Introduction
The emergence of tyrosine kinase inhibitors (TKIs)
has significantly changed chronic myeloid leukemia (CML) treatment
(1–3).
With imatinib, ~40% of chronic-phase (CP) CML patients achieve a
complete molecular response (CMR) within 5 years, as determined by
the sensitive reverse transcription quantitative polymerase chain
reaction (qPCR) analysis (4,5). The estimated overall survival (OS) rate
of CML patients treated with imatinib was reported to be 89 and 85%
at 5 and 8 years, respectively (6).
Next-generation TKIs have also been found to be effective for CML
(7,8).
Although CML patients primarily require lifelong treatment with
TKIs, recent clinical studies demonstrated that approximately half
of the patients in CMR who discontinued TKIs remained in CMR,
whereas the remaining patients lost CMR (9–11).
The high cost of TKIs may be prohibitive for their
administration (12). A significant
proportion of patients have decided to discontinue TKI treatment,
but their outcomes have not been reported. The aim of this study
was to investigate the natural course of patients who voluntarily
discontinue TKI treastment.
Patients and methods
Patients
The medical records from the Hakodate Municipal
Hospital were reviewed to identify all Philadelphia
chromosome-positive CP-CML patients aged >18 years who achieved
CMR with TKIs, such as imatinib, nilotinib and dasatinib, between
August, 2002 and March, 2013. Certain patients had a history of
prior treatment with interferon, hydroxycarbamide or busulfan. Our
study protocol was approved by the Hakodate Municipal Hospital
Institutional Review Board. Based on the Declaration of Helsinki,
written informed consent was obtained from all participating
patients.
Treatment
The patients were treated according to the European
LeukemiaNet recommendations (13). We
monitored the BCR-ABL transcript levels in the peripheral blood
based on the recommendations of the Europe Against Cancer Program
(14). CMR was defined as no
detection of BCR-ABL/ABL transcript. The limit of detection with
this method was <2×105 copy/µgRl. A complete
hematological response (CHR) was defined as a white blood cell
count of <1.0×104/l, a platelet count of
<45×104/l, a proportion of basophils <5%, with no
blast cells in the peripheral blood and no splenomegaly. qPCR
analysis of the peripheral blood was performed once a month for 2
years after the initiation of TKI therapy and once every 3 months
thereafter. Following TKI discontinuation, qPCR analysis was
performed once a month.
Analysis
The OS was defined as the time from the initiation
of TKI treatment until death from any cause or the date of the last
follow-up. In patients who discontinued TKI treatment, event-free
survival (EFS) was defined as the time from TKI discontinuation to
molecular relapse (loss of CMR) or the date of the last molecular
evaluation. These values were estimated using the Kaplan-Meier
method. Statistical analysis was performed using a log-rank test. A
P-value of ≤0.05 was considered to indicate statistically
significant differences.
Results
Patient characteristics
We evaluated a total of 46 newly diagnosed CML-CP
patients who were treated with various TKIs and achieved CMR
(Table I). The first-line TKI
treatment was imatinib in 38, dasatinib in 6 and nilotinib in 2
patients. Prior to TKI treatment, additional cytogenetic
abnormalities were detected by G-band analysis in 2 patients. With
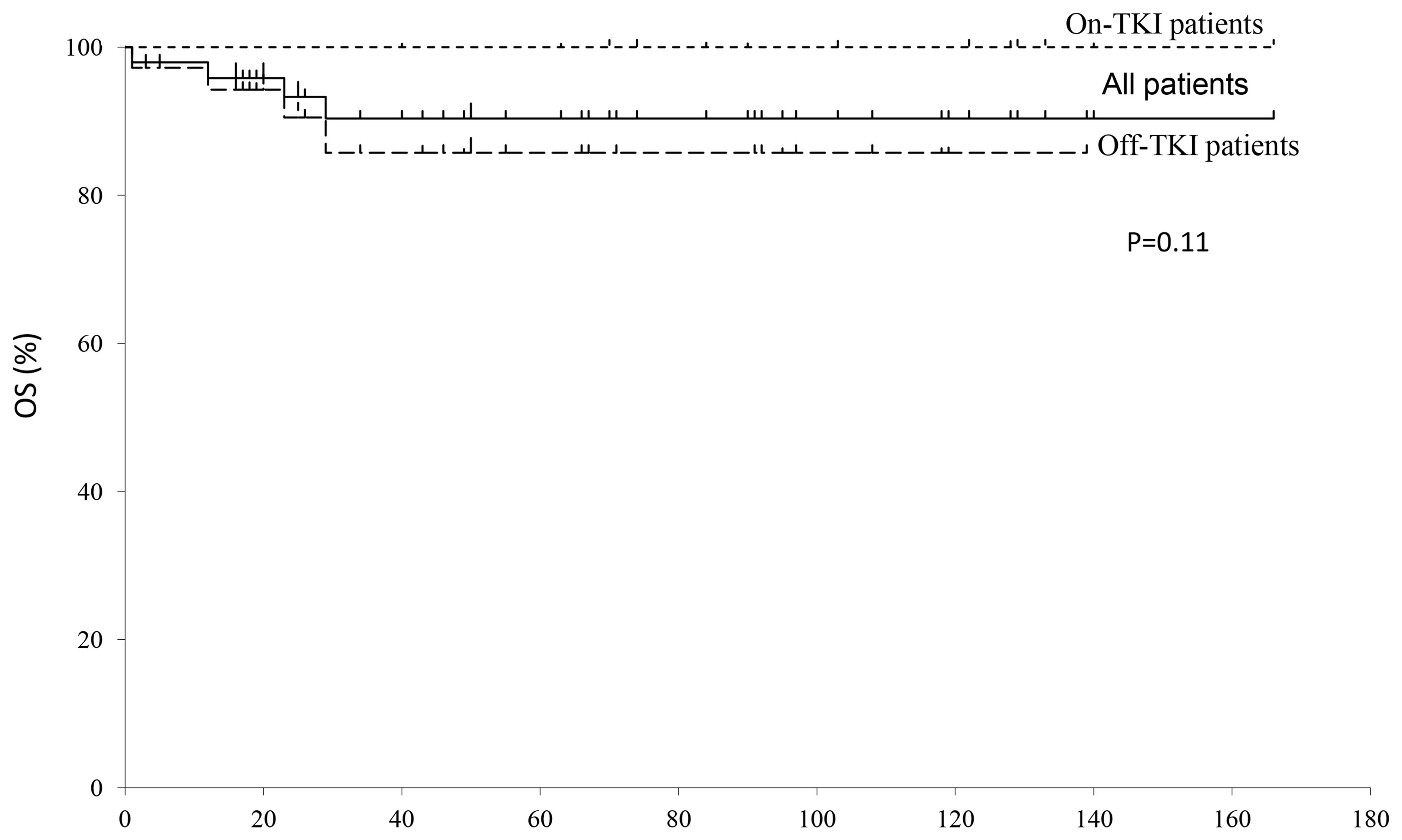
a median follow-up of 66 months, the 8-year OS rate of all the
patients was 83% (Fig. 1). There was
no significant difference in the OS rate between patients who
continued (86%) and those who discontinued TKI treatment
(100%).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristics | On TKIs (n=33) | Off TKIs (n=13) | P-value |
|---|
| Median age, years
(range) | 64 (34–84) | 56 (39–85) | 0.6661a |
| Gender
(male/female) | 24/9 | 10/3 | 0.999b |
| First-line TKI |
|
|
0.0226c |
|
Imatinib | 25 | 13 |
|
|
Dasatinib | 6 | 0 |
|
|
Nilotinib | 6 | 0 |
|
Characteristics of patients who
discontinued TKI treatment
In patients who discontinued TKIs, the median
duration of the treatment until discontinuation was 74 months
(range, 27–158 months) (Table II).
The reasons for treatment disconinuation were as follows: A total
of 5 patients were unable to receive TKIs due to surgery (1 for a
traffic accident and 4 for malignancy) and refused TKI treatment
after recovery. The remaining 8 patients declined TKI treatment due
to their high cost.
 | Table II.Characteristics of patients who
discontinued TKI treatment. |
Table II.
Characteristics of patients who
discontinued TKI treatment.
| Characteristics | No. |
|---|
| Duration of TKI
therapy, months | 74 (27–158) |
| Median
(range) |
|
| Reasons for TKI
discontinuation |
|
| Surgery
for accidents | 1 |
| Surgery
for malignancy | 4 |
| High
cost | 8 |
Patient outcomes
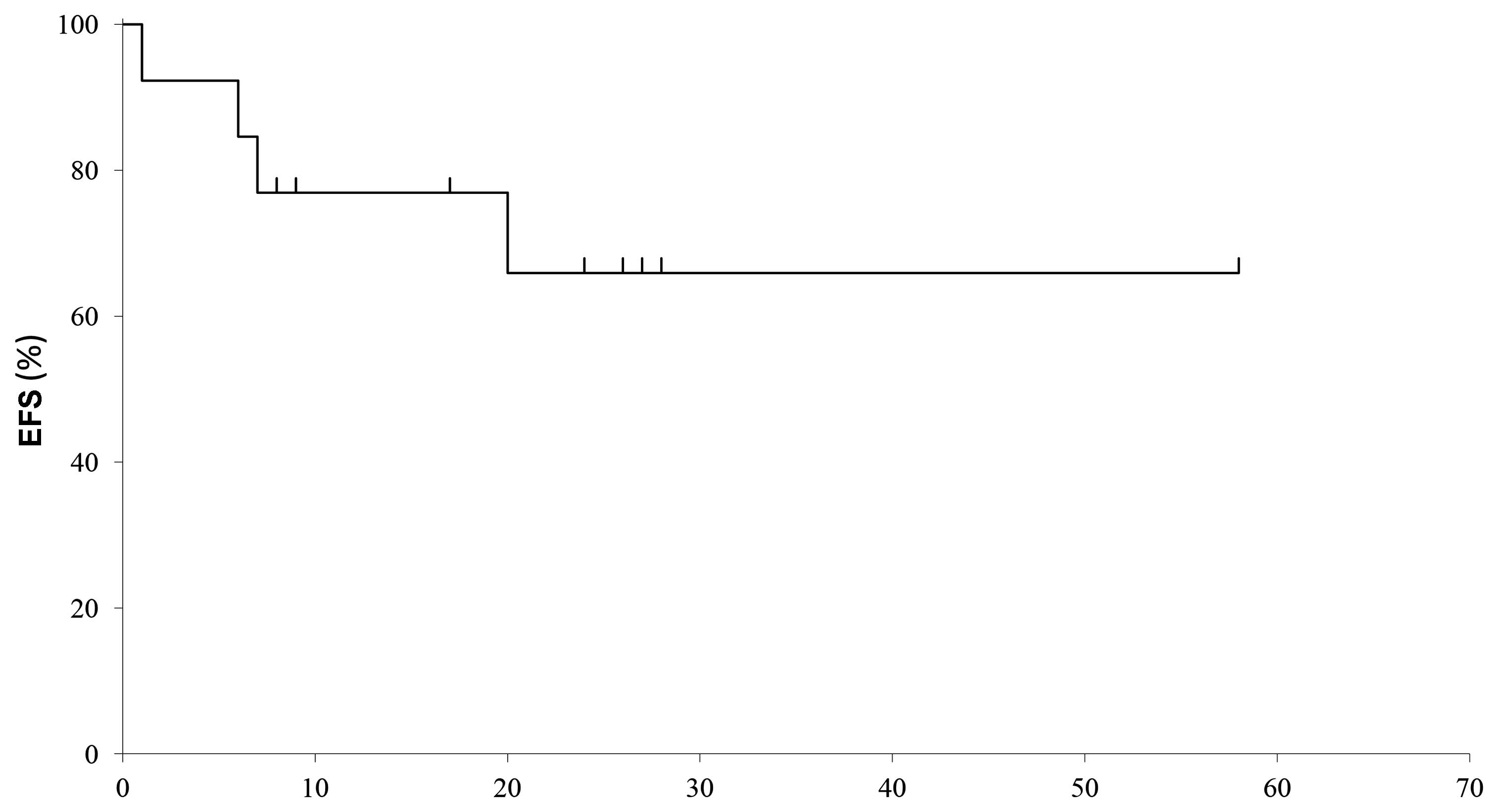
The median follow-up of the patients after TKI
discontinuation was 28 months (range, 10–60 months) (Table III). The 5-year EFS was 67% and the
median duration of CMR was 20 months (range, 1–60 months) (Fig. 2).
 | Table III.Patient outcome. |
Table III.
Patient outcome.
| Outcome | On TKIs (n=33) | Off TKIs (n=13) |
|---|
| Median follow-up,
months (range) | 34 (1–143) | 107 (44–177) |
| Median follow-up
after TKI | – | 28 (10–60) |
| discontinuation,
months (range) |
|
|
| Disease status |
|
|
| First
CMR | 20 | 7 |
| Second
CMR | 1 | 4 |
| CHR | 11 | 2 |
| Disease
progression | 1 | 0 |
| Cause of death | 4 | 0 |
| Lung
cancer | 1 |
|
| Pulmonary
infarction | 1 |
|
| Uterine
cancer | 1 |
|
| CML blast
crisis | 1 |
|
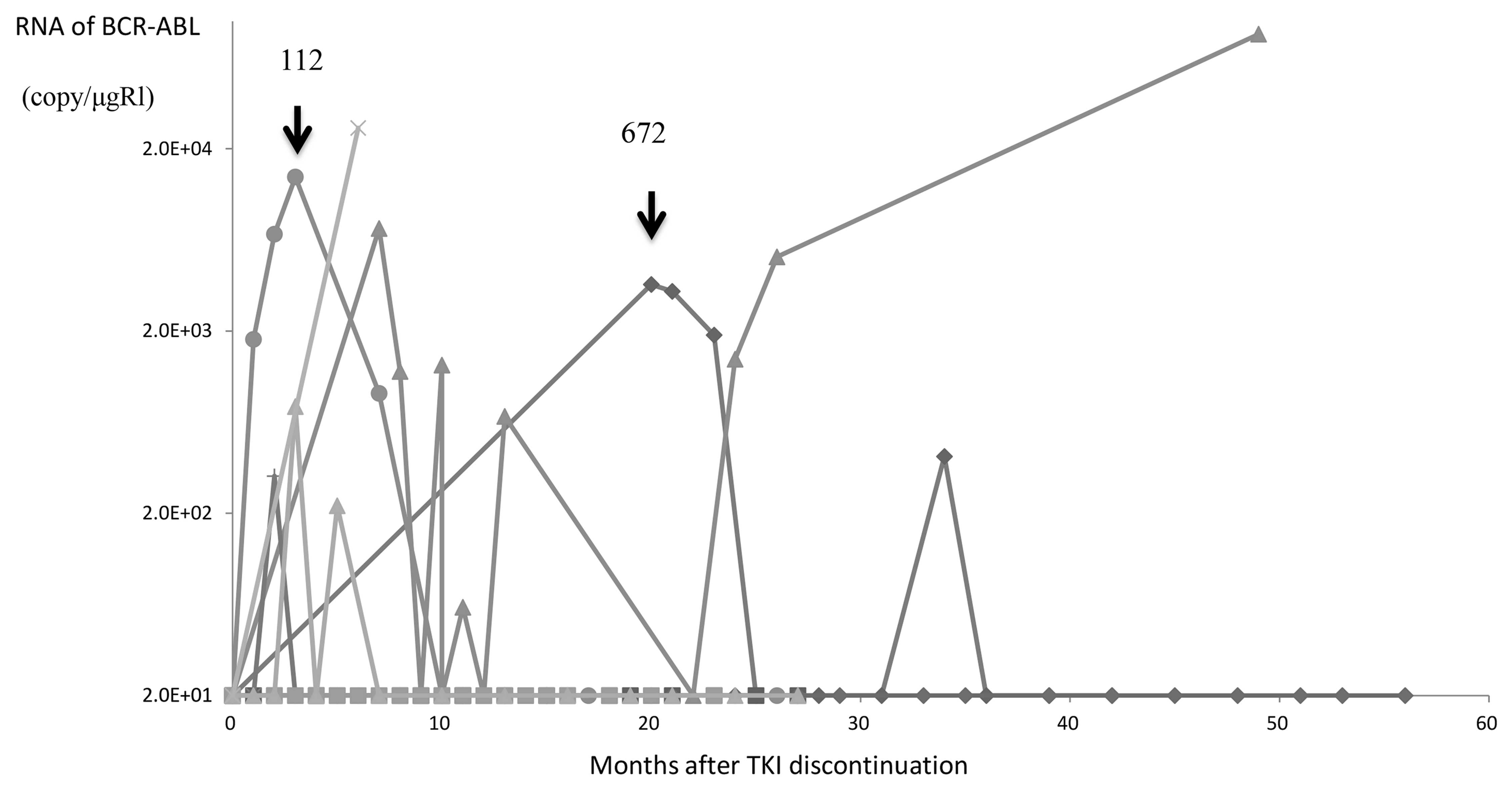
Of the 13 patients who discontinued TKIs, 7 remained
in CMR with a median follow-up of 26 months (range, 10–60 months)
(Table III), whereas the remaining
6 patients lost CMR, with a median follow-up of 29.5 months (range,
10–52 months). The median time to CMR loss after TKI
discontinuation was 6.5 months (range, 1–20 months). The BCR-ABL
levels after TKI discontinuation are presented in Fig. 3. Of the 6 patients who lost CMR, 2
were re-treated with TKIs and attained a second CMR; 2
spontaneously attained a second CMR without TKI treatment; and the
remaining 2 patients lost CMR but remained in CHR without any
treatment for 43–126 months. A total of 4 patients on TKI treatment
who succumbed to the disease were aged >75 years, of whom 2
patients had solid cancers (lung cancer, n=1; and uterine cancer,
n=1); for these 2 patients, the spleen size was not measured at
diagnosis, so the Sokal or Hasford scores could not be calculated.
The remaining 2 patients were classified as a high-risk Sokal score
group; in 1 patient, severe thrombocytosis was found at diagnosis
and led to pulmonary infarction and death; the other patient was
unresponsive to all types of TKIs, developed a blast crisis and
succumbed to CML. There were no mortalities among patients who
discontinued TKI treatment.
Discussion
TKI discontinuation has been investigated in a
number of clinical trials in CML patients who have been in CMR. In
a study by Rousselot et al (9), where imatinib was discontinued in
patients who had been in CMR for >2 years (range, 26–45 months),
approximately half of the patients experienced a molecular relapse
within 6 months following discontinuation. No late relapse was
observed 4 years after TKI discontinuation. In the Stop Imatinib
(STIM) study, 61% of the patients lost CMR, mostly within the first
6 months following treatment discontinuation (15). The predictive factors for
treatment-free remission are the Sokal score and the length of the
imatinib treatment (12,15–17).
However, the Sokal score could not be analysed, as the spleen size
on admission was not recorded for all the patients in this
study.
In our study, the probability of CMR persistence was
41%. The OS in patients who discontinued TKI treatment in our study
almost equaled that in prospective TKI stop studies (15,18–20).
Interestingly, no statistical differences in OS were observed
between patients who continued and those who discontinued TKI
treatment in our analysis, suggesting that survival was not always
compromised by molecular relapse following TKI discontinuation,
even without TKI re-treatment.
A total of 2 patients spontaneously attained a
second CMR without TKI treatment, and 2 patients remained in CHR
without any treatment for 43 and 126 months. These findings suggest
that immediate re-treatment with TKIs may not always be necessary
for patients who had been in CMR for >2 years and lost CMR
following TKI discontinuation. This study was conducted
retrospectively using a single-center analysis, which raises the
risk of bias; thus, additional investigation is required to
elucidate this issue.
References
|
1
|
Druker BJ, Tamura S, Buchdunger E, Ohno S,
Segal GM, Fanning S, Zimmermann J and Lydon NB: Effects of a
selective inhibitor of the Abl tyrosine kinase on the growth of
Bcr-Abl positive cells. Nat Med. 2:561–566. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Deininger MW, Goldman JM and Melo JV: The
molecular biology of chronic myeloid leukemia. Blood. 96:3343–3356.
2000.PubMed/NCBI
|
|
3
|
O'Brien SG, Guilhot F, Larson RA, Gathmann
I, Baccarani M, Cervantes F, Cornelissen JJ, Fischer T, Hochhaus A,
Hughes T, et al: IRIS Investigators: Imatinib compared with
interferon and low-dose cytarabine for newly diagnosed
chronic-phase chronic myeloid leukemia. N Engl J Med. 348:994–1004.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Colombat M, Fort MP, Chollet C, Marit G,
Roche C, Preudhomme C, Reiffers J, Praloran V and Mahon FX:
Molecular remission in chronic myeloid leukemia patients with
sustained complete cytogenetic remission after imatinib mesylate
treatment. Haematologica. 91:162–168. 2006.PubMed/NCBI
|
|
5
|
Branford S, Seymour JF, Grigg A, Arthur C,
Rudzki Z, Lynch K and Hughes T: BCR-ABL messenger RNA levels
continue to decline in patients with chronic phase chronic myeloid
leukemia treated with imatinib for more than 5 years and
approximately half of all first-line treated patients have stable
undetectable BCR-ABL using strict sensitivity criteria. Clin Cancer
Res. 13:7080–7085. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Druker BJ, Guilhot F, O'Brien SG, Gathmann
I, Kantarajian H, Gattermann N, Deininger MW, Silver RT, Goldman
JM, Stone RM, et al: IRIS Investigators: Five-year follow-up of
patients receiving imatinib for chronic myeloid leukemia. N Engl J
Med. 355:2408–2417. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Larson RA, Hochhaus A, Hughes TP, Clark
RE, Etienne G, Kim DW, Flinn IW, Kurokawa M, Moiraghi B, Yu R, et
al: Nilotinib vs imatinib in patients with newly diagnosed
Philadelphia chromosome-positive chronic myeloid leukemia in
chronic phase: ENESTnd 3-year follow-up. Leukemia. 26:2197–2203.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kantarjian HM, Shah NP, Cortes JE,
Baccarani M, Agarwal MB, Undurraga MS, Wang J, Ipiña JJ, Kim DW,
Ogura M, et al: Dasatinib or imatinib in newly diagnosed
chronic-phase chronic myeloid leukemia: 2-year follow-up from a
randomized phase 3 trial (DASISION). Blood. 119:1123–1129. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rousselot P, Huguet F, Rea D, Legros L,
Cayuela JM, Maarek O, Blanchet O, Marit G, Gluckman E, Reiffers J,
et al: Imatinib mesylate discontinuation in patients with chronic
myelogenous leukemia in complete molecular remission for more than
2 years. Blood. 109:58–60. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ross DM, Grigg A, Schwarer A, Arthur C,
Loftus K, Mills AK, et al: The majority of chronic myeloid leukemia
patients who cease imatinib after achieving a sustained complete
molecular response (CMR) remain in CMR, and any relapses occur
early. Blood (ASH Annual Meeting abstracts). 112:402–403. 2008.
|
|
11
|
Mahon FX, Rea D, Guilhot F, Legros L,
Guilhot J, Aton E, Dulucq S, Reiffers J and Rousselot P:
Persistence of complete molecular remission in chronic myeloid
leukemia after imatiib discontinuation: Interim analysis of the
STIM trial. J Clin Oncol. May 20;Supplement. 27:2009.
|
|
12
|
Ross DM and Hughes TP: How I determine if
and when to recommend stopping tyrosine kinase inhibitor treatment
for chronic myeloid leukaemia. Br J Haematol. 166:3–11. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Baccarani M, Cortes J, Pane F,
Niederwieser D, Saglio G, Apperley J, Cervantes F, Deininger M,
Gratwohl A, Guilhot F, et al: European LeukemiaNet: Chronic myeloid
leukemia: An update of concepts and management recommendations of
European LeukemiaNet. J Clin Oncol. 27:6041–6051. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gabert J, Beillard E, van der Velden VHJ,
Bi W, Grimwade D, Pallisgaard N, Barbany G, Cazzaniga G, Cayuela
JM, Cavé H, et al: Standardization and quality control studies of
‘real-time’ quantitative reverse transcriptase polymerase chain
reaction of fusion gene transcripts for residual disease detection
in leukemia-A Europe Against Cancer Program. Leukemia.
17:2318–2357. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mahon FX, Réa D, Guilhot J, Guilhot F,
Huguet F, Nicolini F, Legros L, Charbonnier A, Guerci A, Varet B,
et al: Intergroupe Français des Leucémies Myéloïdes Chroniques:
Discontinuation of imatinib in patients with chronic myeloid
leukaemia who have maintained complete molecular remission for at
least 2 years: The prospective, multicentre Stop Imatinib (STIM)
trial. Lancet Oncol. 11:1029–1035. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yhim HY, Lee NR, Song EK, Yim CY, Jeon SY,
Shin S, Kim JA, Kim HS, Cho EH and Kwak JY: Imatinib mesylate
discontinuation in patients with chronic myeloid leukemia who have
received front-line imatinib mesylate therapy and achieved complete
molecular response. Leuk Res. 36:689–693. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ross DM, Bartley PA, Goyne J, Morley AA,
Seymour JF and Grigg AP: Durable complete molecular remission of
chronic myeloid leukemia following dasatinib cessation, despite
adverse disease features. Haematologica. 96:1720–1722. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ross DM, Branford S, Seymour JF, Schwarer
AP, Arthur C, Bartley PA, Slader C, Field C, Dang P, Filshie RJ, et
al: Patients with chronic myeloid leukemia who maintain a complete
molecular response after stopping imatinib treatment have evidence
of persistent leukemia by DNA PCR. Leukemia. 24:1719–1724. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mahon FX, Nicolini FE, Noel MP, Escoffre
M, Charbonnier A, Rea D, Dubruille V, Varet BR, Legros L, Guerci A,
et al: Preliminary report of the STIM2 study: A multicenter stop
imatinib trial for chronic phase chronic myeloid leukemia de novo
patients on imatinib. Blood. 122:6542013.
|
|
20
|
Rousselot P, Charbonnier A, Cony-Makhoul
P, Agape P, Nicolini FE, Varet B, Gradembas M, Etienne G, Rea D,
Roy L, et al: Loss of major molecular kinase inhibitor therapy in
patients with chronic-phase chronic myelogenous leukemia who have
stopped imatinib after durable undetectable disease. J Clin Oncol.
32:424–430. 2014. View Article : Google Scholar : PubMed/NCBI
|