Introduction
Breast cancer is a leading cause of cancer-related
mortality among women in industrialized countries. Despite advances
in the technologies used for its diagnosis and treatment,
recurrence and metastasis of breast cancer remain serious clinical
issues. Therefore, there is an urgent need to identify biomarkers
or techniques to be used for the early detection of carcinogenesis
or recurrence following curative surgery using minimally invasive
tests.
MicroRNAs (miRs) are small non-coding RNAs
consisting of 20–22 nucleotides. Changes in the levels of miRs are
involved in the initiation and progression of human cancers due to
the altered translation of various target genes (1). The recent increase in miR interest is
attributed to the breakthrough discovery of their role in numerous
pathological processes, including malignant transformation
(2). In fact, miRs have been reported
as potential biomarkers of various malignancies (3,4).
MicroRNA-29b (miR-29b) regulates a number of
important genes that mediate carcinogenesis and tumor development
in breast cancer (5–8). For example, miR-29b targets a
network of pro-metastatic regulators involved in angiogenesis,
collagen remodeling and proteolysis, thereby inhibiting metastasis
(5). Furthermore, miR-29b
directly targets DNA methyltransferase 3A (DNMT3A),
DNMT3B, ten-eleven translocation 1 (TET1) and thymine
DNA glycosylase (TDG), all of which play crucial roles in
the progression and metastasis of various cancers by altering the
DNA methylation status (9–16). Although almost all the studies
investigating the numerous important roles of miR-29b in
breast cancer have been experimental studies conducted in
vitro and in vivo (5–8,16), the clinicopathological significance of
miR-29b in breast cancer cases has not been determined
clinically. The present study evaluated the importance of
miR-29b in breast cancer cases and additionally showed the
associations between miR-29b and several target genes of
miR-29b indicated in the regulation of DNA methylation
status in clinical samples.
Materials and methods
Patients
Breast cancer patients (n=94) who underwent surgical
treatment at several hospitals [National Hospital Organization
Kyushu Cancer Center (Fukuoka, Fukuoka) Kyushu University Beppu
Hospital (Beppu, Oita), Oita Prefectural Hospital (Yufu, Oita) and
Takada-Chuo Hospital (Yokohama, Kanagawa), all in Japan)] between
1990 and 1999 were enrolled in the study. Prior to sample
acquisition, each patient provided written informed consent at the
respective hospital. The study was approved by the ethics
committees of Kyushu University. Patients were excluded who had
been diagnosed with ductal carcinoma in situ. Three patients
who had distant metastasis at first diagnosis received no
neo-adjuvant chemotherapy. Post-operative adjuvant chemotherapy and
endocrine therapy were performed according to the St. Gallen
Consensus Conference guidelines (17). Among the 94 patients, 63 were estrogen
receptor (ER)-positive. The expression levels of the HER2 protein
could not be confirmed in the cases, as the measurements used for
HER2 expression were not common when the surgeries were performed.
The mean observation period ranged from 1 to 124 months (median, 54
months). Among the 94 patients, only 30, 81 and 57 patients were
examined for TET1, TDG and DNMT3A expression,
respectively, due to the deficiency of samples for quantification
of each cDNA by reverse transcription-quantitative polymerase chain
reaction (RT-qPCR).
Total RNA extraction and first-strand
cDNA synthesis
The resected tumor tissue specimens were frozen
immediately in liquid nitrogen and stored at −80°C until analysis.
The total RNA extraction from the primary tumors was performed
according to the ISOGEN-LS (Nippon Gene Co., Ltd., Tokyo, Japan)
manufacturer's instructions. The reverse transcription reactions
and first-strand cDNA synthesis were performed as described
previously (18).
RT-qPCR for miR-29b, TET1, TDG
and DNMT3A
Quantitative analysis was performed of
miR-29b- and RNU6B (internal control)-specific cDNAs
derived from total RNA extracted from resected tumors using
gene-specific primers, according to the TaqMan MicroRNA Assay
protocol (Assay IDs: 000413 for hsa-miR-29b-3p and 001093
for RNU6B; Applied Biosystems, Carslbad, California, USA).
The procedures were as described previously (18). The raw miR expression levels were
normalized to RNU6B expression for calculation of the
relative miR expression values. To determine the relative
expression levels of TET1, TDG and DNMT3A,
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as
the internal control. RT-qPCR was performed using the LightCycler®
480 system and the LightCycler® 480 Probes Master kit (Roche
Applied Science, Penzberg, Germany). The sequences of the primers
for TET1, TDG and DNMT3A were as follows:
TET1 sense, 5′-TCTGTTGTTGTGCCTCTGGA-3′ and antisense,
5′-GCCTTTAAAACTTTGGGCTTC-3′; TDG sense,
5′-ATGCAGCAGTGAACCTTGTG-3′ and antisense,
5′-GTCATCCACTGCCCATTAGG-3′; and DNMT3A sense,
5′-AAGGAGGAGCGCCAAGAG-3′ and antisense, 5′-ATCACCGCAGGGTCCTTT-3′.
The expression of DNMT3B was not detected in the
samples.
Statistical analysis
For miR-29b analysis, differences between
clinicopathological factors were analyzed using χ2 tests
for categorical variables. Disease-free survival (DFS) and overall
survival (OS) times were measured from the time of the first
surgery until the date of mortality or last follow-up. Survival
curves were determined by the Kaplan-Meier method and statistical
significance between groups was assessed using the Wilcoxon test.
Multivariate analysis was performed to assess the relative
influence of prognostic factors on OS using the Cox proportional
hazards model with a forward stepwise procedure. Statistical
analysis was performed by JMP® Pro version 9.0.2 for Mac OS (SAS
Institute Japan, Tokyo, Japan).P<0.05 was considered to indicate
a statistically significant difference.
Results
Low miR-29b expression in
primary tumor tissues is a prognostic factor for breast cancer
patients
miR-29b expression was assessed in primary
tumor tissues from 94 breast cancer patients. Patients were divided
into miR-29b high and low expression groups according to the
median value of miR-29b expression. Clinicopathological
factors were subsequently analyzed in association with
miR-29b levels. The miR-29b low expression group
exhibited a significantly larger tumor size and more advanced
clinical stages compared to the miR-29b high expression
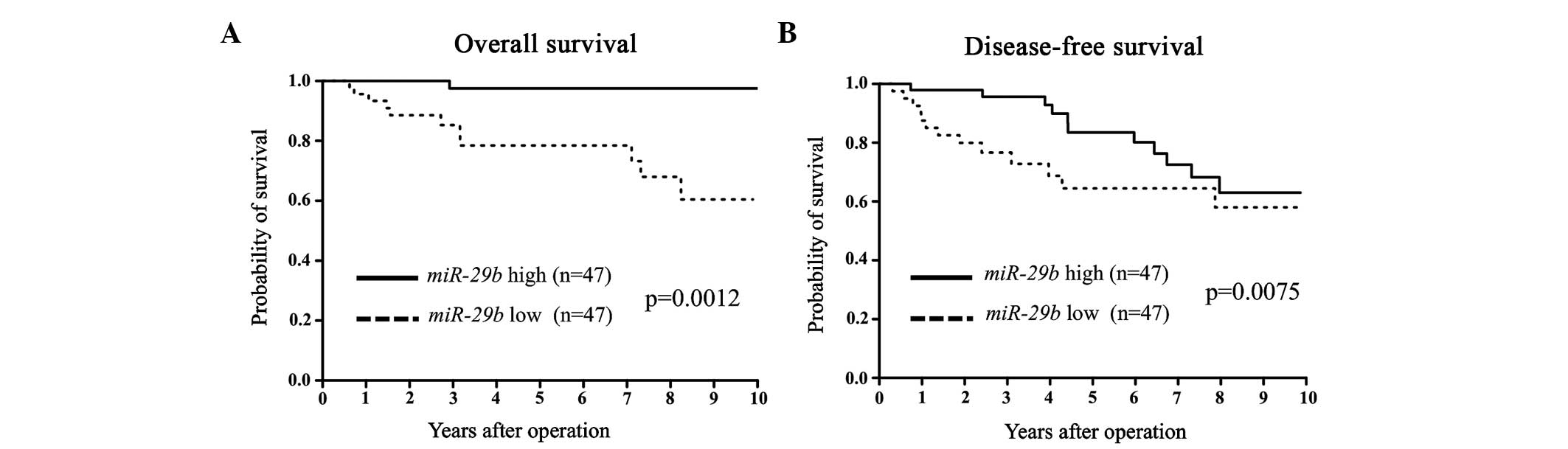
group (Table I). In terms of DFS and
OS, the miR-29b low expression group showed a significantly
poorer prognosis than that of the miR-29b high expression
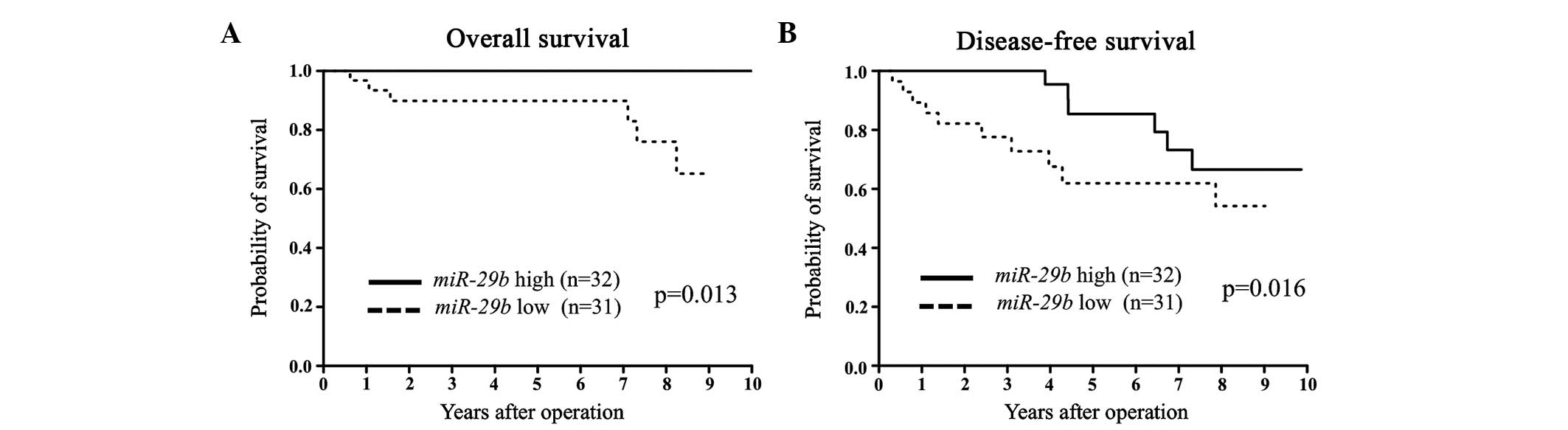
group (Fig. 1). Among the ER-positive
cases, the low miR-29b expression group had significantly
poorer DFS and OS compared to the high miR-29b expression
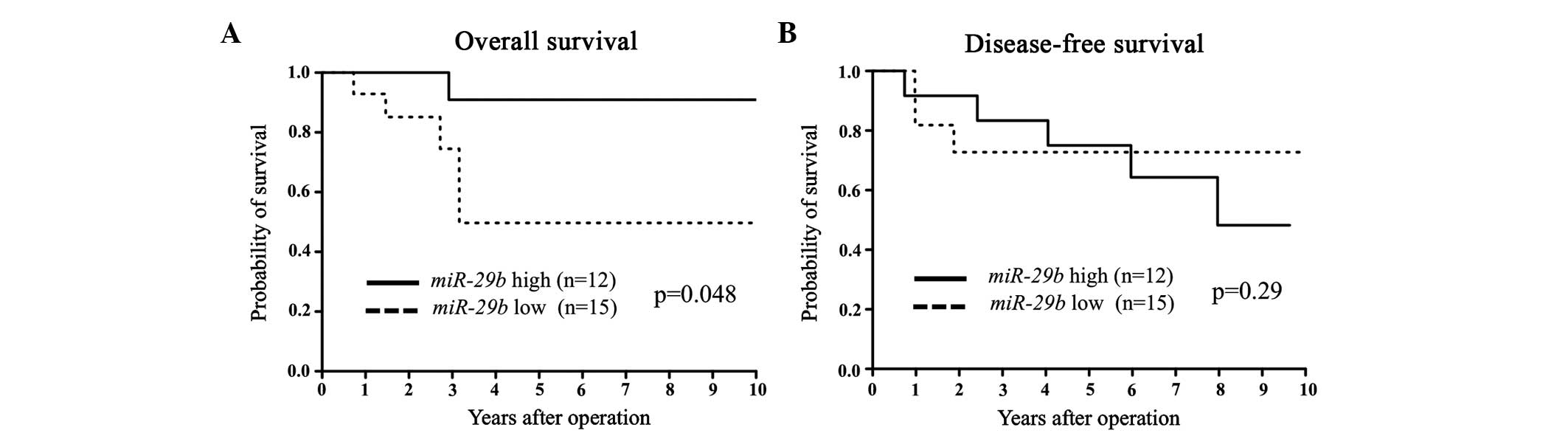
group (Fig. 2). Among the ER-negative
cases, low miR-29b expression correlated with a poorer OS
only (Fig. 3).
 | Table I.miR-29b expression and
clinicopathological factors. |
Table I.
miR-29b expression and
clinicopathological factors.
|
| miR-29b
expression |
|
|---|
|
|---|
| Factors | Low (n=47), no.
(%) | High (n=47), no.
(%) | P-value |
|---|
| Age, mean years ±
SD | 55±11 | 54±11 |
|
| ER |
|
|
|
|
Positive | 31 (66) | 32 (68) | 0.59 |
|
Negative | 15 (32) | 12 (26) |
|
| Progesterone
receptor |
|
|
|
|
Positive | 26 (55) | 31 (66) | 0.24 |
|
Negative | 18 (38) | 13 (28) |
|
| T factors |
|
|
|
| T1 | 13 (28) | 25 (53) | 0.01 |
|
T2–4 | 34 (72) | 22 (47) |
|
| Lymph node
metastasis |
|
|
|
|
Absent | 22 (47) | 27 (57) | 0.31 |
|
Present | 25 (53) | 20 (43) |
|
| Lymphatic
invasion |
|
|
|
|
Absent | 18 (38) | 16 (34) | 0.73 |
|
Present | 23 (49) | 24 (51) |
|
| Venous
invasion |
|
|
|
|
Absent | 33 (70) | 34 (72) | 0.60 |
|
Present | 7 (15) | 6 (13) |
|
| Stage |
|
|
|
| Stage I | 7 (15) | 17 (36) | 0.02 |
| Stages II–IV | 40 (85) | 30 (64) |
|
Multivariate analysis of OS showed that the low
level of miR-29b expression was an independent prognostic
predictor in all patients (Table
II).
 | Table II.Results of multivariate analysis of
clinicopathological factors for overall survival (Cox proportional
hazards model). |
Table II.
Results of multivariate analysis of
clinicopathological factors for overall survival (Cox proportional
hazards model).
|
| Multivariate
analysis |
|---|
|
|---|
| Factors | RR (95% CI) | P-value |
|---|
| T factor
(T1/2–4) | 3.14
(0.39–19.5) | 0.250 |
| Lymph node
metastasis | 1.15
(0.13–24.9) | 0.910 |
| Lymphatic
invasion | 11.4
(0.61–743) | 0.120 |
| Venous
invasion | 2.59
(0.56–12.7) | 0.220 |
| Stage
(I/II–IV) | 2.57
(0.04–211) | 0.650 |
| miR-29b
expression | 15.6
(2.33–348) | 0.003 |
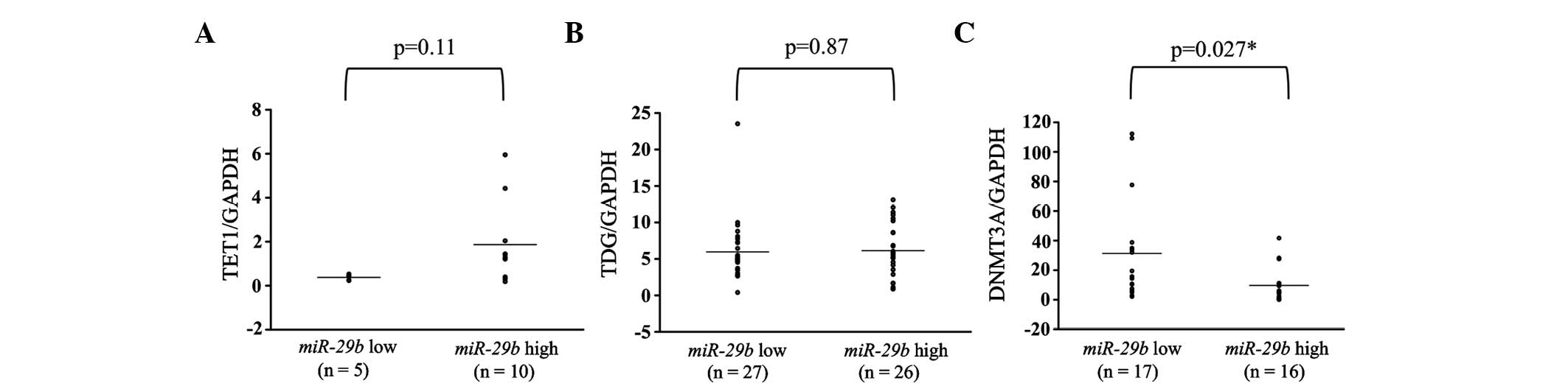
Evaluation of TET1, TDG and DNMT3A
expression levels and their comparison with miR-29b levels
in breast cancer patients
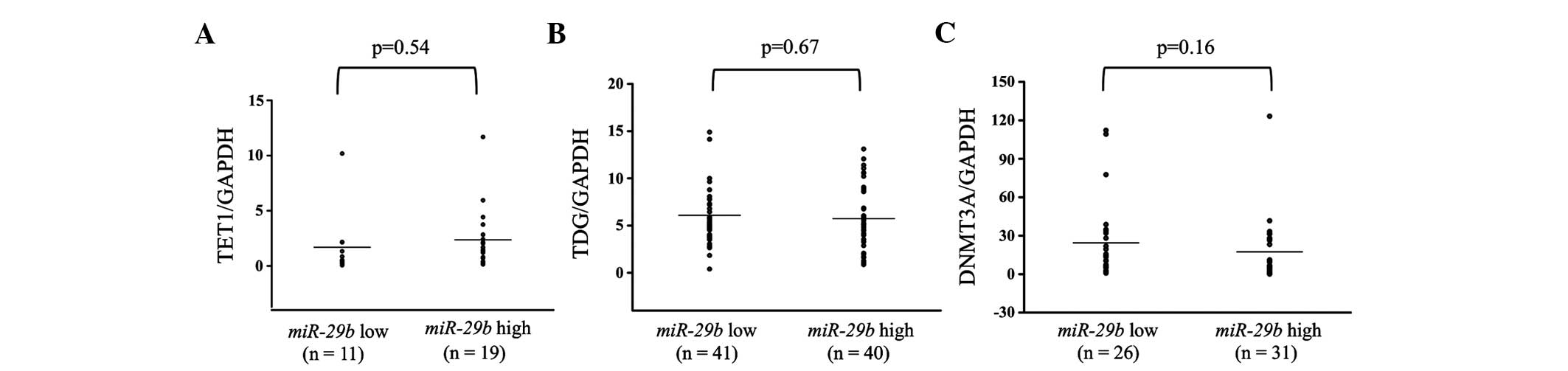
Additionally, the expression levels of TET1,
TDG and DNMT3A were examined in breast cancer primary
tumor tissues. These levels were subsequently compared between the
miR-29b high and low expression groups. There were no
significant differences in any of the patients between the
miR-29b high and low expression groups (Fig. 4). However, in analyses of the
ER-positive patients, DNMT3A showed significantly higher
expression in the miR-29b low expression compared to the
high expression group (P=0.027; Fig.
5).
Discussion
In the present study, low miR-29b expression
in primary breast tumors correlated significantly with poor DFS and
OS in breast cancer patients. This was consistent with previous
in vitro and in vivo findings that miR-29b
acts as a tumor suppressive miR (5–7). For
example, Chou et al (5) showed
that miR-29b was induced by GATA3 and inhibited metastasis
by targeting various genes (ANGPTL4, LOX, MMP
and VEGFA) involved in modifying the tumor
microenvironment.
With respect to the clinicopathological factors,
larger tumor sizes and more advanced stages were detected in the
miR-29b low expression compared to the high expression
group. This finding suggested that the suppression of
miR-29b is associated with tumor progression. To clarify how
miR-29b contributed to breast cancer progression, the study
focused on candidate target genes of miR-29b according to
TarBase 6.0 (19). Among 103 genes,
we were interested in those that regulate epigenetic status, such
as TET1, TDG and DNMT3A. The direct
interactions between miR-29b and TET1, TDG and
DNMT3A were confirmed by luciferase assays and western blot
analysis (16). Although there are
numerous pathways that regulate the levels of TET1 (13,20),
TDG (21) and DNMT3A
(22) in breast cancer, significant
inverse correlations were identified between the expression levels
of miR-29b and DNMT3A in ER-positive patients. The
overexpression of DNMT3A correlates with a poor prognosis in
numerous cancers, including breast cancer (10,23).
Starlard-Davenport et al (24)
demonstrated that transfection of pre-miR-29b into breast
cancer cell lines inhibited cell proliferation, decreased
DNMT3A and DNMT3B mRNA levels and decreased the
promoter methylation status of several tumor suppressor genes.
With respect to breast cancer subtypes, the present
results showed a significant correlation between low miR-29b
expression and poor OS, independent of the ER status. According to
the results of the multivariate analysis, miR-29b is a
powerful biomarker for predicting patient outcomes in all the
subtypes of breast cancer.
In conclusion, miR-29b expression in breast
cancer primary tumors was an independent prognostic factor for OS.
Low miR-29b expression in primary tumors may predict poor OS
and DFS in breast cancer patients. Additionally, in ER-positive
cases, a significant inverse correlation between the expression
levels of miR-29b and DNMT3A was identified.
Acknowledgements
The authors would like to thank Ms. K. Oda, Ms.
Kasagi, Ms. S. Kono and Ms. T. Kawano for their technical
assistance. The present study was supported in part by the Japan
Society for the Promotion of Science (grant no. 25830102).
References
|
1
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Iorio MV, Ferracin M, Liu CG, Veronese A,
Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M,
et al: MicroRNA gene expression deregulation in human breast
cancer. Cancer Res. 65:7065–7070. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang H, Yu J, Wang L, Ding D, Zhang L, Chu
C, Chen Q, Xu Z, Zou Q and Liu X: miR-320a is an independent
prognostic biomarker for invasive breast cancer. Oncol Lett.
8:1043–1050. 2014.PubMed/NCBI
|
|
4
|
Tejero R, Navarro A, Campayo M, Viñolas N,
Marrades RM, Cordeiro A, Ruíz-Martínez M, Santasusagna S, Molins L,
Ramirez J, et al: miR-141 and miR-200c as markers of overall
survival in early stage non-small cell lung cancer adenocarcinoma.
PLoS One. 9:e1018992014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chou J, Lin JH, Brenot A, Kim JW, Provot S
and Werb Z: GATA3 suppresses metastasis and modulates the tumour
microenvironment by regulating microRNA-29b expression. Nat Cell
Biol. 15:201–213. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang T, Liang Y, Lin Q, Liu J, Luo F, Li
X, Zhou H, Zhuang S and Zhang H: miR-29 mediates TGFβ1-induced
extracellular matrix synthesis through activation of PI3K-AKT
pathway in human lung fibroblasts. J Cell Biochem. 114:1336–1342.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park SY, Lee JH, Ha M, Nam JW and Kim VN:
miR-29 miRNAs activate p53 by targeting p85 alpha and CDC42. Nat
Struct Mol Biol. 16:23–29. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pandey M, Sultana S and Gupta KP:
Involvement of epigenetics and microRNA-29b in the urethane induced
inception and establishment of mouse lung tumors. Exp Mol Pathol.
96:61–70. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cao XY, Ma HX, Shang YH, Jin MS, Kong F,
Jia ZF, Cao DH, Wang YP, Suo J and Jiang J: DNA methyltransferase3a
expression is an independent poor prognostic indicator in gastric
cancer. World J Gastroenterol. 20:8201–8208. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ibrahem L, Mahfouz R, Elhelw L, Abdsalam
EM and Soliman R: Prognostic significance of DNMT3A mutations in
patients with acute myeloid leukemia. Blood Cells Mol Dis.
54:84–89. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Niederwieser C, Kohlschmidt J, Volinia S,
Whitman SP, Metzeler KH, Eisfeld AK, Maharry K, Yan P, Frankhouser
D, Becker H, et al: Prognostic and biologic significance of DNMT3B
expression in older patients with cytogenetically normal primary
acute myeloid leukemia. Leukemia. 29:567–575. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hsu CH, Peng KL, Kang ML, Chen YR, Yang
YC, Tsai CH, Chu CS, Jeng YM, Chen YT, Lin FM, et al: TET1
suppresses cancer invasion by activating the tissue inhibitors of
metalloproteinases. Cell Reports. 2:568–579. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun M, Song CX, Huang H, Frankenberger CA,
Sankarasharma D, Gomes S, Chen P, Chen J, Chada KK, He C, et al:
HMGA2/TET1/HOXA9 signaling pathway regulates breast cancer growth
and metastasis. Proc Natl Acad Sci USA. 110:9920–9925. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu HG, Zhan W, Yan L, Qin RY, Yan YP, Yang
ZJ, Liu GC, Li GQ, Wang HF, Li XL, et al: TET1 partially mediates
HDAC inhibitor-induced suppression of breast cancer invasion. Mol
Med Rep. 10:2595–2600. 2014.PubMed/NCBI
|
|
15
|
Xu X, Yu T, Shi J, Chen X, Zhang W, Lin T,
Liu Z, Wang Y, Zeng Z, Wang C, et al: Thymine DNA glycosylase is a
positive regulator of Wnt signaling in colorectal cancer. J Biol
Chem. 289:8881–8890. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Morita S, Horii T, Kimura M, Ochiya T,
Tajima S and Hatada I: miR-29 represses the activities of DNA
methyltransferases and DNA demethylases. Int J Mol Sci.
14:14647–14658. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Goldhirsch A, Wood WC, Senn HJ, Glick JH
and Gelber RD: International Consensus Panel on the Treatment of
Primary Breast Cancer: Fifth International Conference on Adjuvant
Therapy of Breast Cancer, St Gallen, March 1995. Eur J Cancer.
31A:1754–1759. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ota D, Mimori K, Yokobori T, Iwatsuki M,
Kataoka A, Masuda N, Ishii H, Ohno S and Mori M: Identification of
recurrence-related microRNAs in the bone marrow of breast cancer
patients. Int J Oncol. 38:955–962. 2011.PubMed/NCBI
|
|
19
|
Vergoulis T, Vlachos IS, Alexiou P,
Georgakilas G, Maragkakis M, Reczko M, Gerangelos S, Koziris N,
Dalamagas T and Hatzigeorgiou AG: TarBase 6.0: Capturing the
exponential growth of miRNA targets with experimental support.
Nucleic Acids Res. 40:(D1). D222–D229. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Song SJ, Poliseno L, Song MS, Ala U,
Webster K, Ng C, Beringer G, Brikbak NJ, Yuan X, Cantley LC, et al:
MicroRNA-antagonism regulates breast cancer stemness and metastasis
via TET-family-dependent chromatin remodeling. Cell. 154:311–324.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
da Costa NM, Hautefeuille A, Cros MP,
Melendez ME, Waters T, Swann P, Hainaut P and Pinto LF:
Transcriptional regulation of thymine DNA glycosylase (TDG) by the
tumor suppressor protein p53. Cell Cycle. 11:4570–4578. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ng EK, Li R, Shin VY, Siu JM, Ma ES and
Kwong A: MicroRNA-143 is downregulated in breast cancer and
regulates DNA methyltransferases 3A in breast cancer cells. Tumour
Biol. 35:2591–2598. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu Z, Xiao Q, Zhao L, Ren J, Bai X, Sun M,
Wu H, Liu X, Song Z, Yan Y, et al: DNA methyltransferase 1/3a
overexpression in sporadic breast cancer is associated with reduced
expression of estrogen receptor-alpha/breast cancer susceptibility
gene 1 and poor prognosis. Mol Carcinog. Jan 25–2014.(Epub ahead of
print). View Article : Google Scholar
|
|
24
|
Starlard-Davenport A, Kutanzi K, Tryndyak
V, Word B and Lyn-Cook B: Restoration of the methylation status of
hypermethylated gene promoters by microRNA-29b in human breast
cancer: A novel epigenetic therapeutic approach. J Carcinog.
12:152013. View Article : Google Scholar : PubMed/NCBI
|