Introduction
Over the past several decades, improvements in
chemotherapeutic agents and supportive care have resulted in
significant progress in the treatment of patients with acute
myeloid leukemia (AML). However, the treatment options for
high-risk AML patients, particularly elderly, relapsed and
refractory patients, remain limited. The outcomes of these patients
are generally poor due to drug resistance, short durability of
response, poor performance status and serious comorbidities
following standard dose-intensive therapy (1,2).
It was confirmed that epigenetic changes are crucial
for the progression of numerous human neoplasms (3), as they are a universal mechanism of gene
inactivation in malignant cells, possibly exceeding mutational
events (4). Aberrant DNA methylation
in gene promoters has been demonstrated to accompany these
epigenetic changes and is also essential for the maintenance of
altered gene expression status in malignant cells (5). Over the last few years, a growing number
of studies have reported that leukemia is also characterized by
high degrees of epigenetic changes (6,7). These
observations have led to the renewed interest in therapeutic
regimens targeting the aberrant epigenome of cancer cells (8) and DNA methylation inhibitors are
expected to be used as antineoplastic agents (3).
Decitabine (5-aza-2′-deoxycytidine; DAC), a DNA
hypomethylating agent that induces differentiation and apoptosis of
leukemic cells, is a well-tolerated alternative to aggressive
chemotherapy. In a study published by Kantarjian et al
(9), the efficacy and safety of
different therapeutic regimens was compared in 485 elderly patients
with newly diagnosed AML; the complete remission (CR) rate,
including CR with delayed platelet recovery [CRp; platelet (PLT)
count <100×109/l] was 17.8% with DAC vs. 7.8% with
supportive care or cytarabine (Ara-C), without significant
differences in safety. However, DAC monotherapy was associated with
a relatively low rate of CR in AML (10,11).
Several groups have attempted to increase the response rate of
DAC-based therapy by developing combination treatments (12–14). The
aim of the present study was to investigate the effect of DAC
sequentially combined with chemotherapeutic drugs in the HL-60/ADR
multidrug-resistant leukemia cell line and retrospectively analyze
the therapeutic efficacy in 7 high-risk AML patients.
Materials and methods
Reagents
The Cell Counting Kit-8 (CCK-8) was purchased from
Dojindo Laboratories (Tokyo, Japan). DAC was supplied and
formulated by Pharmachemie B.V. (Haarlem, The Netherlands).
Aclacinomycin (ACLA) was purchased from Shenzhen Main Luck
Pharmaceuticals Inc. (Shenzhen, China). Rabbit monoclonal anti-DNA
methyltransferase 1 (DNMT1) antibody (dilution, 1:1,000; cat. no.
5032) and rabbit monoclonal anti-GAPDH antibody (dilution, 1:1,000;
cat. no. 5174) and cell lysis buffer were purchased from Cell
Signaling Technology, Inc. (Beverly, MA, USA). Polyvinylidene
fluoride membranes were purchased from Millipore (Billerica, MA,
USA).
Cell culture
The HL-60/ADR human AML cell line, a
multidrug-resistant leukemia cell line, was obtained from the
Institute of Hematology and Blood Diseases Hospital, Chinese
Academy of Medical Sciences (Beijing, China). The cells were grown
in RPMI-1640 (Invitrogen Life Technologies, Carlsbad, CA, USA)
supplemented with 10% fetal bovine serum (Invitrogen Life
Technologies) in plastic tissue culture plates in a humidified
atmosphere containing 5% CO2 at 37°C.
Quantification of cell proliferation
using the CCK-8 assay
For the growth inhibition assay, HL-60/ADR cells
were cultured at a density of 105 cells/ml and aliquots
(100 µl) per well of the cell suspension were dispensed into
96-well plates. At 24 h after plating, DAC was added to the wells
at concentrations of 0.5 and 1.0 µM. The plates were incubated in a
humidified incubator in 5% CO2 for 72 h at 37°C.
Subsequently, ACLA at varying concentrations was added to the
wells. The cell proliferation was determined using the CCK-8 at 24
h after dosing. The plates were then analyzed on an enzyme-linked
immunosorbent assay plate reader (Bio-Rad 680; Bio-Rad, Hercules,
CA, USA) at 490 nm. All the experiments were performed in
triplicate in at least 3 independent experiments.
Western blot analysis
Following treatment with 1 µmol/l DAC for 72 h, the
HL-60/ADR cells were harvested and lysed in cell lysis buffer. The
proteins were separated by 10% sodium dodecyl
sulphate-polyacrylamide gel electrophoresis and transferred onto
polyvinylidene fluoride membranes. The membranes were blocked with
5% skimmed milk and incubated overnight at 4°C with anti-DNMT1 and
anti-GAPDH antibodies in Tris-buffered saline [10 mm Tris-HCl (pH
8.0), 150 mm NaCl] with 0.1% Tween-20. Following incubation with
peroxidase-conjugated secondary antibodies for 2 h, the blots were
developed using enhanced chemiluminescence (Molecular Imager
ChemiDoc™ XRS; Bio-Rad).
Patients and treatment protocols
Following approval of the study protocol by the
Institutional Review Board of Nanfang Hospital we retrospectively
analyzed 11 high-risk AML patients who were diagnosed according to
the World Health Organization criteria (15) between May, 2012 and November, 2014 at
the Department of Hematology of Nanfang Hospital. The pretreatment
characteristics of the 11 patients are presented in Table I. All the patients received induction
therapy with DAC sequentially combined with CAG agents, which
consisted of DAC at a dose of 20 mg/m2 intravenously
over 1 h daily on days 1–3, ACLA 10 mg by intravenous infusion
daily on days 4–10 or 13 and Ara-C 25 mg subcutaneously twice daily
on days 4–13 or 17. On days 4–13 or 17, granulocyte-colony
stimulating factor (G-CSF) 300 µg subcutaneously daily preceded the
chemotherapeutic injections by ~4–6 h and was discontinued when the
white blood cell count reached >20×109/l. The
treatment protocols for patients following achievement of CR were
not uniform, due to differences in financial conditions (Table I). Complete blood count, hepatic and
renal function tests and electrolyte levels were tested once or
twice weekly during the drug administration period and marrow
aspirates were monitored prior to and 2 weeks after treatment, to
observe the therapeutic efficacy. G-CSF was used during the
myelosuppression period and antibiotic therapy was administered as
clinically indicated.
 | Table I.Data of 11 patients with AML who were
ineligible for intensive chemotherapy and received induction
therapy with DAC sequentially combined with CAG. |
Table I.
Data of 11 patients with AML who were
ineligible for intensive chemotherapy and received induction
therapy with DAC sequentially combined with CAG.
|
|
|
|
|
| Duration (days) |
|
| Follow-up |
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| No. | Gender/age | FAB subtype | Cytogenetics | Courses to CR | PLT <20 G/l | NEU <0.5 G/l | Infection Site | Therapeutic regimen
after CR (x course) | Months | Status |
|---|
| 1a | M/43 | UN | t(1;7); 7q-/-7 | 1 | 23 | 16 | Lung crissum | D+HAG×1,
Allo-HSCT | 6 | CCR |
| 2 | F/74 | M4 | del(7q)/-7;
EGR-1/RAR-α deletion; DNMT3A+ | 1 | 10 | 23 | Lung | D+CAG×1 | 4 | Relapse |
| 3b | M/48 | M2 | del(9) | 1 | 8 | 4 | Lung | IA×2, D+CAG×2 | 6 | CCR |
| 4 | F/62 | M2 | Normal karyotype;
NPM1+ | 2 | 5 | 41 | Lung | D+HAG×3, IA×3 | 12 | CCR |
| 5 | M/62 | M5 | Normal
karyotype | NR | 21 | 0 | Unknown origin | HA, IA
re-induction | 3 |
Deceasedc |
| 6 | M/60 | UN | Normal karyotype;
NPM1+ | 1 | 10 | 23 | Lung | D+HAG×2, IA×3,
MD-Ara-C×1 | 9 | CCR |
| 7a | M/57 | UN | Normal karyotype;
DNMT3A+ | 1 | 13 | 23 | Lung | D+HAG×3, IA×3 | 10 | CCR |
| 8 | M/60 | M5 | Normal karyotype;
FLT3-ITD+ | 1 | 9 | 20 | Lung | D+HAG×2, IA×1 | 5 | CCR |
| 9d | M/43 | M2 | +8 | 1 | 11 | 26 | Lung | D+HAG×2, IA×2,
MD-Ara-C×3 | 18 | CCR |
| 10 | F/67 | M1 | +8,-5
FLT3-ITD+ DNMT3A+ | NR | 8 | 19 | Lung | - | 3 |
Deceasede |
| 11 | F/63 | M4 | −7 | NR | 12 | 27 | - | - | 3 | Lost to
follow-up |
Response criteria and side
effects
Response was assessed based on the criteria of the
International Working Group for Diagnosis, Standardization of
Response Criteria, Treatment Outcomes and Reporting Standards for
Therapeutic Trials in Acute Myeloid Leukemia (16). CR required an absolute neutrophil
(NEU) count of ≥1×109/l, PLT count of
≥100×109/l, marrow blasts ≤5% and disappearance of all
signs and symptoms related to disease. CRp was defined as CR with a
PLT count of <100×109/l. Any other response was
considered as treatment failure. Neutropenia and thrombocytopenia
was defined as NEU count <0.5×109/l and PLT count
<20×109/l, respectively. The reported
non-hematological side effects included nausea, vomiting, diarrhea,
skin rashes, liver or renal dysfunction and fever. The febrile
episodes included fever of unknown origin and documented
infections. The adverse drug reactions were scored according to the
National Cancer Institute Common Toxicity Criteria, version 2.0
(17).
Statistical analysis
The statistical significance of the differences
between the proliferation inhibition rate with DAC at two different
concentrations sequentially combined with ACLA and the control
group was assessed by one-way analysis of variance using the SPSS
13.0 software program (SPSS Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
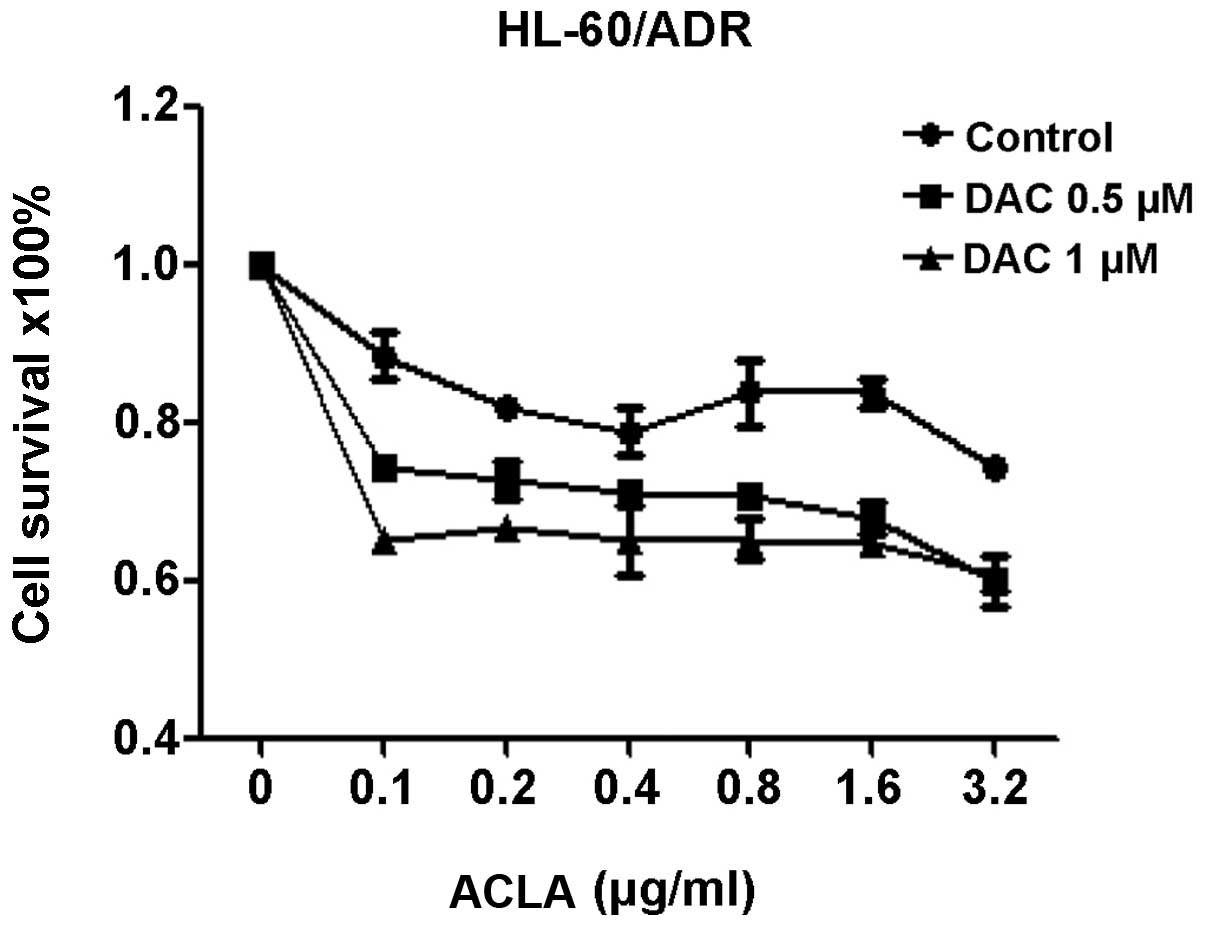
Response of HL-60/ADR cells treated by
DAC sequentially combined with ACLA
To evaluate the effects of sequentially combining
DAC and ACLA on HL-60/ADR cell viability, the cells were treated
with DAC (0.5 and 1.0 µM for 72 h) and sequentially with different
concentrations of ACLA (0.1, 0.2, 0.4, 0.8, 1.6 and 3.2 µg/ml for
24 h). The percentages of live/viable cells in the treated plates
were measured using the CCK-8 proliferation assay. The growth
inhibition rate with DAC at the two different concentrations
sequentially combined with ACLA was significantly higher compared
with that in the control group (P<0.001 for both, Fig. 1). The data revealed that the
proliferation of HL-60/ADR cells was significantly inhibited when
combining DAC and ACLA sequentially, compared with the treatment
groups using ACLA alone.
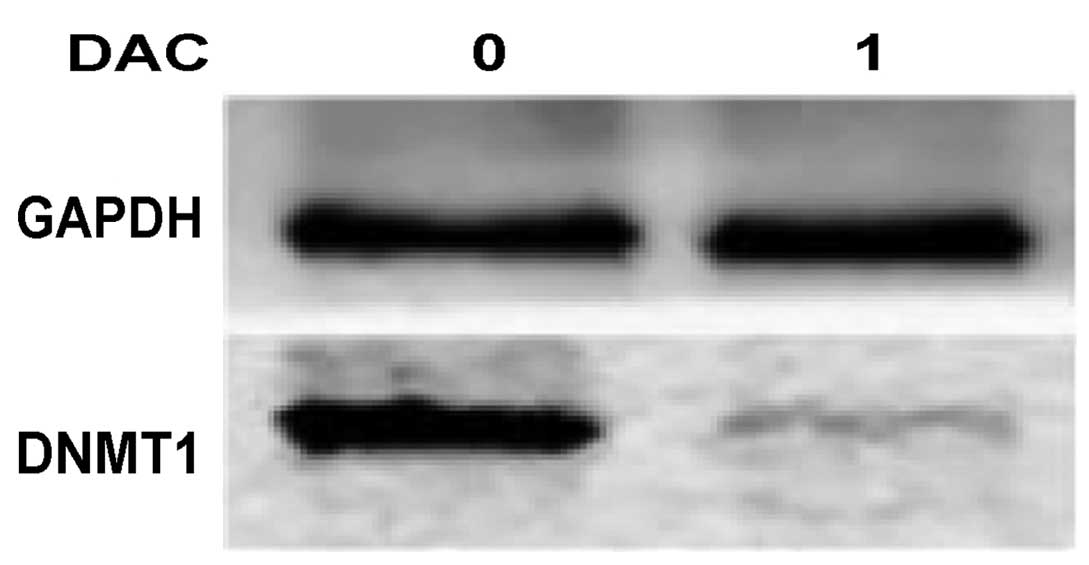
DNMT1 protein expression in HL-60/ADR
cells
DNMT1 protein expression was analyzed in HL-60/ADR
cells following treatment with 1.0 µmol/l DAC for 72 h. The western
blot analysis demonstrated that DNMT1 expression was significantly
repressed following treatment with 1.0 µmol/l DAC in HL-60/ADR
cells (Fig. 2).
Response to treatment
The therapeutic effects in the 11 patients are
detailed in Table I. The median
follow-up time was 6 months (range, 3–18 months) and the median
course of consolidation chemotherapy after CR was 5 months (range,
1–7 months). Of the 11 patients, 7 (63.6%) achieved CR after one
treatment cycle and 1 patient (9.1%) achieved CR after two cycles.
Of the 8 patients achieving CR, 1 patient underwent allogeneic
hematopoietic stem cell transplantation after one cycle of
consolidation therapy with DAC sequentially combined with HAG. One
patient discontinued therapy after one cycle of consolidation
therapy and did not receive any subsequent chemotherapy until he
relapsed 4 months later, at which time re-induction with the
original induction regimen was unsuccessful (non-remission; NR). A
total of 6 patients (5 who achieved CR after one cycle and 1 who
achieved CR after two cycles) received a total of 3–6 courses of
consolidation therapy with DAC combined with CAG or
homoharringtonine + Ara-C + G-CSF (HAG) and IA [idarubicin (IDA) +
Ara-C] regimens. The patients remained in remission at the last
follow-up. Two patients exhibited NR and succumbed to
leukemia-related intracranial hemorrhage and septic shock. One
patient was discharged from the hospital due to therapy
discontinuation.
Side effects
Myelosuppression was observed in all the patients
(Table I). The median duration of
neutropenia was 23 days (range, 0–41 days) and of thrombocytopenia
10 days (range, 5–23 days). During the myelosuppression period, a
febrile episode of unknown origin was reported in 1 patient and
pulmonary infections developed in 9 patients, but the symptoms were
controlled following treatment with hematopoietic stimulating
factor and symptomatic antibiotic support treatment. A total of 3
patients developed nausea and vomiting. Extramedullary toxicity was
generally mild, without grade ≥2 hepatic or renal dysfunction. Of
the 8 patients who achieved CR, 6 patients receiving consolidation
therapy with sequential combination of DAC and CAG or HAG were
analyzable for myelosuppression. The median time to NEU
<0.5×109/l was 8 days (range, 0–10.3 days) and to PLT
<20×109/l 6 days (range, 0–7 days). The duration of
myelosuppression during consolidation therapy was shorter compared
with that during the induction period.
Discussion
Although DAC has been found to be clinically
effective as a single agent in patients with AML, the overall
response rate (ORR) is relatively low, reportedly ranging between
8.5 and 26% (10,16). In order to improve the curative effect
without increasing the toxicity, a therapeutic regimen using DAC
combined with other antileukemic drugs has entered the stage of
experiments in vitro and clinical studies. A number of
hypomethylating combination trials for AML are underway and include
the use of all trans-retinoic acid, Ara-C, IDA, daunorubicin (DNR),
ACLA, thalidomide and homoharringtonine (12,19–21). In
our study, treatment with DAC prior to ACLA inhibited the
proliferation of cultured HL-60/ADR multidrug-resistant cells.
Li et al (12)
reported that the sequential combination of DAC and IDA induced
synergistic cell death in U937 cells and AML cells isolated from
AML patients, while tumor growth inhibition with this sequential
combination was found to be higher compared with single-agent
treatment or controls in vivo. However, other
anthracyclines, including DNR and ACLA, did not exert a synergistic
effect when sequentially combined with DAC, which was not
consistent with our results. Sequential treatment with DAC and
Ara-C was found by Leonard et al (19) to be more effective in reducing tumor
burden compared with treatment with Ara-C alone in xenograft models
of childhood AML.
Clinically, several data also investigated the
strategy of hypomethylating agent-based combinations using DAC. A
study by Zhang et al (22)
compared the clinical efficacy and adverse reactions between
low-dose DAC combined with CAG and CAG alone in intermediate- to
high-risk myelodysplastic syndrome, and found that the CR rate was
higher (75%) in patients treated by the combination regimen
compared with CAG alone (50%), although the difference was not
statistically significant. Jing et al (23) found that DAC combined with modified
CAG regimen for relapsed and refractory AML patients with
AML1-ETO+ displayed higher CR rate and fewer side
effects. Benton et al (24)
conducted a phase 1 study of patients with relapsed/refractory
acute lymphocytic leukemia treated with DAC alone or in combination
with the hyper-CVAD regimen; the ORR (including CR, CRp and marrow
CR) was 21% in the DAC alone group and 56% in the combination
regimen group, and certain patients, who had previously developed
disease progression on Hyper-CVAD alone, achieved a CR when DAC was
added. In the present study, we presented a retrospective analysis
of a single-institution experience with the therapeutic efficacy of
DAC sequentially combined with CAG in newly diagnosed patients with
AML who were considered unfit for intensive chemotherapy. All the
patients were monitored after treatment for the development of
thrombocytopenia, neutropenia and infection, which were
controllable. There was no reported drug-related mortality. The
results were consistent with those reported by Bhatnagar et
al (25).
These findings suggest that DAC sequentially
combined with chemotherapy may enhance the antileukemic effect
in vitro, as well as in clinical trials. Our results also
confirmed that pretreatment with DAC may act as a potential
sensitizer to ACLA, thereby improving its curative efficacy via the
epigenetic modulation of demethylation in HL-60/ADR cells and AML
patients.
Multiple mechanisms may be involved in this
phenomenon. Aberrant expression of DNMTs, which promote DNA
methylation, is recognized as a key factor in the onset of cancer
and drug resistance (26,27). DNMT1 is crucial for the maintenance of
the methylation landscape due to its ability to recognize
hemimethylated DNA and conserve methylation during somatic cellular
division (27). High levels of DNMT1
expression have been reported in cancer patients who are not
responsive to chemotherapy (26). DAC
incorporated into DNA covalently binds to DNMT1, leading to the
reduction of available DNMT1 protein in cells, which in turn
results in DNA demethylation and expression of methylation-silenced
genes (28,29). In the present study, we demonstrated
that DAC decreased DNMT1 protein expression levels, which was
consistent with previous findings.
In conclusion, we retrospectively reported a
single-institution experience using DAC sequentially combined with
CAG in newly diagnosed high-risk AML patients. These findings
suggest clinical potential in the sequential administration of DAC
and CAG regimen for the treatment of elderly and
relapsed/refractory AML patients, or patients with secondary AML.
However, due to the limited number of cases, large-scale
multicenter studies are required to confirm our results.
References
|
1
|
Kantarjian H, Ravandi F, O'Brien S, Cortes
J, Faderl S, Garcia-Manero G, Jabbour E, Wierda W, Kadia T, Pierce,
et al: Intensive chemotherapy does not benefit most older patients
(age 70 years or older) with acute myeloid leukemia. Blood.
116:4422–4429. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schiller GJ: When a gold standard is made
of tin. Blood. 116:4386–4387. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Santini V, Kantarjian HM and Issa JP:
Changes in DNA methylation in neoplasia: Pathophysiology and
therapeutic implications. Ann Intern Med. 134:573–586. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baylin SB and Ohm JE: Epigenetic gene
silencing in cancer - a mechanism for early oncogenic pathway
addiction? Nat Rev Cancer. 6:107–116. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jones PA and Takai D: The role of DNA
methylation in mammalian epigenetics. Science. 293:1068–1070. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Issa JP, Baylin SB and Herman JG: DNA
methylation changes in hematologic malignancies: Biologic and
clinical implications. Leukemia. 11:(Sul 1). S7–S11.
1997.PubMed/NCBI
|
|
7
|
Melki JR and Clark SJ: DNA methylation
changes in leukaemia. Semin Cancer Biol. 12:347–357. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Egger G, Liang G, Aparicio A and Jones PA:
Epigenetics in human disease and prospects for epigenetic therapy.
Nature. 429:457–463. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kantarjian HM, Thomas XG, Dmoszynska A,
Wierzbowska A, Mazur G, Mayer J, Gau JP, Chou WC, Buckstein R,
Cermak J, et al: Multicenter, randomized, open-label, phase III
trial of decitabine versus patient choice, with physician advice,
of either supportive care or low-dose cytarabine for the treatment
of older patients with newly diagnosed acute myeloid leukemia. J
Clin Oncol. 30:2670–2677. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Issa JP, Garcia-Manero G, Giles FJ,
Mannari R, Thomas D, Faderl S, Bayar E, Lyons J, Rosenfeld CS,
Cortes J, et al: Phase 1 study of low-dose prolonged exposure
schedules of the hypomethylating agent 5-aza-2′-deoxycytidine
(decitabine) in hematopoietic malignancies. Blood. 103:1635–1640.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang AS, Doshi KD, Choi SW, Mason JB,
Mannari RK, Gharybian V, Luna R, Rashid A, Shen L, Estecio MR, et
al: DNA methylation changes after 5-aza-2′-deoxycytidine therapy in
patients with leukemia. Cancer Res. 66:5495–5503. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li K, Hu C, Mei C, Ren Z, Vera JC, Zhuang
Z, Jin J and Tong H: Sequential combination of decitabine and
idarubicin synergistically enhances anti-leukemia effect followed
by demethylating Wnt pathway inhibitor promoters and downregulating
Wnt pathway nuclear target. J Transl Med. 12:1672014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gore SD, Baylin S, Sugar E, Carraway H,
Miller CB, Carducci M, Grever M, Galm O, Dauses T, Karp JE, et al:
Combined DNA methyltransferase and histone deacetylase inhibition
in the treatment of myeloid neoplasms. Cancer Res. 66:6361–6369.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Blum W, Klisovic RB, Hackanson B, Liu Z,
Liu S, Devine H, Vukosavljevic T, Huynh L, Lozanski G, Kefauver C,
et al: Phase I study of decitabine alone or in combination with
valproic acid in acute myeloid leukemia. J Clin Oncol.
25:3884–3891. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vardiman JW: The World Health Organization
(WHO) classification of tumors of the hematopoietic and lymphoid
tissues: an overview with emphasis on the myeloid neoplasms.
Chem-Biol Interact. 184:16–20. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheson BD, Bennett JM, Kopecky KJ, Büchner
T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister
TA, et al: International Working Group for Diagnosis,
Standardization of Response Criteria, Treatment Outcomes, and
Reporting Standards for Therapeutic Trials in Acute Myeloid
Leukemia: Revised recommendations of the International Working
Group for Diagnosis, Standardization of Response Criteria,
Treatment Outcomes and Reporting Standards for Therapeutic Trials
in Acute Myeloid Leukemia. J Clin Oncol. 21:4642–4649. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Postma TJ and Heimans JJ: Grading
chemotherapy-induced peripheral neuropathy. Ann Oncol. 11:509–513.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lübbert M, Suciu S, Baila L, Rüter BH,
Platzbecker U, Giagounidis A, Selleslag D, Labar B, Germing U,
Salih HR, et al: Low-dose decitabine versus best supportive care in
elderly patients with intermediate- or high-risk myelodysplastic
syndrome (MDS) ineligible for intensive chemotherapy: Final results
of the randomized phase III study of the European Organisation for
Research and Treatment of Cancer Leukemia Group and the German MDS
Study Group. J Clin Oncol. 29:1987–1996. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Leonard SM, Perry T, Woodman CB and Kearns
P: Sequential treatment with cytarabine and decitabine has an
increased anti-leukemia effect compared to cytarabine alone in
xenograft models of childhood acute myeloid leukemia. PLoS One.
9:e874752014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peng CY, Jiang J, Zheng HT and Liu XS:
Growth-inhibiting effects of arsenic trioxide plus epigenetic
therapeutic agents on leukemia cell lines. Leuk Lymphoma.
51:297–303. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qin T, Youssef EM, Jelinek J, Chen R, Yang
AS, Garcia-Manero G and Issa JP: Effect of cytarabine and
decitabine in combination in human leukemic cell lines. Clin Cancer
Res. 13:4225–4232. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang YP, Wu WZ and Cui GX: Comparison of
clinical efficacy between decitabine combined with CAG regimen and
CAG regimen alone in patients with intermediate to high-risk
myelodysplastic syndromes. J Exp Hematol. 22:1341–1344. 2014.(In
Chinese). PubMed/NCBI
|
|
23
|
Jing Y, Zhu CY, Zhang Q, et al: Clinical
efficacy of decitabine combined with modified CAG regimen for
relapsed-refractory acute myeloid leukemia with
AML1-ETO+. J Exp Hematol. 22:1245–1250. 2014.(In
Chinese). PubMed/NCBI
|
|
24
|
Benton CB, Thomas DA, Yang H, Ravandi F,
Rytting M, O'Brien S, Franklin AR, Borthakur G, Dara S, Kwari M, et
al: Safety and clinical activity of 5-aza-2′-deoxycytidine
(decitabine) with or without Hyper-CVAD in relapsed/refractory
acute lymphocytic leukaemia. Br J Haematol. 167:356–365. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bhatnagar B, Duong VH, Gourdin TS, Tidwell
ML, Chen C, Ning Y, Emadi A, Sausville EA and Baer MR: Ten-day
decitabine as initial therapy for newly diagnosed patients with
acute myeloid leukemia unfit for intensive chemotherapy. Leuk
Lymphoma. 55:1533–1537. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mutze K, Langer R, Schumacher F, Becker K,
Ott K, Novotny A, Hapfelmeier A, Höfler H and Keller G: DNA
methyltransferase 1 as a predictive biomarker and potential
therapeutic target for chemotherapy in gastric cancer. Eur J
Cancer. 47:1817–1825. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Robert MF, Morin S, Beaulieu N, Gauthier
F, Chute IC, Barsalou A and MacLeod AR: DNMT1 is required to
maintain CpG methylation and aberrant gene silencing in human
cancer cells. Nat Genet. 33:61–65. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Daskalakis M, Blagitko-Dorfs N and
Hackanson B: Decitabine. Recent Results Cancer Res. 184:131–157.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oki Y, Aoki E and Issa JP: Decitabine -
bedside to bench. Crit Rev Oncol Hematol. 61:140–152. 2007.
View Article : Google Scholar : PubMed/NCBI
|