Introduction
The systemic renin-angiotensin system (RAS) is
associated with cardiovascular regulation. Angiotensin I-converting
enzyme inhibitors (ACEIs) and angiotensin II type-1 receptor
blockers (ARBs) are among the most widely used antihypertensive
drugs. The local RAS reportedly promotes angiogenesis and vascular
proliferation via expression of vascular endothelial growth factor
(VEGF) or epidermal growth factor receptors (1,2). The use
of ACEIs was associated with a decreased cancer incidence in a
large cohort study, and the potential role of the local RAS in
carcinogenesis has attracted significant attention (3). For example, the growth of gastric cancer
cells was significantly suppressed by treatment with angiotensin II
type-1 receptor (AT1R) antagonists (4). Moreover, AT1R antagonists have been
found to prevent angiogenesis and growth of xenograft tumors
developed by human bladder cancer cells (5). AT1R antagonists induced downregulation
of AT1R expression in the endothelial cells of microvessels in
pancreatic cancer. Such downregulation of AT1R may weaken the
angiogenetic and tumor-proliferative effects of angiotensin
(6). Synergistic inhibition of tumor
growth through suppression of VEGF by combined gemcitabine (GEM)
and losartan treatment has been demonstrated in murine pancreatic
cancer (7). A retrospective analysis
by Nakai et al suggested that ACEIs or ARBs in combination
with GEM may improve clinical outcomes, in terms of overall
survival (OS) and progression-free survival (PFS), in patients with
advanced pancreatic cancer (8).
The systemic administration of oxaliplatin with
5-fluorouracil (5-FU) and leucovorin (FOLFOX) or capecitabine
(XELOX) and bevacizumab (Bev) is the standard first-line
chemotherapeutic regimen in the treatment of metastatic colorectal
cancer (mCRC). We hypothesized that ARBs in combination with
Bev-based chemotherapy may improve clinical outcomes in mCRC
patients. The aim of this study was to retrospectively analyze
clinical outcomes in mCRC patients receiving Bev, in order to
elucidate the effect of ARBs.
Patients and methods
Patients
All mCRC patients receiving first-line Bev-based
chemotherapy at the Department of Gastroenterology, The Cancer
Institute Hospital (Tokyo, Japan) between June, 2007 and September,
2010 were retrospectively investigated. The use of medications to
control hypertension (HT), including ARBs, was retrospectively
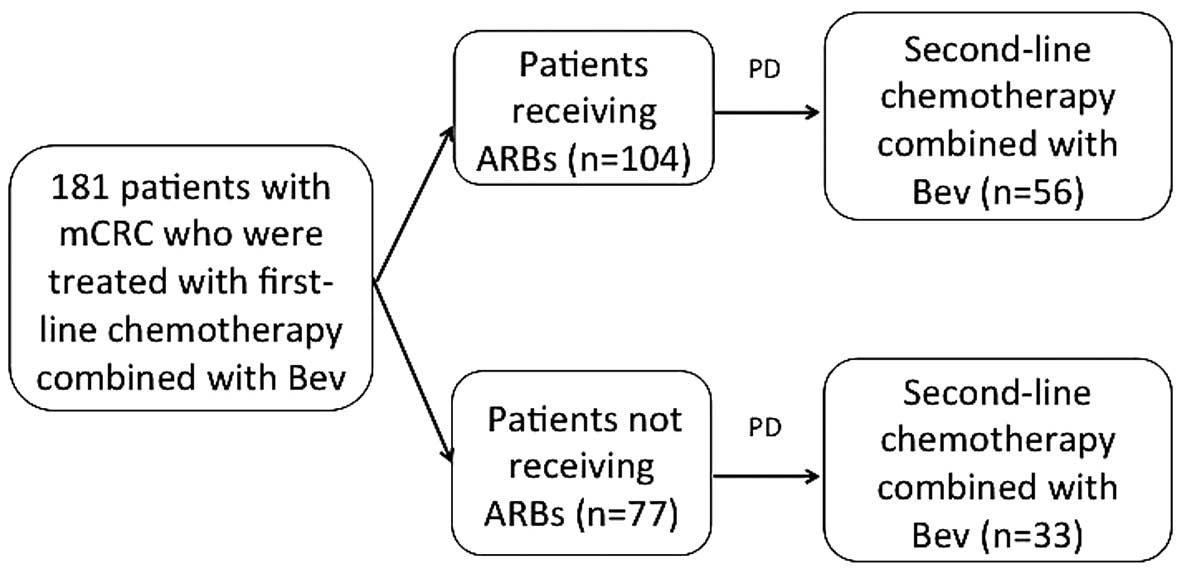
determined from the medical records and the patients were divided
into two groups: An ARB group (patients receiving ARBs as HT
medication), and a non-ARB group (Fig.
1).
This study was approved by the Institutional Review
Board of the Cancer Institute Hospital (registry no. 1244).
Treatment and tumor response
The FOLFOX regimen was administered as follows:
Oxaliplatin on day 1 at a dose of 85 mg/m2 as a 2-h
infusion concurrent with folinic acid 400 mg/m2/day,
followed by bolus 5-FU 400 mg/m2 and a 22-h infusion of
5-FU 2,400 mg/m2 for 2 consecutive days. Bev was
administered at a dose of 5 mg/kg in a 30-min intravenous infusion
on day 1 in 2-week cycles. The XELOX regimen was administered as
follows: Capecitabine 2,000 mg/m2 biweekly, plus
oxaliplatin 130 mg/m2 on day 1. Bev was administered at
a dose of 7.5 mg/kg in a 30-min intravenous infusion on day 1 in
3-week cycles. These regimens were repeated every 2 or 3 weeks,
until disease progression or development of unacceptable toxicity,
or until the patient requested treatment discontinuation. Tumor
response was assessed via computed tomography using the Response
Evaluation Criteria in Solid Tumors (RECIST), version 1.1 (9). The evaluation was repeated every 3 (or
4) courses, or more frequently in patients with clinically
suspected disease progression.
Statistical analysis
OS and PFS were estimated using the Kaplan-Meier
method and compared using the log-rank test. All the reported
P-values were the result of two-sided tests, with P<0.05
considered to indicate statistically significant differences. To
exclude possible confounding factors, a Cox proportional hazards
model was used to estimate hazard ratios (HRs) for the use of ARBs
adjusted for significant prognostic factors. The prognostic factors
included age (<65 or ≥65 years), gender (male or female),
performance status (0–1 or 2), site of metastasis (liver, lung,
lymph nodes, or peritoneum), multiple metastases (yes or no),
ascites (yes or no), treatment group (ARB or non-ARB) and HT (grade
0 or 1/2/3). The prognostic factors with P<0.2 in the univariate
analysis were included in the multivariate analysis.
Results
Patient characteristics
Among the 181 patients who received first-line
Bev-based chemotherapy, 104 received ARBs. The median follow-up
period was 2.2 years (26.7 months). No significant differences were
observed in the baseline clinical characteristics between the two
groups (Table I).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| A, Intention-to-treat
population (n=181) |
|
|
|---|
|
|---|
| Characteristics |
| ARB (n=104) | Non-ARB (n=77) |
|---|
| Gender, no. (%) |
|
|
|
| Male |
| 56 (53.9) | 44 (57.1) |
|
Female |
| 48 (46.1) | 33 (42.9) |
| Age, years [median
(range)] |
| 61.5 (38–75) | 55 (16–74) |
| <65,
no. (%) |
| 61 (58.7) | 61 (79.2) |
| ≥65, no.
(%) |
| 43 (41.3) | 16 (20.8) |
| ECOG PS at baseline,
no. (%) |
|
|
|
| 0 |
| 100 (96.2) | 74 (96.1) |
| 1 |
| 4 (3.8) | 3 (3.9) |
| Metastatic location,
no. (%) |
|
|
|
|
Liver |
| 47 (45.1) | 45 (58.4) |
| Lung |
| 43 (41.3) | 32 (41.5) |
| Lymph
nodes |
| 47 (45.1) | 44 (57.1) |
|
Multiple |
| 59 (56.7) | 55 (71.4) |
|
| B, ARB group |
|
|
|
|
| Characteristics | KRAS WT (n=63) | KRAS MT (n=30) | Unknown (n=11) |
|
| Gender, no. (%) |
|
|
|
| Male | 35 (55.6) | 14 (46.7) | 7 (63.6) |
|
Female | 28 (44.4) | 16 (53.3) | 4 (36.4) |
| Age, years [median
(range)] | 60.31 (38–74) | 64 (48–75) | 61.45 (46–73) |
| <65,
no. (%) | 39 (61.9) | 15 (50.0) | 7 (63.6) |
| ≥65, no.
(%) | 24 (38.1) | 15 (50.0) | 4 (36.4) |
| Metastatic location,
no. (%) |
|
|
|
|
Liver | 30 (47.6) | 12 (40.0) | 5 (45.4) |
| Lung | 23 (36.5) | 16 (53.3) | 4 (36.3) |
| Lymph
nodes | 31 (49.2) | 12 (40.0) | 4 (36.3) |
|
Multiple | 35 (55.5) | 19 (63.3) | 5 (45.4) |
|
| C, Non-ARB group |
|
|
|
|
| Characteristics | KRAS WT (n=47) | KRAS MT (n=16) | Unknown (n=14) |
|
| Gender, no. (%) |
|
|
|
| Male | 23 (48.9) | 12 (75.0) | 7 (50.0) |
|
Female | 24 (51.1) | 4 (25.0) | 7 (50.0) |
| Age, years [median
(range)] | 55.9 (27–73) | 55.6 (39–74) | 65.8 (16–71) |
| <65,
no. (%) | 39 (82.9) | 12 (75.0) | 12 (85.7) |
| ≥65, no.
(%) | 8 (17.1) | 4 (25.0) | 2 (14.3) |
| Metastatic location,
no. (%) |
|
|
|
|
Liver | 33 (70.2) | 7 (43.0) | 5 (35.7) |
| Lung | 21 (44.6) | 4 (25.0) | 7 (50.0) |
| Lymph
nodes | 24 (51.0) | 4 (25.0) | 11 (78.5) |
|
Multiple | 33 (70.2) | 9 (56.2) | 13 (92.8) |
Patient survival
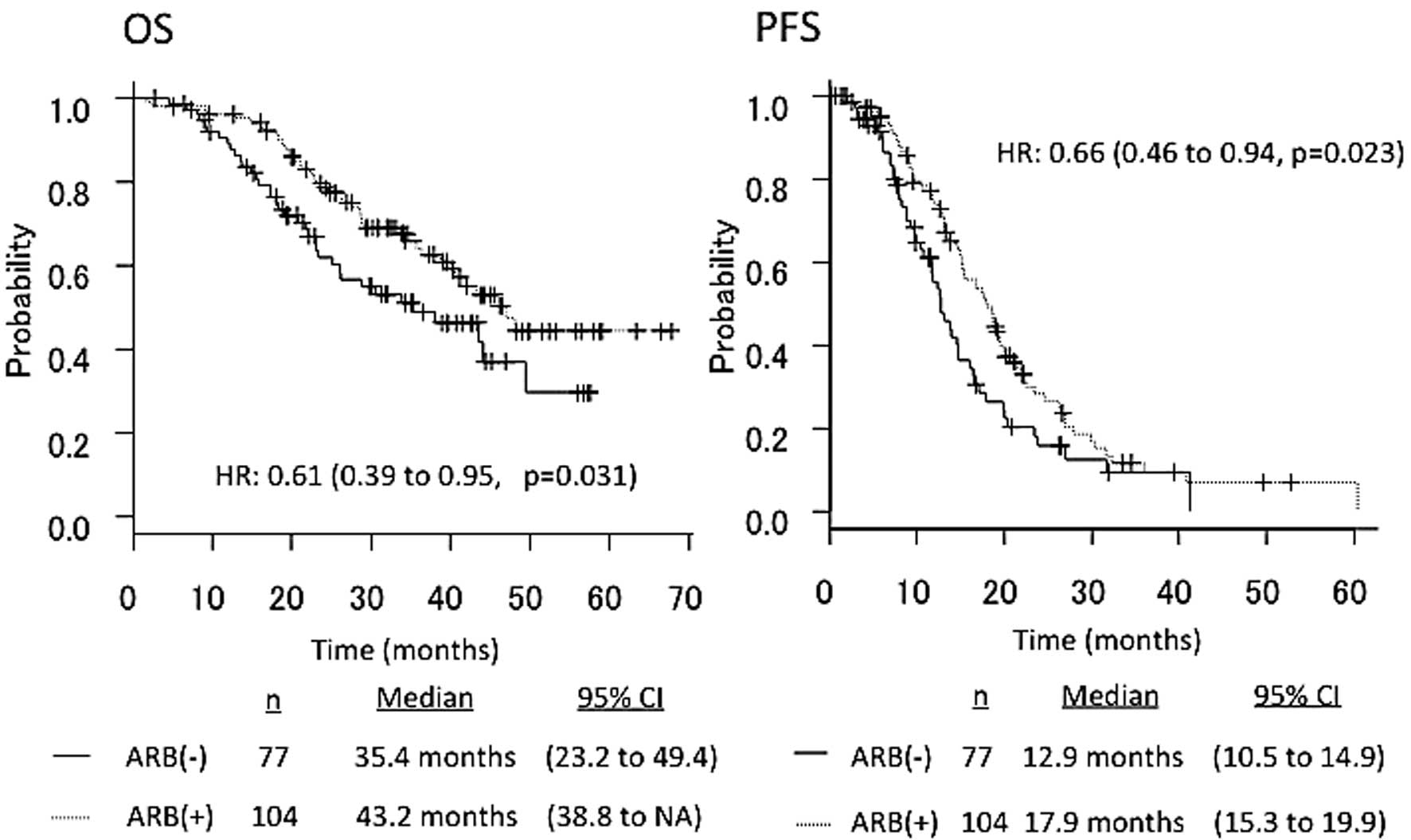
The median PFS in patients receiving ARBs (n=104)
vs. those not receiving ARBs (n=77) was 17.9 vs. 12.9 months,
respectively (HR=0.66, 95% CI: 0.46–0.94, P=0.023). The median OS
in patients receiving ARBs (n=104) vs. those not receiving ARBs
(n=77) was 43.2 vs. 35.4 months, respectively (HR=0.61, 95% CI:
0.39–0.95, P=0.031) (Fig. 2).
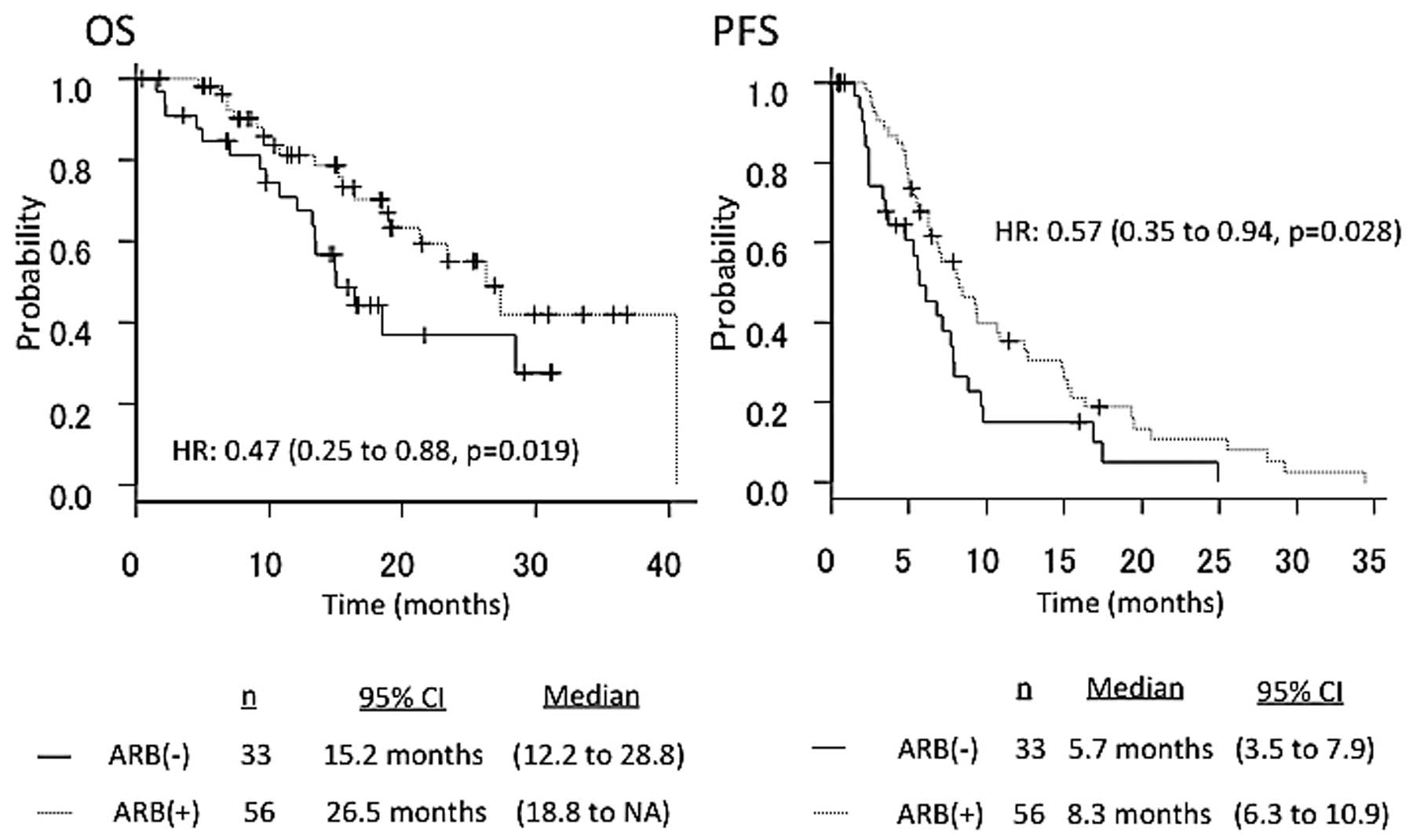
The median PFS in patients who underwent second-line
Bev-based chemotherapy with ARBs (n=56) vs. those without ARBs
(n=33) was 8.3 vs. 5.7 months, respectively (HR=0.57, 95% CI:
0.35–0.94, P=0.028). The median OS in patients who underwent
second-line Bev-based chemotherapy with ARBs (n=56) vs. those
without ARBs (n=33) was 26.5 vs. 15.2 months, respectively
(HR=0.47, 95% CI: 0.25–0.88, P=0.019) (Fig. 3). The overall response rates according
to RECIST were 68.5% (124/181) in total, 74.0% (77/104) in patients
receiving ARBs, and 61.0% (47/77) in patients not receiving ARBs
(Table II). In the multivariate
analysis, the use of ARBs was independently associated with
prolongation of OS and PFS (first- and second-line) (Table III).
 | Table II.Response to treatment in patients
undergoing first- and second-line chemotherapy in combination with
Bev and ARBs. |
Table II.
Response to treatment in patients
undergoing first- and second-line chemotherapy in combination with
Bev and ARBs.
| A, Overall response
rate in patients undergoing first-line chemotherapy in combination
with Bev and ARBs |
|---|
|
|---|
| Best overall
response, no. (%) | ARB (n=104) |
| Non-ARB (n=77) |
|---|
| Complete
response | 8 (7.7) |
| 4 (5.2) |
| Partial
response | 69 (66.3) |
| 44 (57.1) |
| Stable disease | 24 (23.1) |
| 19 (24.7) |
| Progressive
disease | 2 (1.9) |
| 5 (6.5) |
| Not evaluable | 1 (1.0) |
| 5 (6.5) |
| Best overall
response rate |
|
|
|
| All
patients, no. (%) | 77 (74.0) |
| 48 (61.0) |
| Odds
ratio (95% CI) |
| 1.81
(0.91–3.60) |
|
|
P-value |
| 0.075 |
|
|
| B, Disease control
rate in patients undergoing second-line chemotherapy in combination
with Bev and ARBs |
|
| Best overall
response, no. (%) | ARB (n=56) |
| Non-ARB (n=33) |
| Complete
response | 1 (1.8) |
| 0 (0.0) |
| Partial
response | 2 (3.6) |
| 1 (3.0) |
| Stable disease | 44 (78.6) |
| 19 (57.6) |
| Progressive
disease | 8 (14.2) |
| 13 (39.4) |
| Not evaluable | 1 (1.8) |
| 0 (0.0) |
| Disease control
rate |
|
|
|
| All
patients, no. (%) | 47 (83.9) |
| 20 (60.6) |
| Odds
ratio (95% CI) |
| 3.34
(1.11–10.4) |
|
|
P-value |
| 0.021 |
|
 | Table III.Univariate and multivariate
analyses. |
Table III.
Univariate and multivariate
analyses.
|
Characteristics | HR | 95% CI | P-value |
|---|
| First-line |
|
|
|
|
Univariate analysis |
|
|
|
|
OS |
|
|
|
|
Gender | 0.88 | 0.55–1.4 | 0.6 |
|
Age | 0.98 | 0.95–1 | 0.1 |
|
Ascites | 1.7 | 0.9–3.5 | 0.09 |
|
Metastatic
location |
|
|
|
|
Liver | 2.1 | 1.3–3.5 | 0.001 |
|
Lung | 0.95 | 1–1.5 | 0.84 |
|
Lymph
nodes | 2 | 1.2–3.2 | 0.004 |
|
Peritoneum | 1.37 | 0.8–2.1 | 0.18 |
|
Multiple | 2.2 | 1.3–3.8 | 0.001 |
|
Performance
status | 2.7 | 0.8–8.9 | 0.08 |
|
ARB | 0.6 | 0.37–0.96 | 0.03 |
|
Hypertension | 0.79 | 0.38–1.6 | 0.52 |
|
PFS |
|
|
|
|
Gender | 0.55 | 0.25–1.2 | 0.12 |
|
Age | 0.99 | 0.97–1.01 | 0.63 |
|
Ascites | 1.2 | 0.7–2 | 0.4 |
|
Metastatic
location |
|
|
|
|
Liver | 1.9 | 1.3–2.8 | 0.0002 |
|
Lung | 2 | 1.4–2.9 | 0.00007 |
|
Lymph
nodes | 1.04 | 0.73–1.49 | 0.8 |
|
Peritoneum | 1.8 | 1.2–2.6 | 0.002 |
|
Multiple | 1.2 | 0.4–3.5 | 0.72 |
|
Performance
status | 1.05 | 0.38–2.88 | 0.91 |
|
ARB | 0.66 | 0.46–0.94 | 0.02 |
|
Hypertension | 0.81 | 0.47–1.4 | 0.46 |
|
Multivariate analysis |
|
|
|
|
OS |
|
|
|
|
ARB | 0.64 | 0.40–1.0 | 0.056 |
|
Metastatic
location |
|
|
|
|
Liver | 1.92 | 1.21–3.0 | 0.005 |
|
Lymph
nodes | 2.1 | 1.3–3.3 | 0.0016 |
|
PFS |
|
|
|
|
ARB | 0.68 | 0.47–0.98 | 0.043 |
|
Metastatic
location |
|
|
|
|
Lung | 2.2 | 1.5–3.0 | 0.00005 |
|
Liver | 2.08 | 1.45–2.99 | 0.00006 |
| Second-line |
|
|
|
|
Univariate analysis |
|
|
|
|
OS |
|
|
|
|
Gender | 0.92 | 0.48–1.7 | 0.8 |
|
Age | 0.98 | 0.95–1 | 0.43 |
|
Ascites | 1.3 | 0.4–3.7 | 0.6 |
|
Metastatic
location |
|
|
|
|
Liver | 3.3 | 1.6–7 | 0.001 |
|
Lung | 0.54 | 0.27–1 | 0.08 |
|
Lymph
nodes | 2 | 1.2–3.2 | 0.004 |
|
Peritoneum | 1.5 | 0.8–2.9 | 0.18 |
|
Multiple | 3 | 1.3–6.8 | 0.007 |
|
Performance
status | 0.9 | 0.85–1.1 | 0.99 |
|
ARB | 0.47 | 0.25–0.88 | 0.019 |
|
Hypertension | 0.41 | 0.18–0.94 | 0.03 |
|
PFS |
|
|
|
|
Gender | 0.93 | 0.57–1.5 | 0.77 |
|
Age | 0.98 | 0.95–1.01 | 0.31 |
|
Ascites | 1.2 | 0.7–2 | 0.4 |
|
Metastatic
location |
|
|
|
|
Liver | 1.8 | 1.1–3 | 0.01 |
|
Lung | 0.93 | 0.58–1.4 | 0.7 |
|
Lymph
nodes | 1.7 | 1–2.7 | 0.03 |
|
Peritoneum | 1 | 0.66–1.7 | 0.73 |
|
Multiple | 1.8 | 1–3 | 0.025 |
|
Performance
status | 1 | 0.14–7.5 | 0.96 |
|
ARB | 0.57 | 0.35–0.9 | 0.028 |
|
Hypertension | 0.85 | 0.39–1.8 | 0.7 |
|
Multivariate analysis |
|
|
|
|
OS |
|
|
|
|
Metastatic
location |
|
|
|
|
Liver | 2.7 | 1.32–5.8 | 0.007 |
|
Lymph
nodes | 2.8 | 1.3–5.9 | 0.006 |
|
Peritoneum | 2.7 | 1.38–5.5 | 0.003 |
|
ARB | 0.45 | 0.24–0.86 | 0.01 |
|
PFS |
|
|
|
|
ARB | 0.49 | 0.3–0.82 | 0.006 |
|
Liver
metastasis | 2.1 | 1.3–3.5 | 0.002 |
Discussion
The use of ARBs has been associated with longer OS
and PFS in patients with mCRC who undergo first-line Bev-based
chemotherapy. This suggests that the suppression of RAS may inhibit
tumor growth and improve survival. Lever et al(3) reported that the use of ACEIs was
associated with a decreased cancer incidence in a large cohort
study and the potential role of the local RAS in carcinogenesis has
attracted significant attention. The involvement of the local RAS
in pancreatic cancer was suggested due to the expression of AT2 and
the AT1R in human pancreatic cancer (10,11). It
has been demonstrated that ACEIs and ARBs inhibit pancreatic cancer
cell proliferation in vitro and delays murine pancreatic
cancer progression in vivo via downregulation of VEGF
expression (12,13). However, the growth of gastric cancer
cells was significantly suppressed by treatment with AT1R
antagonists. AT1R antagonists were shown to prevent angiogenesis
and the growth of xenograft tumors developed by human bladder
cancer cells (5). The crucial role of
angiogenesis in tumor growth has been widely recognized, and
several reports have revealed that combination treatment with
conventional chemotherapeutic drugs and anti-angiogenic agents
exert synergistic anticancer effects (14). It has been reported that ARBs
clinically exert potent anti-angiogenic activity (7).
GEM exhibits a marked anticancer effect, as a result
of its cytotoxic action, and an anti-angiogenic effect. It has been
reported that GEM inhibited neovascularization in a human
pancreatic tumor in nude mice in a very low-dose metronomic
schedule. The synergistic inhibition of tumor growth through
suppression of VEGF by combined GEM and losartan treatment has been
demonstrated in murine pancreatic cancer. In addition, the
inhibition of RAS was also reported to induce apoptosis in
pancreatic cancer cells (15,16). A retrospective analysis by Nakai et
al suggested that ACEIs or ARBs in combination with GEM improve
clinical outcome in patients with advanced pancreatic cancer
(8).
We retrospectively analyzed the clinical outcome of
mCRC patients who underwent standard chemotherapy with Bev to
elucidate the effect of ARBs. The results demonstrated that the
presence of ARBs prior to the initiation of second-line
chemotherapy prolonged OS and PFS (first- and second-line). The
induction rate of second-line chemotherapy was similar between the
two groups (Table IV). The
development of Bev-induced arterial HT has recently been suggested
as a potential predictive marker. Certain studies have reported
that HT may predict Bev treatment efficacy, regardless of the
analyzed endpoint (OS, PFS, or response rate) (17–21). In
the present study, second-line OS tended to be longer in patients
developing HT. However, there was no significant difference between
the two groups in the multivariate analysis.
 | Table IV.Second-line anticancer treatment. |
Table IV.
Second-line anticancer treatment.
| Agents | ARB, no. (%) | Non-ARB, no.
(%) |
|---|
| Cetuximab | 44 (42.3) | 33 (42.8) |
| Panitummab | 5 (4.8) | 5 (6.4) |
| Bevacizumab | 58 (55.7) | 38 (49.3) |
| Irinotecan | 67 (64.4) | 51 (66.2) |
| Oxaliplatin | 5 (4.8) | 0 (0.0) |
| Capecitabine | 5 (4.8) | 5 (6.4) |
| 5-FU/FA | 60 (57.6) | 44 (57.1) |
| Other | 5 (4.8) | 1 (1.2) |
In conclusion, this study demonstrated that OS and
PFS were longer in mCRC patients who underwent Bev-based
chemotherapy with ARBs, compared with those who did not receive
ARBs. However, further prospective clinical trials are required to
verify this hypothesis.
Acknowledgements
S. Matsusaka received a commercial research grant
from Taiho Pharmaceutical Co., Ltd.; E. Shinozaki received
honoraria from the Speakers Bureau from Taiho Pharmaceutical Co.,
Ltd., Chugai Pharmaceutical Co., Ltd., Yakult Honsha Co., Ltd.,
Bristol-Myers Squibb and Takeda Pharmaceutical Co., Ltd.; N.
Mizunuma received commercial research grants from Taiho
Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Yakult
Honsha Co., Ltd., Bristol-Myers Squibb, Takeda Pharmaceutical Co.,
Ltd., Merck Serono Co., Ltd., ONO Pharmaceutical CO., Ltd. and
Bayer Yakuhin CO., Ltd.
References
|
1
|
Ager EI, Neo J and Christophi C: The
renin-angiotensin system and malignancy. Carcinogenesis.
29:1675–1684. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Khakoo AY, Sidman RL, Pasqualini R and
Arap W: Does the renin-angiotensin system participate in regulation
of human vasculogenesis and angiogenesis? Cancer Res. 68:9112–9115.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lever AF, Hole DJ, Gillis CR, McCallum IR,
McInnes GT, MacKinnon PL, Meredith PA, Murray LS, Reid JL and
Robertson JW: Do inhibitors of angiotensin-I-converting enzyme
protect against risk of cancer? Lancet. 352:179–184. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang W, Wu YL, Zhong J, Jiang FX, Tian XL
and Yu LF: Angiotensin II type 1 receptor antagonist suppress
angiogenesis and growth of gastric cancer xenografts. Dig Dis Sci.
53:1206–1210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kosugi M, Miyajima A, Kikuchi E, Kosaka T,
Horiguchi Y, Murai M and Oya M: Angiotensin II type 1 receptor
antagonist enhances cis-dichlorodiammineplatinum-induced
cytotoxicity in mouse xenograft model of bladder cancer. Urology.
73:655–660. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fujita M, Hayashi I, Yamashina S, Itoman M
and Majima M: Blockade of angiotensin AT1a receptor signaling
reduces tumor growth, angiogenesis and metastasis. Biochem Biophys
Res Commun. 294:441–447. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Noguchi R, Yoshiji H, Ikenaka Y, Namisaki
T, Kitade M, Kaji K, Yoshii J, Yanase K, Yamazaki M, Tsujimoto T,
et al: Synergistic inhibitory effect of gemcitabine and angiotensin
type-1 receptor blocker, losartan, on murine pancreatic tumor
growth via anti-angiogenic activities. Oncol Rep. 22:355–360.
2009.PubMed/NCBI
|
|
8
|
Nakai Y, Isayama H, Ijichi H, Sasaki T,
Sasahira N, Hirano K, Kogure H, Kawakubo K, Yagioka H, Yashima Y,
et al: Inhibition of renin-angiotensin system affects prognosis of
advanced pancreatic cancer receiving gemcitabine. Br J Cancer.
103:1644–1648. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ohta T, Amaya K, Yi S, Kitagawa H,
Kayahara M, Ninomiya I, Fushida S, Fujimura T, Nishimura G, Shimizu
K, et al: Angiotensin converting enzyme-independent, local
angiotensin II-generation in human pancreatic ductal cancer
tissues. Int J Oncol. 23:593–598. 2003.PubMed/NCBI
|
|
11
|
Fujimoto Y, Sasaki T, Tsuchida A and
Chayama K: Angiotensin II type 1 receptor expression in human
pancreatic cancer and growth inhibition by angiotensin II type 1
receptor antagonist. FEBS Lett. 495:197–200. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arafat HA, Gong Q, Chipitsyna G, Rizvi A,
Saa CT and Yeo CJ: Antihypertensives as novel antineoplastics:
angiotensin-I-converting enzyme inhibitors and angiotensin II type
1 receptor blockers in pancreatic ductal adenocarcinoma. J Am Coll
Surg. 204:996–1006. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fendrich V, Chen NM, Neef M, Waldmann J,
Buchholz M, Feldmann G, Slater EP, Maitra A and Bartsch DK: The
angiotensin-I-converting enzyme inhibitor enalapril and aspirin
delay progression of pancreatic intraepithelial neoplasia and
cancer formation in a genetically engineered mouse model of
pancreatic cancer. Gut. 59:630–637. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yanase K, Yoshiji H, Ikenaka Y, Noguchi R,
Kitade M, Kaji K, Yoshii J, Namisaki T, Yamazaki M, Asada K, et al:
Synergistic inhibition of hepatocellular carcinoma growth and
hepatocarcinogenesis by combination of 5-fluorouracil and
angiotensin-converting enzyme inhibitor via anti-angiogenic
activities. Oncol Rep. 17:441–446. 2007.PubMed/NCBI
|
|
15
|
Amaya K, Ohta T, Kitagawa H, Kayahara M,
Takamura H, Fujimura T, Nishimura G, Shimizu K and Miwa K:
Angiotensin II activates MAP kinase and NF-κB through angiotensin
II type I receptor in human pancreatic cancer cells. Int J Oncol.
25:849–856. 2004.PubMed/NCBI
|
|
16
|
Gong Q, Davis M, Chipitsyna G, Yeo CJ and
Arafat HA: Blocking angiotensin II type 1 receptor triggers
apoptotic cell death in human pancreatic cancer cells. Pancreas.
39:581–594. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Österlund P, Soveri LM, Isoniemi H, Poussa
T, Alanko T and Bono P: Hypertension and overall survival in
metastatic colorectal cancer patients treated with
bevacizumab-containing chemotherapy. Br J Cancer. 104:599–604.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tahover E, Uziely B, Salah A, Temper M,
Peretz T and Hubert A: Hypertension as a predictive biomarker in
bevacizumab treatment for colorectal cancer patients. Med Oncol.
30:3272013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dewdney A, Cunningham D, Barbachano Y and
Chau I: Correlation of bevacizumab-induced hypertension and outcome
in the BOXER study, a phase II study of capecitabine, oxaliplatin
(CAPOX) plus bevacizumab as peri-operative treatment in 45 patients
with poor-risk colorectal liver-only metastases unsuitable for
upfront resection. Br J Cancer. 106:1718–1721. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu Ryanne R, Lindenberg PA, Slack R, Noone
AM, Marshall JL and He AR: Evaluation of hypertension as a marker
of bevacizumab efficacy. J Gastrointest Cancer. 40:101–108. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Scartozzi M, Galizia E, Chiorrini S,
Giampieri R, Berardi R, Pierantoni C and Cascinu S: Arterial
hypertension correlates with clinical outcome in colorectal cancer
patients treated with first-line bevacizumab. Ann Oncol.
20:227–230. 2009. View Article : Google Scholar : PubMed/NCBI
|