Introduction
Epidermal growth factor receptor (EGFR) gene
mutation is an important predictor of the response to EGFR-tyrosine
kinase inhibitor (EGFR-TKI) treatment. Gefitinib treatment yields a
longer progression-free survival (PFS) rate than cytotoxic drug
therapy in patients with non-small cell lung cancer (NSCLC)
harboring EGFR gene mutations, including exon 19 deletion
and exon 21 L858R (1–3). However, an important clinical problem is
that in the majority of cases, the tumor cells soon acquire
resistance to gefitinib.
Several mechanisms of acquired resistance to
gefitinib have been proposed. Hepatocyte growth factor has been
shown to induce resistance to gefitinib of lung adenocarcinomas
harboring EGFR gene mutations (4). An investigation of 37 cases with
acquired resistance to gefitinib revealed the presence of the
EGFR T790 mutation, MET amplification, transition from NSCLC
to small cell lung cancer (SCLC), or PIK3CA mutation in
these cases (5).
Blood levels of pro-gastrin releasing peptide
(pro-GRP) and neuron-specific enolase (NSE) have been reported to
be frequently elevated in patients with SCLC (6), and their measurement has been utilized
for diagnosis or evaluation of the treatment outcomes in these
patients. Furthermore, elevation of the pretreatment plasma NSE
level has also been associated with poor outcomes in patients with
NSCLC (7–11).
We hypothesized that the blood level of pro-GRP
and/or NSE may be associated with the clinical course following
initiation of gefitinib therapy in patients with NSCLC harboring
EGFR gene mutations. To endorse its validity, the present
retrospective study was conducted.
Patients and methods
Patient selection
The medical records of patients with NSCLC diagnosed
between 2004 and 2012 were reviewed. The inclusion criteria were as
follows: i) Patients with cytologically or histologically confirmed
NSCLC harboring activating EGFR gene mutations and ii)
patients treated with gefitinib. Patients who had received another
EGFR-TKI prior to the initiation of treatment with gefitinib were
excluded. The study was approved by the Ethics Committee of the
University of Toyama (Toyama, Japan).
Analysis for EGFR gene mutations,
immunohistochemistry and clinical information
The presence/absence of EGFR gene mutations
was analyzed by the PCR-invader assay (BML, Inc., Tokyo, Japan).
Plasma NSE and serum pro-GRP were measured by a commercial
laboratory (SRL Inc., Tokyo, Japan). Plasma NSE was measured by a
radioimmunoassay method before December 2013 and by an
electrochemiluminescence method from December 2013. Therefore, the
values measured by radioimmunoassay were revised using the
following equation: y = 1.060x + 0.665 (y, value measured by the
electrochemiluminescence method; x, value measured by the
radioimmunoassay method), developed by SRL, Inc. (http://www.srl.info/srlinfo/srlnews/2011/pdf/2011-24.pdf).
The serum pro-GRP level was measured by a chemiluminescent enzyme
immunoassay method.
From the medical records, the clinical information
regarding the patients was reviewed, including the age, gender,
performance status (PS) and disease stage. Stage was classified as
postoperative recurrence and as stage I–IV according to the
tumor-node-metastasis classification. Determination of disease
progression was based on computed tomography, according to the
Response Evaluation Criteria in Solid Tumors, version 1.1.
Immunohistochemical (IHC) staining of the tumor
specimens was performed for NSE and cluster of differentiation 56
(CD56). NSE staining was performed using the anti-rabbit NSE
polyclonal antibody (rabbit polyclonal, prediluted; Nichirei
Biosciences, Inc., Tokyo, Japan) and CD56 staining used the
anti-mouse monoclonal antibody (Clone 1B6, prediluted; Nichirei
Biosciences, Inc.). Immunoperoxidase reactions were performed using
the Ventana BenchMark GX automated immunostainer (Ventana Medical
System, Tucson, AZ, USA), in accordance with the manufacturer's
instructions. The pathological diagnoses and results of assessment
of the tumor immunoreactivity were reviewed by two investigators
and reported by consensus.
Statistical analysis
Survival curves were drawn using the Kaplan-Meier
method to analyze the PFS and overall survival (OS) rate of the
patients. The PFS was calculated as the time from the initiation of
treatment with gefitinib to the date of mortality or detection of
PD and censored at the date of the last visit of the patients not
confirmed to have PD. The OS was calculated from the initiation of
treatment with gefitinib to the date of mortality and censored at
the date of the last visit of the patients who had not succumbed.
The PFS and OS were compared by the log-rank test. Patients were
divided based on the median tumor marker levels. Independent
associations were analyzed using a Cox proportional hazards
regression model adjusted for age, gender, PS, EGFR status and the
disease stage. Statistical analysis was performed using the
statistical package JMP 10.0.2 (SAS Institute, Cary, NC, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patient characteristics
A total of 554 patients were diagnosed as having
NSCLC between 2004 and 2012 at the First Department of Internal
Medicine, University of Toyama. Of these, 74 patients were treated
with gefitinib, of whom 41 with activating EGFR gene
mutations were included in this study.
Table I shows the
patient characteristics. Seventeen patients (41.5%) were male and
19 (46.3%) had a history of smoking. The histological diagnosis was
adenocarcinoma in 38 patients (92.7%). In regard to the EGFR
gene mutation, exon 19 deletion was detected in 23 (56.1%) tumors,
exon 21 L858R point mutation in 15 (36.6%) cases and exon 18 point
mutation in 3 (7.3%) patients. Of the patients, 32 (78.0%) were
classified as having stage IIIB or IV disease and 9 (22.0%) were
classified as having postoperative recurrence. Gefitinib was
administered as the first-line therapy in 34 (82.9%) patients and
as second-line therapy in seven (17.1%). The median (interquartile
range) serum value of pro-GRP in 31 patients was 31.4 (25.8–44.8)
pg/ml and the median (interquartile range) plasma value of NSE in
the 22 patients was 12.1 (9.8–16.0) ng/ml. The patients were
divided into three groups according to the median levels of the
tumor markers, as follows: i) High level group, comprising those
with high serum levels of the tumor markers, ii) low level group,
comprising those with low serum levels of the tumor markers and
iii) an ‘unknown’ group, comprising patients whose tumor marker
levels were unknown.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristics | Values |
|---|
| Patients, n | 41 |
| Age, median years
(interquartile range) | 68 (64–79.5) |
| ≥70, n
(%) | 18 (43.9) |
| Gender, n (%) |
|
| Male | 17 (41.5) |
|
Female | 24 (58.5) |
| Histology, n (%) |
|
|
Adenocarcinoma | 38 (92.7) |
|
Non-adenocarcinoma | 3 (7.3) |
| EGFR status, n
(%) |
|
| Major mutation |
|
| Exon 19
del | 23 (56.1) |
| Exon 21
L858R | 15 (36.6) |
| Minor mutation |
|
| Exon 18
point mutation | 3 (7.3) |
| PS, n (%) |
|
| 0–1 | 28 (68.3) |
| ≥2 | 13 (31.7) |
| Smoking status, n
(%) |
|
| Yes | 19 (46.3) |
| No | 22 (53.7) |
| Stage, n (%) |
|
|
IIIB/IV | 32 (78.0) |
|
Postoperative recurrence | 9 (22.0) |
| Prior regimen, n
(%) |
|
| 0 | 34 (83.0) |
| 1 | 7 (17.0) |
Survival rates
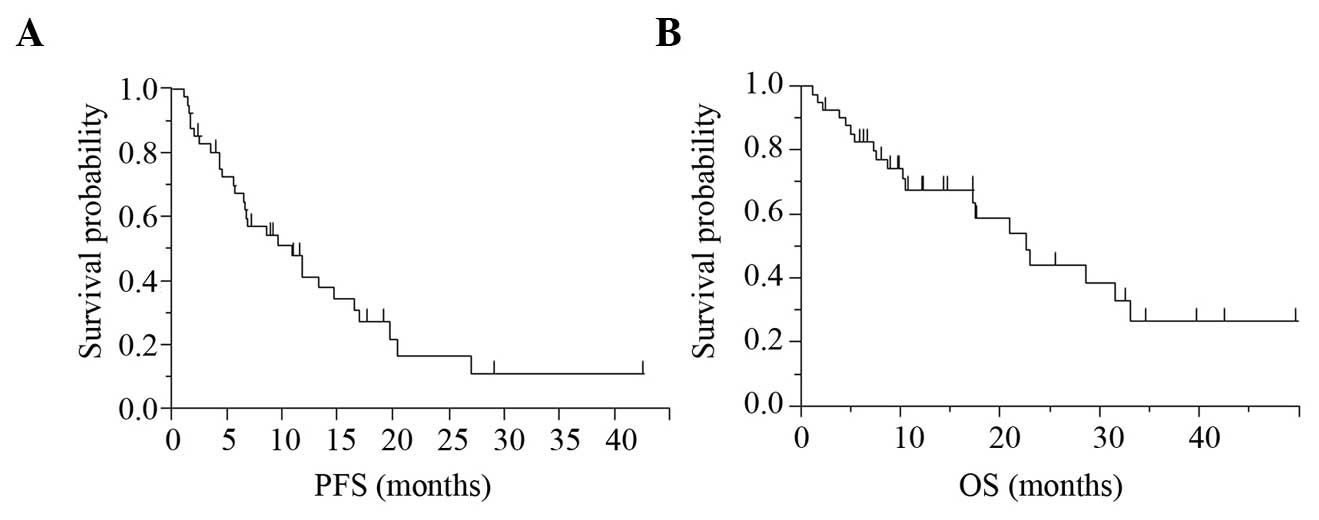
The PFS and OS in the 41 patients were 9.6 and 22.6
months, respectively (Fig. 1).
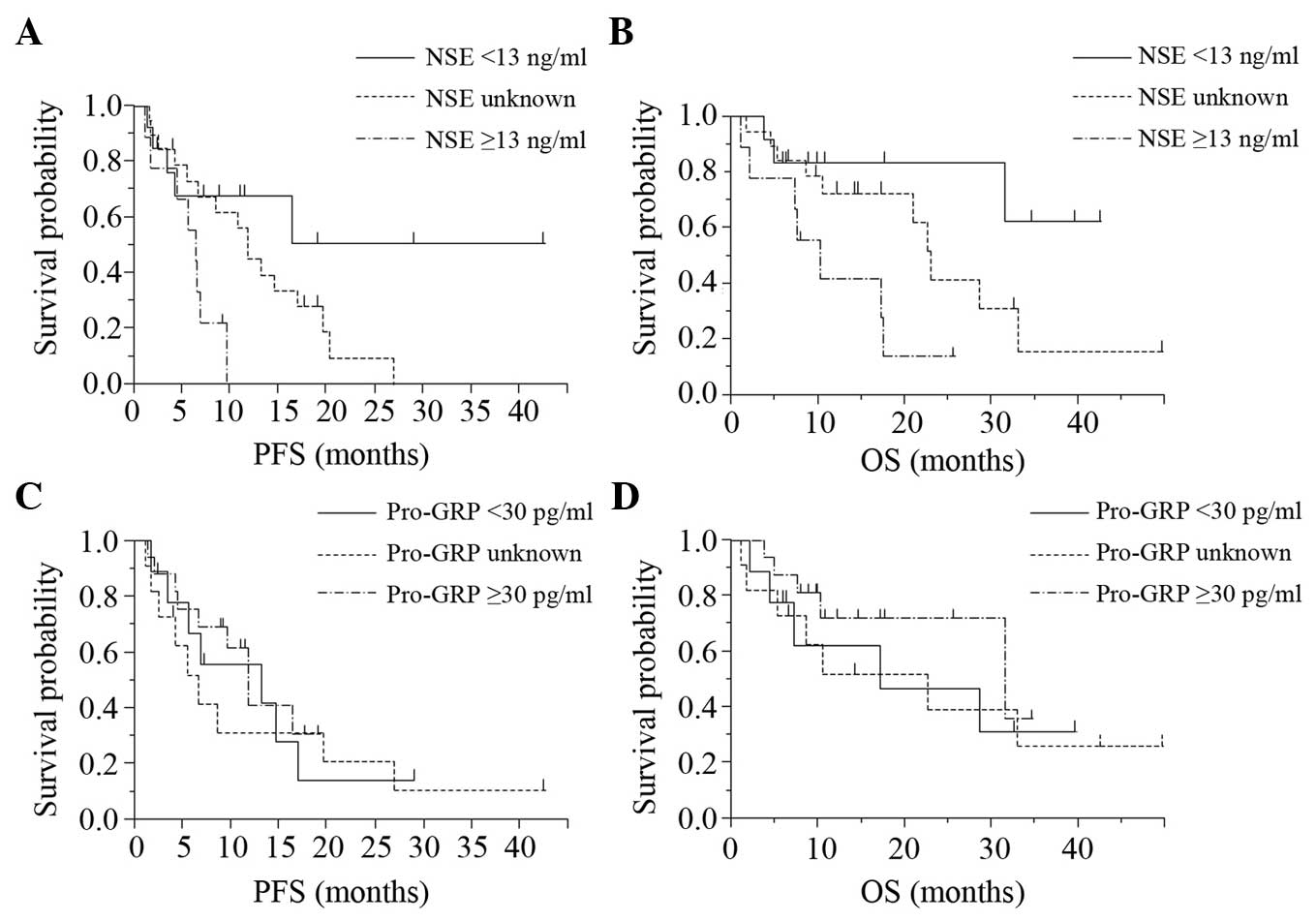
Patients with higher NSE levels (high level group) showed shorter
PFS (P=0.013) and OS (P=0.014) values according to the log-rank
test, than those of the patients with lower NSE levels (low level
group) and the group in which the NSE levels were unknown (‘unknown
group), whereas no association was observed between the serum
pro-GRP level and the PFS or OS (Fig.
2). Table II shows the results
of analysis using the Cox proportional hazards regression model.
This analysis identified pretreatment NSE as being significantly
associated with the PFS (P=0.021) and OS (P=0.0024), independent of
the age, gender, EGFR status, PS or disease stage.
 | Table II.Analysis using a Cox proportional
hazards regression model for assessing the association between
plasma NSE and survival rate. |
Table II.
Analysis using a Cox proportional
hazards regression model for assessing the association between
plasma NSE and survival rate.
|
| PFS | OS |
|---|
|
|
|---|
| Characteristics | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, years |
|
|
|
|
|
|
| ≥70 | 0.41 | 0.15–1.10 | 0.079 | 1.25 | 0.39–3.87 | 0.700 |
|
<70 | Reference |
|
| Reference |
|
|
| Gender |
|
|
|
|
|
|
| Male | 1.84 | 0.71–4.83 | 0.209 | 1.37 | 0.44–4.29 | 0.583 |
|
Female | Reference |
|
| Reference |
|
|
| EGFR status |
|
|
|
|
|
|
|
Major | 0.89 | 0.18–5.23 | 0.891 | 0.70 | 0.09–6.71 | 0.740 |
|
Minor | Reference |
|
| Reference |
|
|
| PS |
|
|
|
|
|
|
| ≥2 | 3.23 | 1.17–9.07 | 0.024 | 8.74 | 2.35–35.7 | 0.0013 |
|
0–1 | Reference |
|
| Reference |
|
|
| Stage |
|
|
|
|
|
|
|
Recurrence | 0.47 | 0.10–1.81 | 0.285 | 0.30 | 0.02–1.94 | 0.230 |
|
IIIB/IV | Reference |
|
| Reference |
|
|
| NSE |
|
|
|
|
|
|
|
≥13 | 4.69 | 1.27–19.12 | 0.021 | 10.31 | 2.26–59.2 | 0.0024 |
|
Unknown | 2.72 | 0.87–10.14 | 0.088 | 3.08 | 0.80–15.33 | 0.103 |
|
<13 | Reference |
|
| Reference |
|
|
IHC staining
IHC staining was performed in 27 cases. All 27
showed positive staining for NSE and negative staining for CD56.
Thus, there were no significant associations between the plasma NSE
levels and the findings of IHC.
Discussion
In the present study, the univariate and
multivariate analyses revealed the existence of an association
between the pretreatment plasma NSE level and patient survival
rate. However, no association was observed between the serum
pro-GRP level and survival rate. To investigate the neuroendocrine
properties of the tumor cells, IHC analysis was conducted.
NSE is an isozyme of the intracytoplasmic enzyme
enolase, which was first identified in an extract of mouse brain
tissue (12) and later shown to be
elevated in neuroendocrine tumors, including SCLC. By contrast, in
patients with NSCLC, the plasma NSE level has been shown to be
associated with the patient survival rate. This association was
observed in patients with NSCLC, regardless of whether they were
treated by surgical resection, radiation therapy or chemotherapy
(7–11). Furthermore, recently it has been
reported that a higher pretreatment NSE level was associated with a
shorter survival duration following the initiation of gefitinib in
non-selective patients with NSCLC (13). In the present study the association
between the plasma NSE level and the prognosis was examined in a
specific patient population and confirmed the existence of the same
association in patients with NSCLC harboring EGFR mutations and
receiving gefitinib treatment. This association is noteworthy, in
view of transition to SCLC being reported as one of the mechanisms
of acquisition of resistance to gefitinib (5).
According to previous studies, plasma NSE elevation
in NSCLC patients may reflect the heterogeneity of NSCLC and a
neuroendocrine nature of the tumor. However, consistent with
previous studies (14,15), no association was found between the
plasma NSE level and the presence of neuroendocrine markers,
including NSE and CD56, as determined by IHC. Although the reason
for this inconsistency is unclear, it could be attributable to the
difficulty in quantitative measurements by IHC. Indeed, although
neuroendocrine markers have been detected in a considerable
proportion of NSCLCs by IHC (16–25),
positive (18–22) and negative findings (23–25) have
been reported in terms of the association between the IHC
characteristics and the prognosis. Another explanation is that NSE
expression is not identical to NSE secretion into the blood
circulation, as NSE expression has also been detected by
immunohistochemistry in various normal human tissues other than
nervous and neuroendocrine tissues, including type II alveolar
epithelial cells (26). It is
possible that in all the patients in the present study, the tumor
expressed NSE, as the tumor was an adenocarcinoma in the majority
of patients. However, the mechanism of NSE secretion remains
unclear.
Pro-GRP is superior in sensitivity for the diagnosis
of SCLC, but its association with the prognosis is weak (27,28). The
present finding of a lack of any association between the serum
pro-GRP level and survival rate is in line with previous
studies.
There were several limitations of the present study.
Pretreatment plasma NSE was not measured in certain patients and
the study sample was very small in size. Although we conducted
multivariate analyses to adjust for confounding factors, it may be
difficult to exclude the influences of factors other than the
plasma NSE level on the patient survival rate. These findings
should be interpreted with caution and further study in a larger
study population is necessary. Second, we cannot speculate on the
mechanism of elevation of the plasma NSE, as the IHC analysis
revealed no significant findings. Third, although the pretreatment
plasma NSE level is likely to be a prognostic factor in patients
with NSCLC based on previous studies, we cannot conclude from our
observations whether it may be a predictive factor in patients with
NSCLC receiving gefitinib treatment.
In conclusion, the results of the present study
indicate the existence of an association between the pretreatment
NSE level and survival rate in NSCLC patients receiving treatment
with gefitinib. In the practical treatment of NSCLC, gefitinib
therapy is one of the important treatment options for patients with
NSCLC harboring EGFR gene mutations, regardless of the
plasma NSE level. However, these findings suggest that measurement
of the pretreatment plasma NSE level can contribute to follow-up
planning in patients receiving gefitinib treatment.
References
|
1
|
Mok TS, Wu YL, Thongprasert S, Yang CH,
Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et
al: Gefitinib or carboplatin-paclitaxel in pulmonary
adenocarcinoma. N Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al: North-East Japan Study Group: Gefitinib or chemotherapy for
non-small-cell lung cancer with mutated EGFR. N Engl J Med.
362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mitsudomi T, Morita S, Yatabe Y, Negoro S,
Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, et
al: West Japan Oncology Group: Gefitinib versus cisplatin plus
docetaxel in patients with non-small-cell lung cancer harbouring
mutations of the epidermal growth factor receptor (WJTOG3405): An
open label, randomised phase 3 trial. Lancet Oncol. 11:121–128.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yano S, Wang W, Li Q, Matsumoto K,
Sakurama H, Nakamura T, Ogino H, Kakiuchi S, Hanibuchi M, Nishioka
Y, et al: Hepatocyte growth factor induces gefitinib resistance of
lung adenocarcinoma with epidermal growth factor
receptor-activating mutations. Cancer Res. 68:9479–9487. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sequist LV, Waltman BA, Dias-Santagata D,
Digumarthy S, Turke AB, Fidias P, Bergethon K, Shaw AT, Gettinger
S, Cosper AK, et al: Genotypic and histological evolution of lung
cancers acquiring resistance to EGFR inhibitors. Sci Transl Med.
3:75ra262011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Molina R, Auge JM, Filella X, Viñolas N,
Alicarte J, Domingo JM and Ballesta AM: Pro-gastrin-releasing
peptide (proGRP) in patients with benign and malignant diseases:
Comparison with CEA, SCC, CYFRA 21-1 and NSE in patients with lung
cancer. Anticancer Res. 25:1773–1778. 2005.PubMed/NCBI
|
|
7
|
van Zandwijk N, Jassem E, Bonfrer JM, Mooi
WJ and van Tinteren H: Serum neuron-specific enolase and lactate
dehydrogenase as predictors of response to chemotherapy and
survival in non-small cell lung cancer. Semin Oncol. 19:(Suppl 2).
37–43. 1992.PubMed/NCBI
|
|
8
|
Diez M, Torres A, Ortega L, Maestro M,
Hernando F, Gomez A, Picardo A, Granell J and Balibrea JL: Value of
serum neuron-specific enolase in nonsmall cell lung cancer.
Oncology. 50:127–131. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pujol JL, Boher JM, Grenier J and Quantin
X: Cyfra 21-1, neuron specific enolase and prognosis of non-small
cell lung cancer: Prospective study in 621 patients. Lung Cancer.
31:221–231. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ferrigno D, Buccheri G and Giordano C:
Neuron-specific enolase is an effective tumour marker in non-small
cell lung cancer (NSCLC). Lung Cancer. 41:311–320. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Viñolas N, Molina R, Galán MC, Casas F,
Callejas MA, Filella X, Grau JJ, Ballesta AM and Estape J: Tumor
markers in response monitoring and prognosis of non-small cell lung
cancer: Preliminary report. Anticancer Res. 18:631–634.
1998.PubMed/NCBI
|
|
12
|
Rider CC and Taylor CB: Evidence for a new
form of enolase in rat brain. Biochem Biophys Res Commun.
66:814–820. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fiala O, Pesek M, Finek J, Benesova L,
Minarik M, Bortlicek Z and Topolcan O: The role of neuron-specific
enolase (NSE) and thymidine kinase (TK) levels in prediction of
efficacy of EGFR-TKIs in patients with advanced-stage NSCLC.
Anticancer Res. 34:5193–5198. 2014.PubMed/NCBI
|
|
14
|
Slodkowska J, Zych J, Szturmowicz M,
Demkow U, Rowinska-Zakrzewska E and Roszkowski-Sliz K:
Neuroendocrine phenotype of non-small cell lung carcinoma:
Immunohistological evaluation and biochemical study. Int J Biol
Markers. 20:217–226. 2005.PubMed/NCBI
|
|
15
|
Sørhaug S, Steinshamn S, Haaverstad R,
Nordrum IS, Martinsen TC and Waldum HL: Expression of
neuroendocrine markers in non-small cell lung cancer. APMIS.
115:152–163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Broers JL, Rot MK, Oostendorp T, Huysmans
A, Wagenaar SS, Wiersma-van Tilburg AJ, Vooijs GP and Ramaekers FC:
Immunocytochemical detection of human lung cancer heterogeneity
using antibodies to epithelial, neuronal, and neuroendocrine
antigens. Cancer Res. 47:3225–3234. 1987.PubMed/NCBI
|
|
17
|
Pujol JL, Simony J, Laurent JC, Richer G,
Mary H, Bousquet J, Godard P and Michel FB: Phenotypic
heterogeneity studied by immunohistochemistry and aneuploidy in
non-small cell lung cancers. Cancer Res. 49:2797–2802.
1989.PubMed/NCBI
|
|
18
|
Kibbelaar RE, Moolenaar KE, Michalides RJ,
Van Bodegom PC, Vanderschueren RG, Wagenaar SS, Dingemans KP,
Bitter-Suermann D, Dalesio O, Van Zandwijk N, et al: Neural cell
adhesion molecule expression, neuroendocrine differentiation and
prognosis in lung carcinoma. Eur J Cancer. 27:431–435. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Graziano SL, Mazid R, Newman N, Tatum A,
Oler A, Mortimer JA, Gullo JJ, DiFino SM and Scalzo AJ: The use of
neuroendocrine immunoperoxidase markers to predict chemotherapy
response in patients with non-small-cell lung cancer. J Clin Oncol.
7:1398–1406. 1989.PubMed/NCBI
|
|
20
|
Pujol JL, Simony J, Demoly P, Charpentier
R, Laurent JC, Daurès JP, Lehmann M, Guyot V, Godard P and Michel
FB: Neural cell adhesion molecule and prognosis of surgically
resected lung cancer. Am Rev Respir Dis. 148:1071–1075. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hiroshima K, Iyoda A, Shibuya K, Toyozaki
T, Haga Y, Fujisawa T and Ohwada H: Prognostic significance of
neuroendocrine differentiation in adenocarcinoma of the lung. Ann
Thorac Surg. 73:1732–1735. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pelosi G, Pasini F, Sonzogni A, Maffini F,
Maisonneuve P, Iannucci A, Terzi A, De Manzoni G, Bresaola E and
Viale G: Prognostic implications of neuroendocrine differentiation
and hormone production in patients with Stage I nonsmall cell lung
carcinoma. Cancer. 97:2487–2497. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Howe MC, Chapman A, Kerr K, Dougal M,
Anderson H and Hasleton PS: Neuroendocrine differentiation in
non-small cell lung cancer and its relation to prognosis and
therapy. Histopathology. 46:195–201. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Linnoila RI, Piantadosi S and Ruckdeschel
JC: Impact of neuroendocrine differentiation in non-small cell lung
cancer. The LCSG experience. Chest. 106:(Suppl 6). 367S–371S. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hage R, Elbers HR, Brutel de la Rivière A
and van den Bosch JM: Neural cell adhesion molecule expression:
Prognosis in 889 patients with resected non-small cell lung cancer.
Chest. 114:1316–1320. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Haimoto H, Takahashi Y, Koshikawa T,
Nagura H and Kato K: Immunohistochemical localization of
gamma-enolase in normal human tissues other than nervous and
neuroendocrine tissues. Lab Invest. 52:257–263. 1985.PubMed/NCBI
|
|
27
|
Shibayama T, Ueoka H, Nishii K, Kiura K,
Tabata M, Miyatake K, Kitajima T and Harada M: Complementary roles
of pro-gastrin-releasing peptide (ProGRP) and neuron specific
enolase (NSE) in diagnosis and prognosis of small-cell lung cancer
(SCLC). Lung Cancer. 32:61–69. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nisman B, Biran H, Ramu N, Heching N,
Barak V and Peretz T: The diagnostic and prognostic value of ProGRP
in lung cancer. Anticancer Res. 29:4827–4832. 2009.PubMed/NCBI
|