Introduction
Ovarian high-grade serous carcinoma (HGSC) is the
most common type of epithelial ovarian carcinoma, with the highest
mortality rate among gynecological malignancies. Following
debulking surgery and adjuvant carboplatin and paclitaxel
chemotherapy, a significantly high percentage of cases eventually
recur, with resistance to systemic therapy. The availability of
targeted therapies is currently limited and death typically occurs
within 5 years after tumor recurrence and development of
chemoresistance (1).
Recently, the Cancer Genome Atlas (TCGA) project has
characterized the genomic landscape of HGSC and highlighted a
prevalence of somatic tumor protein p53 gene mutations and numerous
DNA amplifications and deletions (2).
Similar to previous studies, the TCGA study additionally confirmed
that a significant number of HGSCS exhibited genetic alterations in
the breast cancer 1, early onset (BRCA1) and BRCA2,
cyclin E and MYC pathways, with extensive gene copy number
alterations. However, the absence of high-frequency oncogenic point
mutations in drug target genes has precluded the development of
targeted therapeutics for HGSC (2–5).
The goal of this study was to investigate whether
novel therapeutic target pathways with available inhibitors may be
associated with specific survival outcomes in patients with HGSC.
In recent years, chromatin regulators, such as
bromodomain-containing protein 4 (BRD4), a member of the
bromodomain and extra terminal domain family, which is associated
with acetylated chromatin and transcriptional activation, have
become attractive targets for cancer therapy. BRD4 has been shown
to selectively regulate the transcription of key oncogenic drivers,
such as CMYC, in several tumor types (6–9), whereas
more recent analyses using epigenetic datasets have demonstrated
that BRD4 specifically targets cell-type specific enhancer
sequences (i.e., super enhancers) (10). The aim of the present study, was to
determine whether the BRD4 gene, which is a druggable gene
product, is amplified in a subset of HGSCS, and whether its
amplification is associated with worse prognosis and survival.
Patients and methods
Survival analysis
Clinical data from newly diagnosed patients with
ovarian carcinoma from the TCGA study (2), specifically 489 cases with copy number
alterations data, were integrated with BRD4 amplification
data from the TCGA data portal. Kaplan-Meier overall and
disease-free survival analysis was performed with the cBio Cancer
Genomics Portal (http:cbioportal.org; Memorial Sloan Kettering Cancer
Center, New York, NY, USA); significance was estimated using the
log-rank test (11,12).
Review of clinical and pathological
data
Pathology reports were downloaded from the Cancer
Genome Atlas data portal control-access directory (https://tcga-data.nci.nih.gov/tcga) or via the
cBio portal. Quality control hematoxylin and eosin images from
frozen and permanent sections were analyzed via the TCGA BioSig
website hosted at Lawrence Berkeley National Laboratory (http:tcga.lbl.gov:8080/biosig/tcgadownload.do) and via
the Cancer Digital Slide Archive (http:cancer.digitalslidearchive.net; Emory University,
Atlanta, GA, USA).
This study was conducted in accordance with the TCGA
publication policy for ovarian carcinomas (http:cancergenome.nih.gov) and was approved by the
Beth Israel Deaconess Medical Center HCC Institutional Review
Board.
Results
Cases and survival
Clinical data from newly diagnosed patients with
ovarian carcinoma from the TCGA study, specifically 489 cases with
copy number alterations data, were integrated with BRD4
amplification data from the TCGA data portal via the cBio Cancer
Genomics Portal (2,11,12).
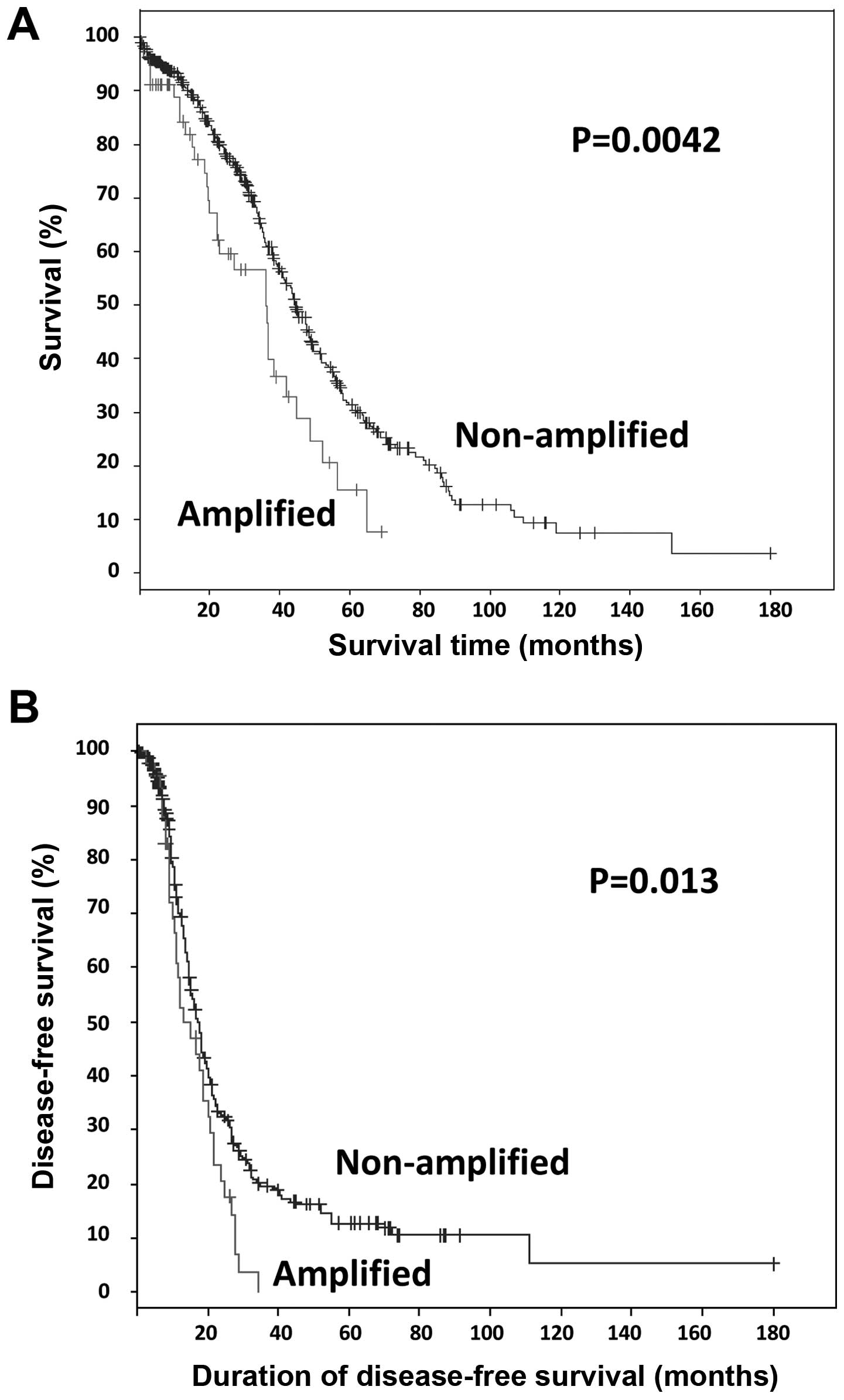
Somatic amplification of BRD4 was identified in 12% (57/489)
of the cases. Survival analysis of all TCGA tumors with ovarian
HGSC (n=489), revealed that patients with somatic BRD4
amplification exhibited significantly worse overall and
progression-free survival compared with those with non-amplified
BRD4 (log-rank test P=0.0042 and P=0.013, respectively;
Fig. 1). The median number of months
until relapse was 13 for cases with amplification, compared with 17
for cases without amplification. Patients with BRD4
amplification exhibited a median survival OF 36 months, compared
with 44 months for patients without amplification.
mRNA data
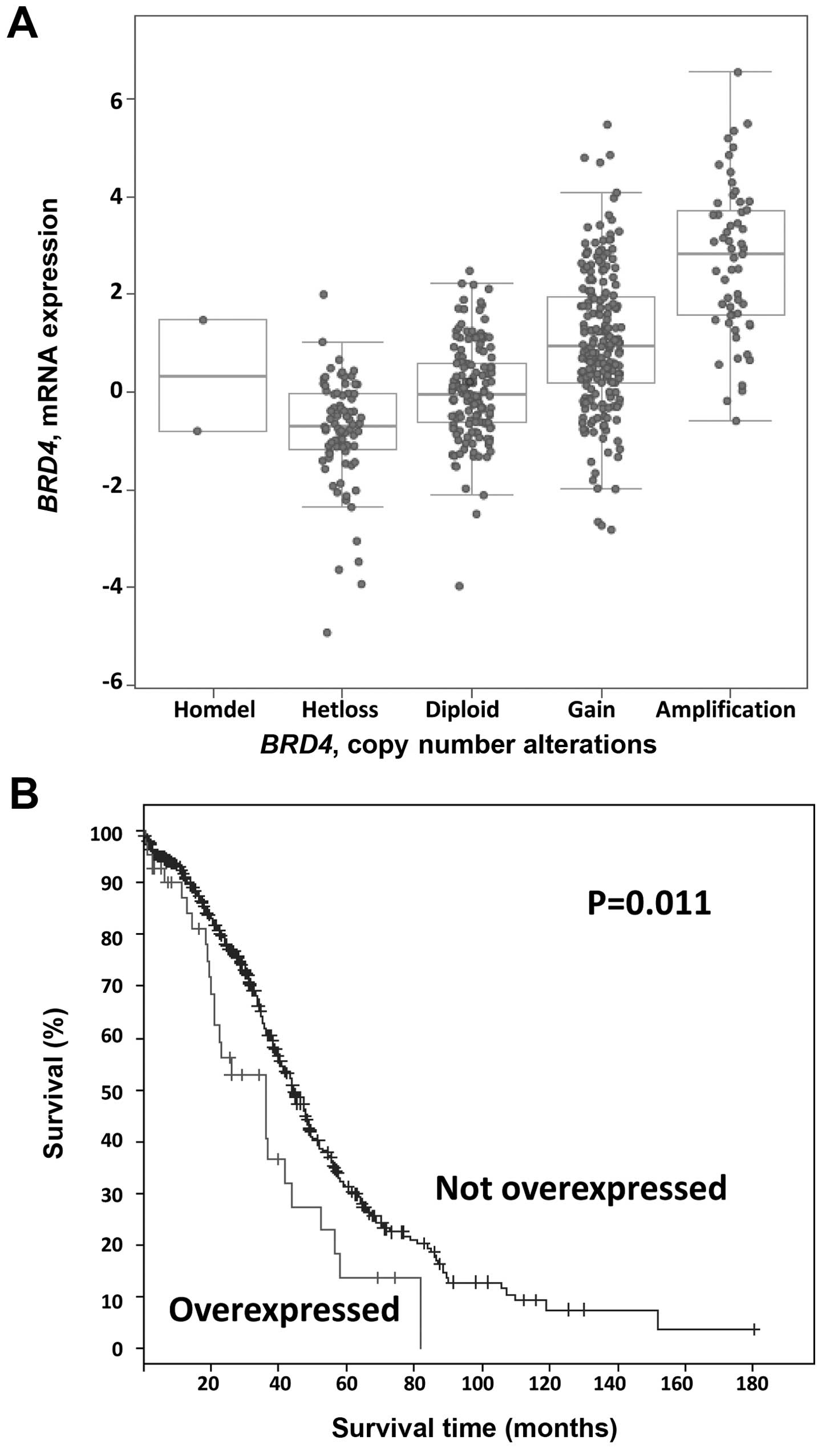
We next examined the mRNA data for these cases. As
shown in Fig. 2A, BRD4 mRNA
levels tended to increase with copy number. Using a stringent Z
score threshold of +3.0 to define significant upregulation,
BRD4 mRNA was increased in 9% (43/489) of the cases, and
increased BRD4 mRNA levels were associated with worse
overall survival (log-rank test P=0.01; Fig. 2B). By contrast, there were no
significant differences in progression-free survival in cases with
BRD4 mRNA upregulation (log-rank test P=0.59). Again using a
Z score threshold of +3.0, in 45% of the amplified cases (26/57),
BRD4 amplification resulted in mRNA upregulation.
Conversely, 60% (26/43) of tumors with high mRNA levels harbored an
underlying amplification.
Genes differentially expressed in
BRD4-amplified tumors
To elucidate the mechanism through which BRD4
amplification may affect patient survival, we assessed which genes
may be differentially expressed in BRD4-amplified tumors,
including CMYC or NMYC, which are known downstream
targets of BRD4 (7,13,14). In
the TCGA cohort, a subset of BRD4-amplified tumors exhibited
high CMYC and NMYC mRNA levels; however, there was
only a weak positive association between BRD4 copy number
and CMYC mRNA (Pearson, 0.130; and Spearman, 0.072), or
NMYC mRNA levels (Pearson, 0.119; and Spearman, 0.063) (data
not shown). By contrast, there was a strong association between
BRD4 amplification and increased mRNA levels of other genes
located in close proximity to BRD4, such as WIZ,
PRKACA, NOTCH3 and SMARCA4 (Table I), suggesting a potential
collaborative regional amplification effect. Future studies with
independent cohorts, cell culture and/or animal model systems are
required to address the potential mechanisms through which
BRD4 amplification contributes to the pathogenesis and
prognosis of ovarian HGSCS.
 | Table I.Differentially expressed genes (mRNA)
in bromodomain-containing protein 4 gene-amplified tumors. |
Table I.
Differentially expressed genes (mRNA)
in bromodomain-containing protein 4 gene-amplified tumors.
| Genes | Pearson | Spearman |
|---|
| WIZ | 0.79 | 0.80 |
| AKAP8 | 0.74 | 0.72 |
| CHERP | 0.69 | 0.70 |
| AKAP8L | 0.67 | 0.64 |
| SIN3B | 0.66 | 0.66 |
| DCAF15 | 0.66 | 0.58 |
| MYO9B | 0.65 | 0.65 |
| MED26 | 0.65 | 0.66 |
| PRKACA | 0.64 | 0.60 |
| CCDC130 | 0.62 | 0.58 |
| NACC1 | 0.61 | 0.60 |
| PKN1 | 0.60 | 0.55 |
| CC2D1A | 0.60 | 0.58 |
| AP1M1 | 0.59 | 0.58 |
| C19ORF44 | 0.59 | 0.57 |
| EPS15L1 | 0.59 | 0.59 |
| TECR | 0.58 | 0.56 |
| MAP1S | 0.57 | 0.56 |
| ZNF333 | 0.57 | 0.53 |
| NOTCH3 | 0.56 | 0.55 |
| SLC35E1 | 0.56 | 0.56 |
| TRMT1 | 0.55 | 0.50 |
| GTPBP3 | 0.55 | 0.53 |
| KRI1 | 0.55 | 0.53 |
| TNPO2 | 0.54 | 0.50 |
| JUND | 0.53 | 0.57 |
| RAD23A | 0.53 | 0.51 |
| SMARCA4 | 0.53 | 0.52 |
| TYK2 | 0.53 | 0.49 |
| ILF3 | 0.52 | 0.47 |
Discussion
BRD4 is a bromodomain protein and part of a
chromatin remodeling complex, which regulates gene expression by
binding acetylated chromatin at specific promoters and enhancers.
Via its bromodomains, BRD4 recruits positive transcription
elongation factor b and triggers the release of RNA polymerase II
from promoters, thereby resulting in productive transcriptional
elongation and active gene expression of genes such as CMYC
(13,15).
We exploited the TCGA data portal on ovarian
carcinomas to interrogate specific genetic events of druggable
genes associated with survival and clinicopathological
characteristics, as well as to generate hypotheses for future
testing. We herein propose that BRD4 amplification in
ovarian HGSCS may be associated with unfavorable prognosis and
survival. However, due to the limitations of the TCGA study (i.e.,
relatively short follow-up time and lack of information on other
comorbidities), these findings are exploratory and
hypothesis-generating in nature rather than definitive. Future
studies with independent patient cohorts are required to validate
the TGCA findings.
Furthermore, the mechanism through which BRD4
amplification may affect patient prognosis and survival remains
unclear. Amplification of BRD4 appears to segregate
preferentially to the non-BRCAness molecular subgroup of ovarian
HGSCS (16). A recently published
study suggested that BRD4 inhibitors may be effective in a subset
of ovarian HGSCS that exhibit high CMYC or MYCN levels (17). In the TCGA cohort, there was a weak
positive association between BRD4 copy number alterations
and CMYC or MYCN mRNA levels, suggesting that
survival effects may be MYC-independent. By contrast, there was a
strong association between BRD4 amplification and increased
mRNA levels of other genes located in close proximity to
BRD4, such as NOTCH3 and SMARCA4, suggesting a
potential collaborative regional amplification effect. Supporting
this hypothesis, SMARCA4 (a member of the SWI/SNF chromatin
remodeling complex, also known as Brg1) has been found to occupy
the same MYC enhancer sites as BRD4, thereby
enhancing MYC expression (18). In addition, NOTCH1 has been
shown to enhance MYC expression in T-cell leukemia
independently of BRD4 (19).
In ovarian HGSC, our finding that worse overall survival is also
observed with increased BRD4 mRNA levels in addition to
BRD4 amplification suggests that this survival effect may be
specific to BRD4 function.
Finally, we hypothesized that BRD4-amplified
tumors may exhibit increased sensitivity to newly developed BRD4
small-molecule inhibitors. Future studies, including animal
xenografts or cell culture models, are required to further
elucidate the mechanisms through which BRD4 amplification
and overexpression may affect tumor progression and patient
survival. In summary, we herein presented evidence that the
BRD4 gene is amplified and overexpressed in a subset of
HGSCS, and that its amplification and overexpression are associated
with worse prognosis and survival. Our findings suggest that
targeted inhibition of BRD4 with specific inhibitors should be
tested in a subset of ovarian carcinoma patients.
References
|
1
|
Romero I and Bast RC Jr: Minireview: Human
ovarian cancer: Biology, current management, and paths to
personalizing therapy. Endocrinology. 153:1593–1602. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cancer Genome Atlas Research Network, .
Integrated genomic analyses of ovarian carcinoma. Nature.
474:609–615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
BastRC Jr, Hennessy B and Mills GB: The
biology of ovarian cancer: New opportunities for translation. Nat
Rev Cancer. 9:415–428. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pal T, PermuthWey J, Betts JA, Krischer
JP, Fiorica J, Arango H, LaPolla J, Hoffman M, Martino MA, Wakeley
K, et al: BRCA1 and BRCA2 mutations account for a large proportion
of ovarian carcinoma cases. Cancer. 104:2807–2816. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Etemadmoghadam D, AuYeung G, Wall M,
Mitchell C, Kansara M, Loehrer E, Batzios C, George J, Ftouni S,
Weir BA, et al: Resistance to CDK2 inhibitors is associated with
selection of polyploid cells in CCNE1-amplified ovarian cancer.
Clin Cancer Res. 19:5960–5971. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dawson MA, Prinjha RK, Dittmann A,
Giotopoulos G, Bantscheff M, Chan WI, Robson SC, Chung CW, Hopf C,
Savitski MM, et al: Inhibition of BET recruitment to chromatin as
an effective treatment for MLL-fusion leukaemia. Nature.
478:529–533. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Delmore JE, Issa GC, Lemieux ME, Rahl PB,
Shi J, Jacobs HM, Kastritis E, Gilpatrick T, Paranal RM, Qi J, et
al: BET bromodomain inhibition as a therapeutic strategy to target
c-Myc. Cell. 146:904–917. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mertz JA, Conery AR, Bryant BM, Sandy P,
Balasubramanian S, Mele DA, Bergeron L and Sims RJ III: Targeting
MYC dependence in cancer by inhibiting BET bromodomains. Proc Natl
Acad Sci USA. 108:16669–16674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zuber J, Shi J, Wang E, Rappaport AR,
Herrmann H, Sison EA, Magoon D, Qi J, Blatt K, Wunderlich M, et al:
RNAi screen identifies Brd4 as a therapeutic target in acute
myeloid leukaemia. Nature. 478:524–528. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lovén J, Hoke HA, Lin CY, Lau A, Orlando
DA, Vakoc CR, Bradner JE, Lee TI and Young RA: Selective inhibition
of tumor oncogenes by disruption of super-enhancers. Cell.
153:320–334. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao J, Aksoy BA, Dogrusoz U, Dresdner G,
Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al:
Integrative analysis of complex cancer genomics and clinical
profiles using the cBioPortal. Sci Signal. 6:p112013. View Article : Google Scholar
|
|
13
|
Fu LL, Tian M, Li X, Li JJ, Huang J,
Ouyang L, Zhang Y and Liu B: Inhibition of BET bromodomains as a
therapeutic strategy for cancer drug discovery. Oncotarget.
6:5501–5516. 2015.PubMed/NCBI
|
|
14
|
Puissant A, Frumm SM, Alexe G, Bassil CF,
Qi J, Chanthery YH, Nekritz EA, Zeid R, Gustafson WC, Greninger P,
et al: Targeting MYCN in neuroblastoma by BET bromodomain
inhibition. Cancer Discov. 3:308–323. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi J and Vakoc CR: The mechanisms behind
the therapeutic activity of BET bromodomain inhibition. Mol Cell.
54:728–736. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Goundiam O, Gestraud P, Popova T, De la
Motte Rouge T, Fourchotte V, Gentien D, Hupé P, Becette V, Houdayer
C, RomanRoman S, et al: Histo-genomic stratification reveals the
frequent amplification/overexpression of CCNE1 and BRD4 genes in
non-BRCAness high grade ovarian carcinoma. Int J Cancer Apr.
17:2015.(Epub ahead of print).
|
|
17
|
Baratta MG, Schinzel AC, Zwang Y,
Bandopadhayay P, BowmanColin C, Kutt J, Curtis J, Piao H, Wong LC,
Kung AL, et al: An in-tumor genetic screen reveals that the BET
bromodomain protein, BRD4, is a potential therapeutic target in
ovarian carcinoma. Proc Natl Acad Sci USA. 112:232–237. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shi J, Whyte WA, ZepedaMendoza CJ, Milazzo
JP, Shen C, Roe JS, Minder JL, Mercan F, Wang E, EckersleyMaslin
MA, et al: Role of SWI/SNF in acute leukemia maintenance and
enhancer-mediated Myc regulation. Genes Dev. 27:2648–2662. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
YashiroOhtani Y, Wang H, Zang C, Arnett
KL, Bailis W, Ho Y, Knoechel B, Lanauze C, Louis L, Forsyth KS, et
al: Long-range enhancer activity determines Myc sensitivity to
Notch inhibitors in T cell leukemia. Proc Natl Acad Sci USA.
111:E4946–E4953. 2014. View Article : Google Scholar : PubMed/NCBI
|