Introduction
The epidermal growth factor receptor (EGFR) is
abundantly expressed in a broad spectrum of carcinomas, including
colorectal adenocarcinoma. EGFR represents one of most promising
molecules for targeted therapy of carcinomas. Cetuximab is a
chimeric IgG1 monoclonal antibody that competitively binds to the
EGFR extracellular domain with a higher affinity compared with
endogenous ligands. Cetuximab mediates antibody-dependent
cytotoxicity against human tumor cells. Several in vitro,
in vivo and clinical studies have demonstrated that the
efficacy of anti-EGFR monoclonal antibodies depends on the lack of
v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS)
mutations. KRAS, a small G protein, is an essential component of
the Ras/Raf/mitogen-activated protein kinase pathway, which may
acquire oncogenic mutations that produce a constitutively active
protein. Approximately 30–50% of patients with metastatic
colorectal cancer (mCRC) have a KRAS mutation, which is a major
predictive biomarker of resistance to anti-EGFR therapies. The most
frequent mutations occur in codons 12 (G12D, 13%; and G12V, 9%) or
13 (G13D, 8%) (1). Previous studies
have suggested that patients who have tumors with KRAS G13D
mutations are likely to respond better to cetuximab compared with
those who have other KRAS mutations (2). In vitro as well as in vivo
treatment with cetuximab significantly inhibited the growth of
tumors formed by wild-type or KRAS p.G13D mutant cells (3). By contrast, the growth of tumors formed
by KRAS p.G12V cells was not significantly affected by cetuximab
treatment (3).
As regards the level of activation of EGFR and its
downstream effectors, in the presence of cetuximab, p.G12V-mutated
cells were seemingly still able to activate the extracellular
signal-regulated kinase pathway, whereas p.G13D-mutated cells were
not. Of note, the levels of activated KRAS were similar in p.G12V-
and p.G13D-mutated cells. Overall, these results indicate that the
KRAS p.G12V and p.G13D mutations differently affect the response to
cetuximab in preclinical models (3).
Similarly, KRAS codon 12 was reported to be a poor-prognosis gene
in the PETTAC8 clinical trial, whereas codon 13 is apparently
associated with a less poor prognosis (4). Anti-EGFR inhibitors may thus constitute
new treatment options for patients with tumors harboring KRAS G13D
mutations. The purpose of this retrospective analysis was to assess
the prevalence of KRAS p.G13D mutations and evaluate the
effectiveness of treatment with cetuximab in Japanese mCRC patients
who harbor KRAS p.G13D or other KRAS mutations.
Patients and methods
Patients
The clinical records of 98 mCRC patients who had
genotyped KRAS mutations and were treated between August, 2004 and
January, 2011 in four hospitals located in Tokyo and Kyushu Island,
were reviewed to study the subtypes of KRAS mutations and patient
characteristics. Of the 98 patients, 31 received regimens combining
cetuximab with irinotecan, and their KRAS mutation status was
evaluated by Luminex assays. In the combined regimen, cetuximab was
administered at an initial dose of 400 mg/m2 followed by
weekly intravenous infusions of 250 mg/m2, whereas
irinotecan was administered once every 2 weeks as an intravenous
infusion of 150 mg/m2, in accordance with the package
insert for irinotecan in Japan. One treatment cycle in our study
comprised cetuximab administered twice weekly plus irinotecan
administered once every 2 weeks by intravenous infusion. Patients
who met all of the following inclusion criteria were
retrospectively included in analyses: ⅰ) histologically confirmed
diagnosis of adenocarcinoma of the colon or rectum with evaluated
KRAS status and ⅱ) presence of unresectable metastatic disease.
This study was conducted following approval of the
Science Review Board of the Cancer Institute Hospital (Tokyo,
Japan).
KRAS testing
KRAS status was evaluated by Luminex assays. KRAS
testing by Luminex assay and direct sequencing was performed by
clinical testing companies (MBL, Nagoya, Japan; and SRL, Tokyo,
Japan). The sensitivity of KRAS testing by Luminex has been
reported to be 10% (5).
Statistical analysis
Progression-free survival (PFS) was defined as the
time from the first day of treatment with cetuximab plus
irinotecan, to either the first objective evidence of disease
progression or death from any cause. Overall survival (OS) was
defined as the time from the first day of treatment with cetuximab
plus irinotecan to death from any cause. The principal
investigators at our institution re-evaluated PFS and OS for all
the patients. Fisher's exact tests were used to compare patient
characteristics. OS and PFS were estimated using the Kaplan-Meier
method, and differences among groups categorized according to KRAS
status were compared using the log-rank test. All the reported
P-values were derived by two-sided tests, with P<0.05 considered
to indicate statistically significant differences. The prognostic
factors included age (<65 or ≥65 years), gender (male or
female), performance status (PS; 0 or 1), primary site (colon,
rectum and multiple), pathology (well- or moderately vs. poorly
differentiated), and KRAS mutations (G13D or others). Prognostic
factors with P<0.2 on univariate analysis were included in
multivariate analysis. The statistical analyses were performed
using IBM SPSS statistics 18 software (SPSS Inc., Tokyo,
Japan).
Results
Patient characteristics
Between September, 2008 and April, 2010, 98 patients
met the inclusion criteria and were included in the analysis. Of
the 98 patients, 23 (23.5%) had tumors with KRAS p.G13D mutations,
whereas the remaining 75 (76.5%) had tumors with other mutations.
Of the 98 patients, 31 received cetuximab: 9 (29.0%) had KRAS
p.G13D mutations and the remaining 22 (71.0%) patients harbored
other mutations. The frequency of tumors with KRAS p.13D mutations
tended to be higher in female patients and in the colon. There were
no significant differences in age, gender, primary site,
pathological type, history of chemotherapy, or the combined use of
irinotecan between either of these subgroups of patients. The
characteristics of the patients are summarized in Table I.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristics | p.G13D mutation, no.
(%) n=23 (23.5) | Other mutations, no.
(%) n=75 (76.5) | P-value |
|---|
| Age (years) | 0.14 |
| Median
(range) | 64.5 (44.0–76.0) | 60.0 (39.0–81.0) |
|
<65 | 11 (47.8) | 50 (66.7) |
|
| ≥65 | 12 (52.2) | 25 (33.3) |
|
| Gender | 0.05 |
| Male | 8 (34.8) | 45 (60.0) |
|
|
Female | 15 (65.2) | 30 (40.0) |
| Primary site | 0.08 |
|
Colon | 17 (73.9) | 39 (52.0) |
|
|
Rectum/anus | 5 (21.7) | 34 (45.3) |
|
|
Multiple | 1 (4.4) | 2 (2.7) |
|
| Differentiation | 0.97 |
| High | 8 (34.8) | 22 (29.3) |
|
|
Moderate | 13 (56.6) | 43 (57.3) |
|
| Poor | 1 (4.3) | 5 (6.7) |
|
|
Unknown | 1 (4.3) | 5 (6.7) |
|
Frequency of p.G13D mutations
A total of 24 patients had KRAS codon 13 mutations;
23 of these patients (95.8%) had a KRAS p.G13D mutation, and only 1
patient (4.2%) had a KRAS p.G13C mutation (Table II).
 | Table II.KRAS mutations. |
Table II.
KRAS mutations.
| Codon 12, no. (%)
n=74 (75.5) | Codon 13, no. (%)
n=24 (24.5) |
|---|
| G12D (GAT), 38
(51.4) | G13D (GAC), 23
(95.8) |
| G12S (AGT), 6
(8.1) | G13S (AGC), 0
(0.0) |
| G12C (TGT), 6
(8.1) | G13C (TGC), 1
(4.2) |
| G12R (CGT), 1
(1.3) | G13R (CGC), 0
(0.0) |
| G12V (GTT), 21
(28.4) | G13V (GTC), 0
(0.0) |
| G12A (GCT), 2
(2.7) | G13A (GCC), 0
(0.0) |
Between-study comparison of mutation
frequency
The prevalence of KRAS p.G13D mutations was 23.5% in
this study. There were no significant differences compared with the
results of previous studies (Table
III).
 | Table III.Comparison with previous studies. |
Table III.
Comparison with previous studies.
| Mutation type | NCI
CTG/AGITGa (n=164) | Leuvenb (n=122) | Italianc (n=24) | This study (Japanese)
(n=98) | P-value |
|---|
| p.G13D | 20 (12.2) | 20 (16.4) | 5 (20.8) | 23 (23.5) | P=0.11 |
| Other | 144 (87.8) | 102 (83.6) | 19 (79.2) | 75 (76.5) |
Characteristics of patients
administered cetuximab as first-line treatment
Of the 31 patients who received cetuximab as
first-line treatment, 9 (29%) had a KRAS p.G13D mutation and 22
(71%) had another type of KRAS mutation. There were no significant
differences in the baseline clinical characteristics between these
two groups (Table IV).
 | Table IV.Patient characteristics. |
Table IV.
Patient characteristics.
| Characteristics | p.G13D mutations, no.
(%) n=9 (29.0) | Other mutations, no.
(%) n=22 (71.0) | P-value |
|---|
| Age (years) |
|
| 1.00 |
| Median
(range) | 61.0 (44.0–72.0) | 64.0 (50.0–79.0) |
|
|
<65 | 5 (55.6) | 13 (59.1) |
|
| ≥65 | 4 (44.4) | 9 (40.9) |
|
| Gender |
|
| 0.43 |
| Male | 3 (33.3) | 12 (54.5) |
|
|
Female | 6 (66.7) | 10 (45.5) |
|
| ECOG PS |
|
| 0.65 |
| 0 | 6 (66.7) | 17 (77.2) |
|
| 1 | 3 (33.3) | 5 (22.8) |
|
| 2 | 0 (0.0) | 0 (0.0) |
|
Overall survival (OS) and
progression-free survival (PFS) according to the presence of p.G13D
vs. other mutations
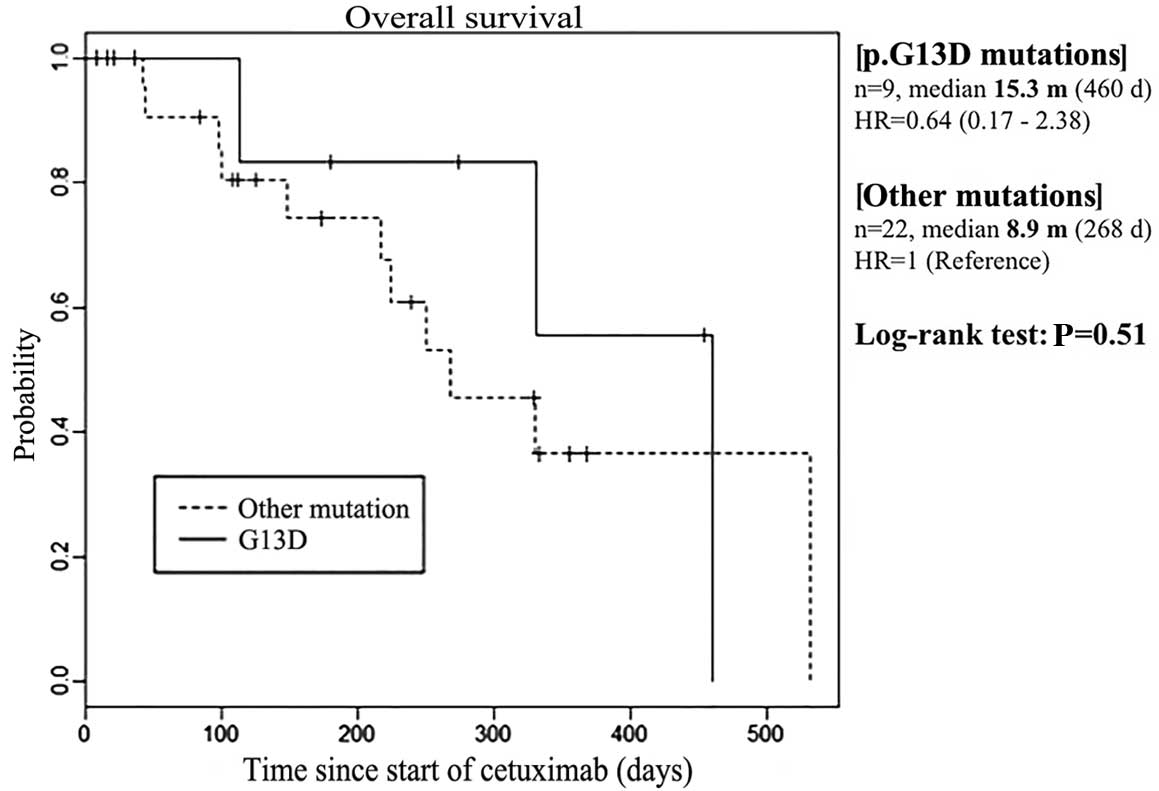
The median OS was 15.3 months in patients with KRAS
p.G13D mutations (n=9), compared with 8.9 months in those with
other KRAS mutations (n=22) (P=0.51; Fig.
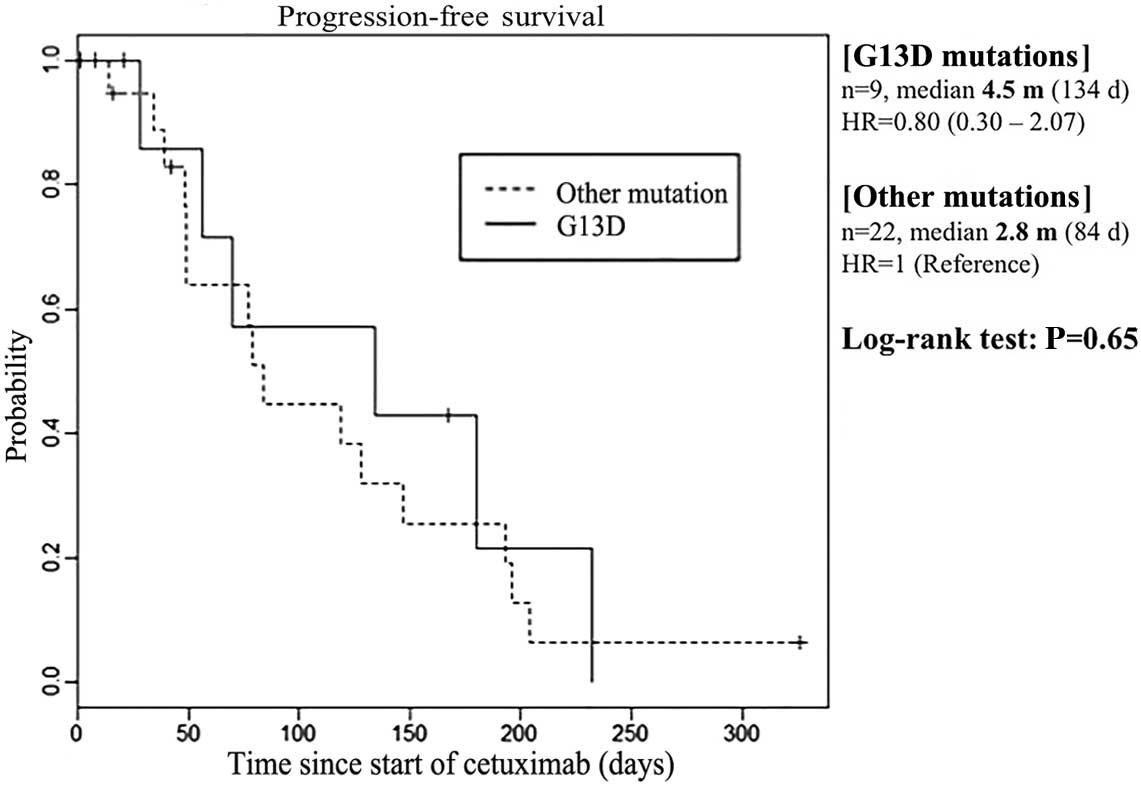
1). The median PFS was 4.5 months in patients with KRAS p.G13D
mutations (n=9), compared with 2.8 months in those with other KRAS
mutations (n=22) (P=0.65; Fig.
2).
Cox regression analysis of OS and
PFS
On the multivariate analysis, Eastern Cooperative
Oncology Group PS was found to be independently associated with
prolongation of OS and PFS. In addition, OS and PFS tended to be
prolonged in patients with KRAS p.G13D mutations. However, the
difference between patients with KRAS p.G13D mutations and those
with other genotypes did not reach statistical significance (OS,
P=0.13; and PFS, P=0.07) (Table
V).
 | Table V.Murtivariate analysis. |
Table V.
Murtivariate analysis.
|
| HR | Lower 95% CI | Upper 95% CI | P-value |
|---|
| Overall survival |
| Age,
years (<65 vs. ≥65) | 1.86 | 0.50 | 6.84 | 0.35 |
| Gender (M
vs. F) | 1.50 | 0.39 | 5.78 | 0.55 |
| ECOG-PS
(0 vs. 1) | 5.29 | 1.39 | 20.2 | <0.01 |
| KRAS
(other vs. G13D) | 0.23 | 0.04 | 1.54 | 0.13 |
| Progression-free
survival |
| Age,
years (<65 vs. ≥65) | 1.05 | 0.41 | 2.72 | 0.91 |
| Gender
(M vs. F) | 1.28 | 0.42 | 3.96 | 0.66 |
| ECOG-PS
(0 vs. 1) | 5.41 | 1.71 | 17.2 | <0.01 |
| KRAS
(other vs. G13D) | 0.29 | 0.08 | 1.10 | 0.07 |
Discussion
Anti-EGFR therapy is currently approved for use only
in patients with wild-type mCRC tumors. OS is >30 months in
patients with KRAS wild-type mCRC, as compared with only 20.3
months in patients with mCRC harboring KRAS exon 2 mutations
(6). The results of further clinical
trials of TAS-102 (7) or other new
drugs for the treatment of mCRC with KRAS mutations are therefore
eagerly awaited.
In this study, KRAS p.G13D mutations accounted for
23.5% of all KRAS mutations, which is consistent with the results
of previous studies. However, the prevalence of KRAS p.G13D
mutations was higher in female patients, which is not similar to
the results of studies performed by another Japanese group
(8) and other groups in Europe
(1,3).
In patients who received cetuximab, OS and PFS were marginally but
not significantly longer in patients with KRAS p.G13D mutations
compared with patients with other KRAS mutations.
A pooled exploratory analysis of the Cetuximab
Combined with Irinotecan in First-Line Therapy for Metastatic
Colorectal Cancer (CRYSTAL) study and the Oxaliplatin and Cetuximab
in First-Line Treatment of Metastatic Colorectal Cancer (OPUS)
study recently reported the effect of KRAS p.G13D mutations on
outcome in patients with mCRC who received first-line chemotherapy
with or without cetuximab (1).
Patients with p.G13D-mutant tumors appeared to benefit from
first-line treatment with cetuximab, although there were no
significant differences. On the other hand, in a subanalysis of the
Medical Research Council COIN trial, a phase III trial evaluating
the benefits of adding cetuximab to oxaliplatin-based first-line
combination chemotherapy, in the PRIME study and studies 20050181
and 20020408, which were phase III trials evaluating the
effectiveness of adding panitumumab to combination chemotherapy,
patients with p.G13D-mutant tumors did not appear to benefit from
first-line treatment with an anti-EGFR inhibitor (9).
A prospective phase II trial titled ‘A Trial of
Cetuximab with or without Irinotecan for Advanced Bowel Cancer (ICE
CREAM)’ is currently ongoing in the United Kingdom. The aim of this
trial is to validate that cetuximab alone or combined with
irinotecan is effective for advanced bowel cancer with p.G13D
mutations, and the results are eagerly awaited. However, it remains
unclear whether we should use an anti-EGFR inhibitor to treat
patients who have mCRC with KRAS p.G13D mutations. Therefore, we do
not recommend an anti-EGFR inhibitor for such patients at
present.
An important topic for future research is vertical
blockade of the Ras pathway. Previous in vitro studies
reported that, given the higher efficacy of triple-drug therapy [a
mitogen-activated protein kinase kinase (MEK) and phosphoinositide
3-kinase (PI3K)/mammalian target of rapamycin (mTOR) inhibitor
combined with cetuximab], periodic administration of anti-EGFR
antibodies may more effectively delay tumor progression (10). Concomitant blockade of MEK and
PI3K/mTOR induced high rates of disease stabilization in
patient-derived mCRC xenografts harboring KRAS, neuroblastoma RAS
viral oncogene homolog (NRAS), BRAF and/or PIK3 catalytic subunit α
mutations. These findings suggest potential therapeutic
opportunities to delay disease progression by several alternative
treatment options in patients who have tumors with KRAS or other
mutations (10).
The present study had several limitations. Most
importantly, it was a retrospective study and the sample size was
small. In addition, we did not evaluate other biomarkers, such as
KRAS exon 3 or 4, NRAS, or BRAF mutations. Although cetuximab may
be more clinically beneficial for mCRC patients with KRAS p.G13D
mutations compared with those harboring other mutations, further
studies are required to elucidate the potential benefits of
cetuximab treatment for mCRC patients with KRAS p.G13D
mutations.
In conclusion, the use of an anti-EGFR antibody is
not currently recommended for patients with mCRC associated with
KRAS p.G13D mutations; however, as for the combination with a drug
blocking the tumor growth pathway perpendicularly, it it likely to
be effective.
References
|
1
|
Tejpar S, Celik I, Schlichting M,
Sartorius U, Bokemeyer C and Van Cutsem E: Association of KRAS G13D
tumor mutations with outcome in patients with metastatic colorectal
cancer treated with first-line chemotherapy with or without
cetuximab. J Clin Oncol. 30:3570–3577. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mao C, Huang YF, Yang ZY, Zheng DY, Chen
JZ and Tang JL: KRAS p.G13D mutation and codon 12 mutations are not
created equal in predicting clinical outcomes of cetuximab in
metastatic colorectal cancer: a systematic review and
meta-analysis. Cancer. 119:714–721. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
De Roock W, Jonker DJ, Di Nicolantonio F,
Sartore-Bianchi A, Tu D, Siena S, Lamba S, Arena S, Frattini M,
Piessevaux H, et al: Association of KRAS p.G13D mutation with
outcome in patients with chemotherapy-refractory metastatic
colorectal cancer treated with cetuximab. JAMA. 304:1812–1820.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Blons H, Emile JF, Le Malicot K, Julié C,
Zaanan A, Tabernero J, Mini E, Folprecht G, Van Laethem JL, Thaler
J, et al: Prognostic value of KRAS mutations in stage III colon
cancer: Post hoc analysis of the PETTAC8 phase III trial dataset.
Ann Oncol. 25:2378–2385. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fukushima Y, Yanaka S, Murakami K, Abe Y,
Koshizaka T, Hara H, Samejima C, Kishi Y, Kaneda M and Yoshino T:
High-throughput screening method of KRAS mutations at codons 12 and
13 in formalin-fixed paraffin-embedded tissue specimens of
metastatic colorectal cancer. Cancer & chemotherapy.
38:1825–1835. 2011.(In Japanese).
|
|
6
|
Heinemann V, von Weikersthal LF, Decker T,
Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller
C, Kahl C, Seipelt G, et al: FOLFIRI plus cetuximab versus FOLFIRI
plus bevacizumab as first-line treatment for patients with
metastatic colorectal cancer (FIRE-3): A randomised, open-label,
phase 3 trial. Lancet Oncol. 15:1065–1075. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yoshino T, Mizunuma N, Yamazaki K, Nishina
T, Komatsu Y, Baba H, Tsuji A, Yamaguchi K, Muro K, Sugimoto N, et
al: TAS-102 monotherapy for pretreated metastatic colorectal
cancer: A double-blind, randomised, placebo-controlled phase 2
trial. Lancet Oncol. 13:993–1001. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bando H, Yoshino T, Yuki S, Shinozaki E,
Nishina T, Kadowaki S, Yamazaki K, Kajiura S, Tsuchihara K, Fujii
S, et al: Clinical outcome of Japanese metastatic colorectal cancer
patients harbouring the KRAS p.G13D mutation treated with cetuximab
+ irinotecan. Jpn J Clin Oncol. 42:1146–1151. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peeters M, Douillard JY, Van Cutsem E,
Siena S, Zhang K, Williams R and Wiezorek J: Mutant KRAS codon 12
and 13 alleles in patients with metastatic colorectal cancer:
Assessment as prognostic and predictive biomarkers of response to
panitumumab. J Clin Oncol. 31:759–765. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Migliardi G, Sassi F, Torti D, Galimi F,
Zanella ER, Buscarino M, Ribero D, Muratore A, Massucco P, Pisacane
A, et al: Inhibition of MEK and PI3K/mTOR suppresses tumor growth
but does not cause tumor regression in patient-derived xenografts
of RAS-mutant colorectal carcinomas. Clin Cancer Res. 18:2515–2525.
2012. View Article : Google Scholar : PubMed/NCBI
|