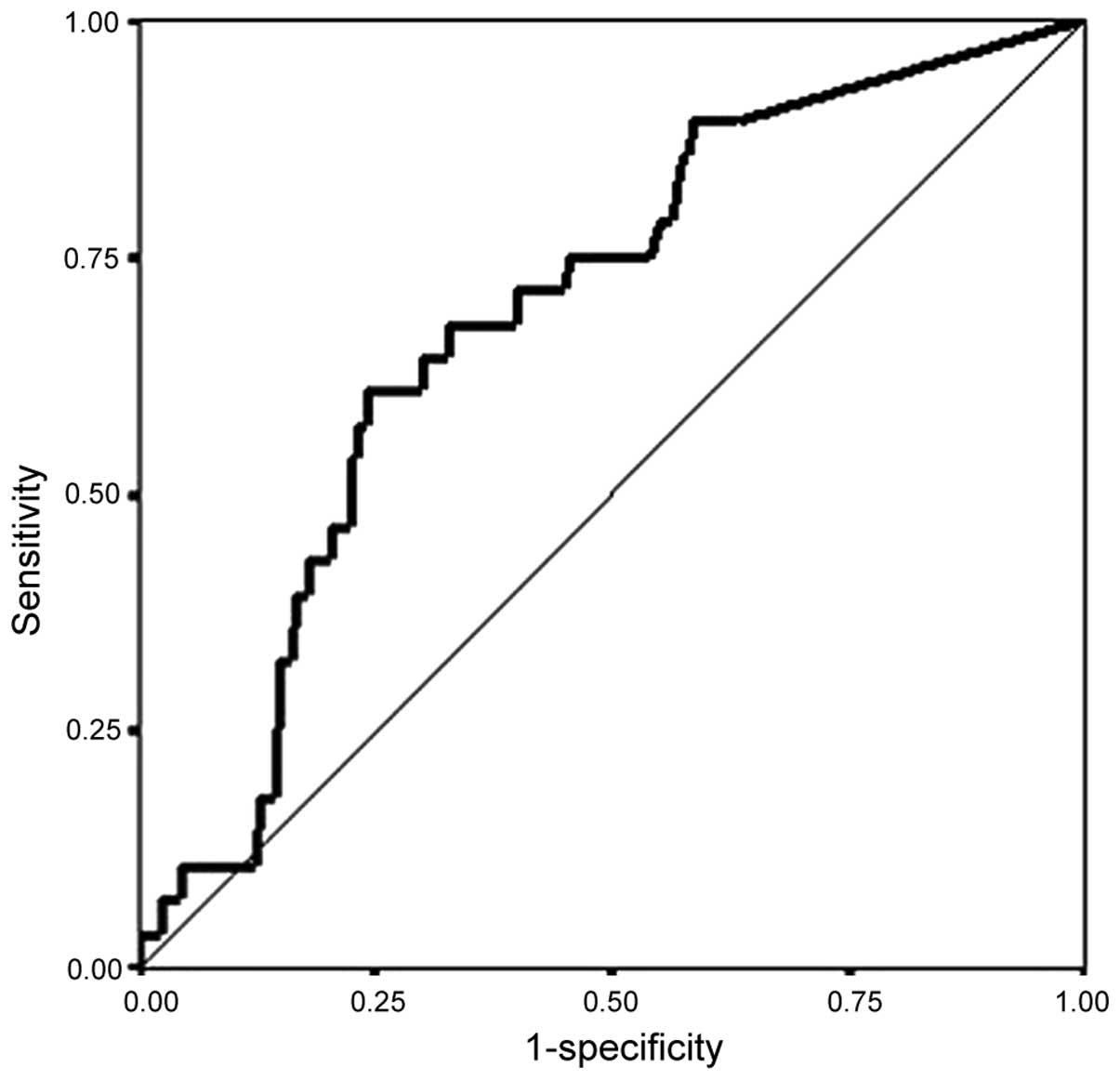
The area under the ROC curve of the RI count was
0.69 [95% confidence interval (CI): 0.60–0.78; Fig. 1]. We set the cut-off values at 100,
200, and 300 in view of the inflection point of the ROC curve. The
sensitivity was 71% when the cut-off value was set at 100 and 61%
when the cut-off value was set at 300 (Fig. 1, Table
IV).
 | Table IV.Diagnostic characteristics of the
radioactivity count of the hot nodes (RI count). |
Table IV.
Diagnostic characteristics of the
radioactivity count of the hot nodes (RI count).
| RI count | Sensitivity (95%
CI) | Specificity (95%
CI) | Positive likelihood
ratio (95% CI) | Negative likelihood
ratio (95% CI) |
|---|
| 100 | 0.71 (0.53–0.85) | 0.58 (0.54–0.61) | 1.68 (1.31–2.16) | 0.50 (0.28–0.90) |
| 200 | 0.64 (0.46–0.79) | 0.68 (0.64–0.71) | 1.99 (1.48–2.68) | 0.53 (0.32–0.87) |
| 300 | 0.61 (0.42–0.76) | 0.75 (0.72–0.78) | 2.46 (1.78–3.40) | 0.52 (0.33–0.83) |
Examinations required to detect
metastatic SNs
There were 19 metastatic SNs cases in this study.
Although there were three cases with insufficient data (nos. 7, 9
and 15), it was clear that, in order to detect metastatic SNs, we
only had to preferentially examine 1–2 HNs and GNs with high RI
counts, with the exception of case no. 8 (Table V).
 | Table V.Examinations required for the
detection of metastatic SNs. |
Table V.
Examinations required for the
detection of metastatic SNs.
| Case number | Total SNs | Metastatic SNs | Number of
examinations required to detect metastatic SNsa |
|---|
| 1 | 2 | 1 | 1 |
| 2 | 3 | 1 | 2 |
| 3 | 10 | 2 | 1 |
| 4 | 7 | 1 | 1 |
| 5 | 5 | 2 | 1 |
| 6 | 16 | 5 | 2 |
| 7 | 6 | 4 | Not clear |
| 8 | 8 | 1 | 7 or 8 |
| 9 | 21 | 1 | Not clear |
| 10 | 18 | 2 | 1 |
| 11 | 11 | 1 | 1 |
| 12 | 9 | 3 | 2 |
| 13 | 5 | 1 | 1 |
| 14 | 13 | 1 | 1 |
| 15 | 16 | 1 | Not clear |
| 16 | 3 | 3 | 1 |
| 17 | 6 | 3 | 1 |
| 18 | 1 | 1 | 1 |
| 19 | 7 | 1 | 1 |
Discussion
In this study, we attempted to determine the
priority with which SNs should be examined during SNNS for gastric
cancer. During surgery, it is difficult to examine and assess
multiple SNs promptly and accurately. Therefore, it is crucial to
determine which SNs are the best candidates for metastasis based on
size and the accumulation of tracers to ensure successful SNNS in
gastric cancer.
We also investigated the possibility of establishing
a diagnosis of metastasis based on the RI count. Although
metastatic SNs exhibited higher RI counts compared with
non-metastatic SNs, the area under the ROC curve and the diagnostic
characteristics of the RI count were insufficient for setting firm
cut-off values; thus, further evaluation of additional cases is
required to explore this methodology (Fig. 1, Table
IV). The diagnostic role of radioactivity in SNs for breast
cancer is, to a certain degree, established (11–13);
however, although the SNs with the highest counts are positive in
the majority of breast cancer patients with multiple SNs, a
consistent and relatively high RI count does not predict SN
positivity in all breast cancer patients (11–13).
Similar results have been reported in melanoma and head and neck
cancer (14,15).
Although these findings indicate that it is
difficult to diagnose metastasis by RI count, RI counts may be of
value in the preferential selection of SNs. Based on the review of
metastatic SN cases in the present study, we concluded that, in
order to determine the presence or absence of metastatic SNs, only
1–2 SNs must be examined during surgery, even if >10 SNs are
detected; however, it should be noted that 3 cases presented with
insufficient data, whereas 1 case (no. 8) required the examination
of 7–8 nodes, thus contradicting this theory (Table V). Case no. 8 had advanced gastric
cancer with T2 of the posterior side on the upper portion with a
30-mm tumor and had metastasis with GNs in all 8 SNs (6 hot and
green nodes and 2 GNs) distributed around the right paracardial and
lesser curvature. It has not been elucidated why this case only
metastasized to the GNs; however, this suggests that it is crucial
to limit SNNS to early gastric cancer.
There has been some debate over the actual
procedures for the clinical application of SNNS in gastric cancer,
including the type of tracer to be used, the injection site, how to
detect and harvest SNs and how to detect metastatic SNs (16). As regards the detection of metastatic
SNs, researchers tend to focus on diagnostic methods, such as
molecular techniques, rather than frozen section diagnoses with
hematoxylin and eosin (H&E), which tend to be inaccurate. We
previously reported that the one-step nucleic acid amplification
(OSNA) method, which is a semi-automated molecular-based rapid
diagnostic method, has the same diagnostic ability as the final
H&E-based histopathological examination and that it should be
applied for intraoperative diagnoses in SNNS for gastric cancer
(17). The OSNA method may be used to
diagnose SN metastasis within 30 min, although it is difficult to
simultaneously examine numerous SNs during surgery; a maximum of 4
SNs may be examined using the OSNA measurement equipment that is
currently available. Therefore, when assessing several SNs, we
recommend that SNs that have high RI counts with both ‘hot’ and
‘green’ status are preferentially examined.
Although multicenter trials with a larger number of
cases are required to confirm our results, we are convinced that
the prioritization described herein will speed up intraoperative
diagnosis, enabling the wider application of SNNS in clinical
practice. However, further accumulation of cases is required to set
the cut-off values for the diagnosis of metastasis based on the RI
count.
Glossary
Abbreviations
Abbreviations:
|
SN
|
sentinel node
|
|
SNNS
|
SN navigation surgery
|
|
HNs
|
hot nodes
|
|
GNs
|
green nodes
|
|
RI
|
radioisotope
|
|
RI count
|
radioactivity count of the HNs
|
|
SSs
|
SN stations
|
|
H&E
|
hematoxylin and eosin
|
|
OSNA
|
one-step nucleic acid
amplification
|
References
|
1
|
Morton DL, Wen DR, Wong JH, Economou JS,
Cagle LA, Storm FK, Foshag LJ and Cochran AJ: Technical details of
intraoperative lymphatic mapping for early stage melanoma. Arch
Surg. 127:392–399. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Krag D, Weaver D, Ashikaga T, et al: The
sentinel node in breast cancer - a multicenter validation study. N
Engl J Med. 339:941–946. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Veronesi U, Paganelli G, Viale G, et al: A
randomized comparison of sentinel-node biopsy with routine axillary
dissection in breast cancer. N Engl J Med. 349:546–553. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ross G, Shoaib T, Soutar DS, Camilleri IG,
Gray HW, Bessent RG, Robertson AG and MacDonald DG: The use of
sentinel node biopsy to upstage the clinically N0 neck in head and
neck cancer. Arch Otolaryngol Head Neck Surg. 128:1287–1291. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ross GL, Soutar DS, Gordon MacDonald D, et
al: Sentinel node biopsy in head and neck cancer: preliminary
results of a multicenter trial. Ann Surg Oncol. 11:690–696. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ohdaira H, Nimura H, Mitsumori N,
Takahashi N, Kashiwagi H and Yanaga K: Validity of modified
gastrectomy combined with sentinel node navigation surgery for
early gastric cancer. Gastric Cancer. 10:117–122. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ichikura T, Sugasawa H, Sakamoto N,
Yaguchi Y, Tsujimoto H and Ono S: Limited gastrectomy with
dissection of sentinel node stations for early gastric cancer with
negative sentinel node biopsy. Ann Surg. 249:942–947. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miyashiro I, Hiratsuka M, Sasako M, Sano
T, Mizusawa J, Nakamura K, Nashimoto A, Tsuburaya A and Fukushima
N: Gastric Cancer Surgical Study Group (GCSSG) in the Japan
Clinical Oncology Group (JCOG): High false-negative proportion of
intraoperative histological examination as a serious problem for
clinical application of sentinel node biopsy for early gastric
cancer: final results of the Japan clinical oncology group
multicenter trial JCOG0302. Gastric Cancer. 17:316–323. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kitagawa Y, Takeuchi H, Takagi Y, et al:
Sentinel node mapping for gastric cancer: a prospective multicenter
trial in Japan. J Clin Oncol. 31:3704–3710. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Morton DL, Thompson JF, Essner R, et al:
Validation of the accuracy of intraoperative lymphatic mapping and
sentinel lymphadenectomy for early-stage melanoma: a multicenter
trial. multicenter selective lymphadenectomy trial group. Ann Surg.
230:63–67. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Martin RC, Fey J, Yeung H, Borgen PI and
Cody HS III: Highest isotope count does not predict sentinel node
positivity in all breast cancer patients. Ann Surg Oncol.
8:592–597. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bourgeois P, Nogaret JM, Veys I, Hertens
D, Dagnelie J, Vanhaudenaerde C, Verdebout JM and Larsimont D: How
‘hot’ is the pathologically positive sentinel lymph node in breast
cancer patients? Nucl Med Commun. 24:513–518. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Morota S, Koizumi M, Koyama M, et al:
Radioactivity thresholds for sentinel node biopsy in breast cancer.
Eur J Surg Oncol. 32:1101–1104. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Carlson GW, Murray DR, Thourani V, Hestley
A and Cohen C: The definition of the sentinel lymph node in
melanoma based on radioactive counts. Ann Surg Oncol. 9:929–933.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kovacs AF, Dobert N, Walendzik H,
Zaplatnikov K and Landes CA: The diagnostic role of radioactivity
in sentinel nodes in oral and oropharyngeal cancer. Cancer Biother
Radiopharm. 21:535–543. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miyashiro I: What is the problem in
clinical application of sentinel node concept to gastric cancer
surgery? J Gastric Cancer. 12:7–12. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yaguchi Y, Sugasawa H, Tsujimoto H, et al:
One-step nucleic acid amplification (OSNA) for the application of
sentinel node concept in gastric cancer. Ann Surg Oncol.
18:2289–2296. 2011. View Article : Google Scholar : PubMed/NCBI
|