Introduction
Although epithelial ovarian cancer (EOC) accounts
for only a relatively small proportion of cancers among women, it
is the leading cause of death from gynecological malignancies
(1). Despite the advances in surgery
and chemotherapy, the 5-year survival rate remains only ~30%,
mainly due to the fact that these tumors are commonly diagnosed at
an advanced stage (2). Thus, further
investigation of the molecular mechanism underlying EOC metastasis
is crucial.
Zinc finger E-box-binding homeobox 1 (ZEB1) is known
to be an important regulator of epithelial-to-mesenchymal
transition (EMT), which is required for cancer development and
metastasis (3). ZEB1 promotes EMT by
repressing genes, such as E-cadherin, which are involved in
maintaining the epithelial phenotype, and activating those required
for transformation to the mesenchymal phenotype (4,5). ZEB1 is a
190–210-kD protein, previously described as a transcriptional
factor, repressor of cell adhesion molecules and cell
polarity-associated genes (6).
Aberrant expression of ZEB-1 in numerous cancers has been
associated with aggressive disease, poor differentiation, rapid
development of metastases and poor clinical outcome (7–11).
Furthermore, high levels of ZEB1 promote the progression of
gynecological cancer (12). However,
the expression status of the ZEB1 protein in human ovarian
carcinoma tissues and its role in clinical outcome requires further
elucidation.
To investigate the expression pattern of ZEB1 in EOC
tissues and evaluate its association with tumor progression and
patient prognosis, we evaluated the expression of ZEB1 in 238 cases
of ovarian cancer by immunohistochemistry (IHC) and analyzed the
association between ZEB1 expression and clinicopathological
parameters, including survival probability of EOC.
Patients and methods
Ethics statement
This study was approved by the Regional Committee
for Medical Research Ethics South of Norway (S-06277a), The Social-
and Health Directorate (06/3280) and The Data Inspectorate
(06/5345).
Patients and materials
This study included 238 patients with EOC. All the
patients underwent surgery at the Norwegian Radium Hospital, Oslo
University Hospital (Oslo, Norway) between March, 1983 and May,
2001. Informed consent was obtained according to the institutional
and national guidelines. The median age of the patients was 58
years (range, 19–89 years). The patients were followed up until
January 1st, 2012. All the patients were clinically staged
according to the International Federation of Gynecologists and
Obstetricians (FIGO) staging system (13). The primary tumors were histologically
graded as well-, moderately and poorly differentiated, according to
the recommendations of the World Health Organisation (13). Disease progression was determined
based on the definitions outlined by the Gynecologic Cancer
Intergroup (14). Paraffin-embedded
ovarian carcinoma tissues were obtained from the Department of
Pathology, and 3-µm sections were cut and used for morphological
examination and IHC.
IHC
Paraffin sections were immunostained by Dako
Autostainer using Dako Envision™ FLEX+ system (K8012; Dako,
Glostrup, Denmark) as described in our previous study (15). Briefly, the sections were
deparaffinized, epitopes were unmasked in PT-link with low pH
target retrieval solution (Dako) and then blocked with peroxidase
blocking solution (Dako) for 5 min. The slides were incubated at
room temperature with polyclonal rabbit anti-human ZEB1 antibody
(cat. no. HPA027524; 1:500; Sigma-Aldrich, St. Louis, USA),
followed by incubation with rabbit linker for 15 min and
horseradish peroxidase for 30 min at room temperature. The slides
were subsequently stained with 3,3′-diaminobenzidine
tetrahydrochloride for 10 min and counter-stained with hematoxylin,
dehydrated, and mounted in Richard-Allan Scientific Cytoseal XYL
(Thermo Fisher Scientific, Waltham, MA, USA). A known ZEB1-positive
human esophageal carcinoma slide was used as positive control.
Serial negative controls were tested by the same concentration of
normal rabbit serum as a substitute for the rabbit anti-human ZEB1
antibody.
IHC scoring method
The immunostaining of ZEB1 was evaluated by two
pathologists (Z.S. and J.M.N.) from the Norwegian Radium Hospital.
Only nuclear staining of ZEB1 was considered in this study. The
case was classified as positive if immunostaining was observed in
>10% of the tumor cells, as described in our previous study
(16).
Statistical analysis
SPSS software, version 18.0 (SPSS Inc., Chicago, IL,
USA) was used for all the data analyses. Associations between
categorical variables were analyzed by Chi-square tests (Pearson's
and linear-by-linear, as appropriate). The Kaplan-Meier method was
used for survival analysis and groups were compared with log-rank
tests. For all the analyses, P<0.05 was considered as
statistically significant.
Results
ZEB1 expression in tumor samples
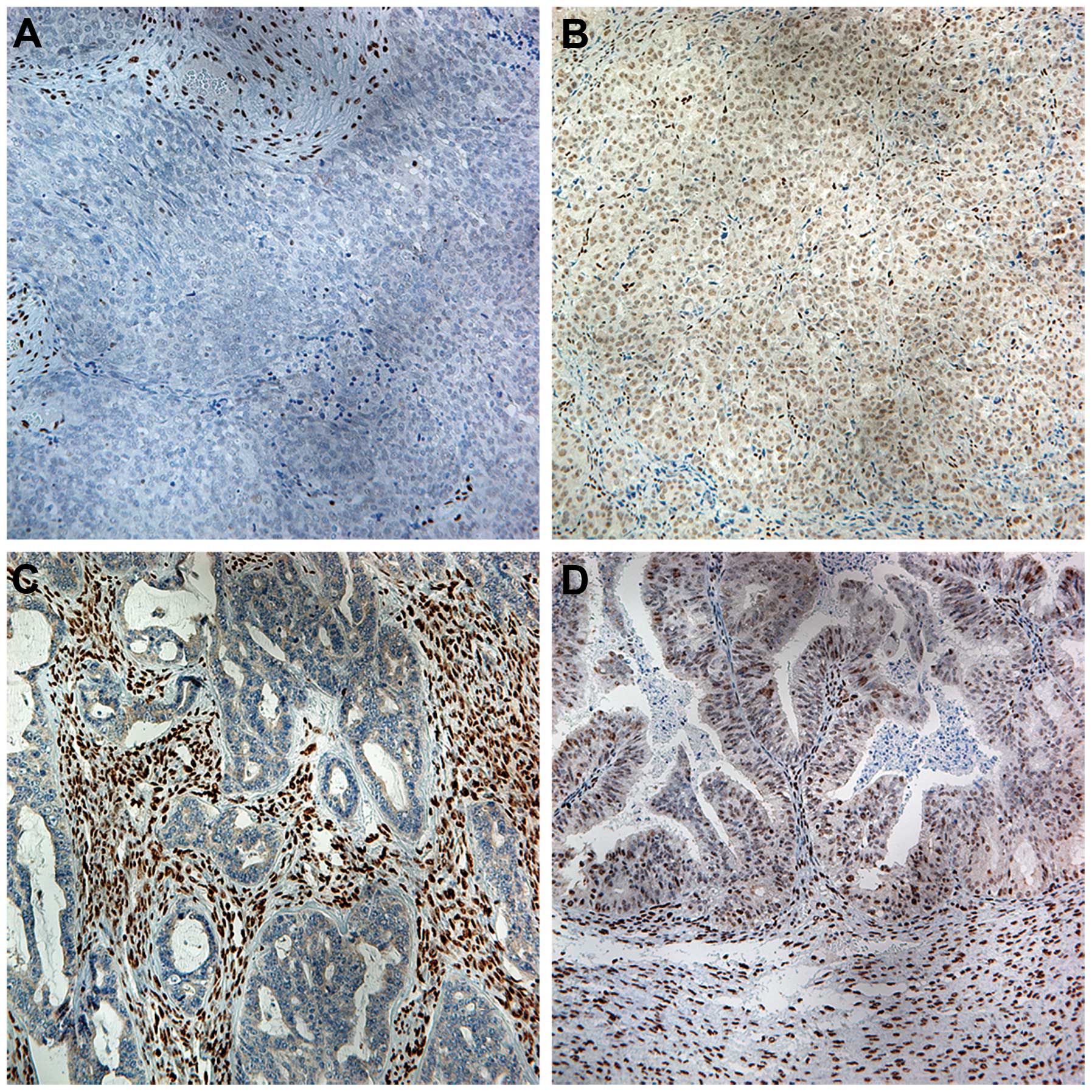
Variable nuclear immunoreactivity for ZEB1 was
detected in ovarian carcinoma cells and the stromal cells in the
ovarian primary tumor samples (Fig.
1). Of the 238 samples, 78 were positive for ZEB1 and the
remaining 160 were negative (Table
I). Compared with the tumor cells, ZEB1 expression in stromal
cells was generally strong.
 | Table I.Association of ZEB1 expression in
ovarian carcinoma with clinicopathological characteristics. |
Table I.
Association of ZEB1 expression in
ovarian carcinoma with clinicopathological characteristics.
|
|
| ZEB1 expression in
tumor cells by IHC |
|
|---|
|
|
|
|
|
|---|
| Characteristics | Total (n=238) | Negative, n (%)
(n=160) | Positive, n (%)
(n=78) | P-value |
|---|
| Age (years) |
|
|
| 0.423 |
| ≤39 | 16 | 9
(56.2) | 7
(43.8) |
|
|
40–49 | 38 | 26 (68.4) | 12 (31.6) |
|
|
50–59 | 61 | 40 (65.6) | 21 (34.4) |
|
|
60–69 | 69 | 46 (66.7) | 23 (33.3) |
|
| ≥70 | 39 | 28 (71.8) | 11 (28.2) |
|
|
Missing | 15 |
|
|
|
| Histological
subtype |
|
|
| 0.278 |
| Serous
carcinoma | 157 | 97 (61.8) | 60 (38.2) |
|
| Mucinous
carcinoma | 17 | 14 (82.4) | 3
(17.6) |
|
|
Endometrioid carcinoma | 19 | 16 (84.2) | 3
(15.8) |
|
|
Clear-cell carcinoma | 10 | 7
(70.0) | 3
(30.0) |
|
| Mixed
epithelial tumor | 11 | 9
(81.8) | 2
(18.2) |
|
|
Undifferentiated tumor |
5 | 3
(60.0) | 2
(40.0) |
|
|
Unclassified tumor and
others | 19 |
|
|
|
| FIGO stage |
|
|
| 0.011 |
| I+II | 43 | 32 (74.4) | 11 (25.6) |
|
| III | 113 | 81 (71.7) | 32 (28.3) |
|
| IV | 76 | 41 (53.9) | 35 (46.1) |
|
| Not
staged or missing |
6 |
|
|
|
| Histological
differentiation |
|
|
| 0.249 |
| High | 19 | 13 (68.4) | 6
(31.6) |
|
|
Moderate | 61 | 45 (73.8) | 16 (26.2) |
|
| Poor | 126 | 79 (62.7) | 47 (37.3) |
|
| Not
graded or missing | 32 |
|
|
|
ZEB1 expression and its
clinicopathological associations
The association of ZEB1 expression with age,
histological subtype, tumor differentiation grade and FIGO stage
were investigated (Table I). ZEB1
expression in ovarian carcinoma cells was associated with advanced
FIGO stage, but not with age, histological subtype or tumor
differentiation.
ZEB1 expression and survival
probability
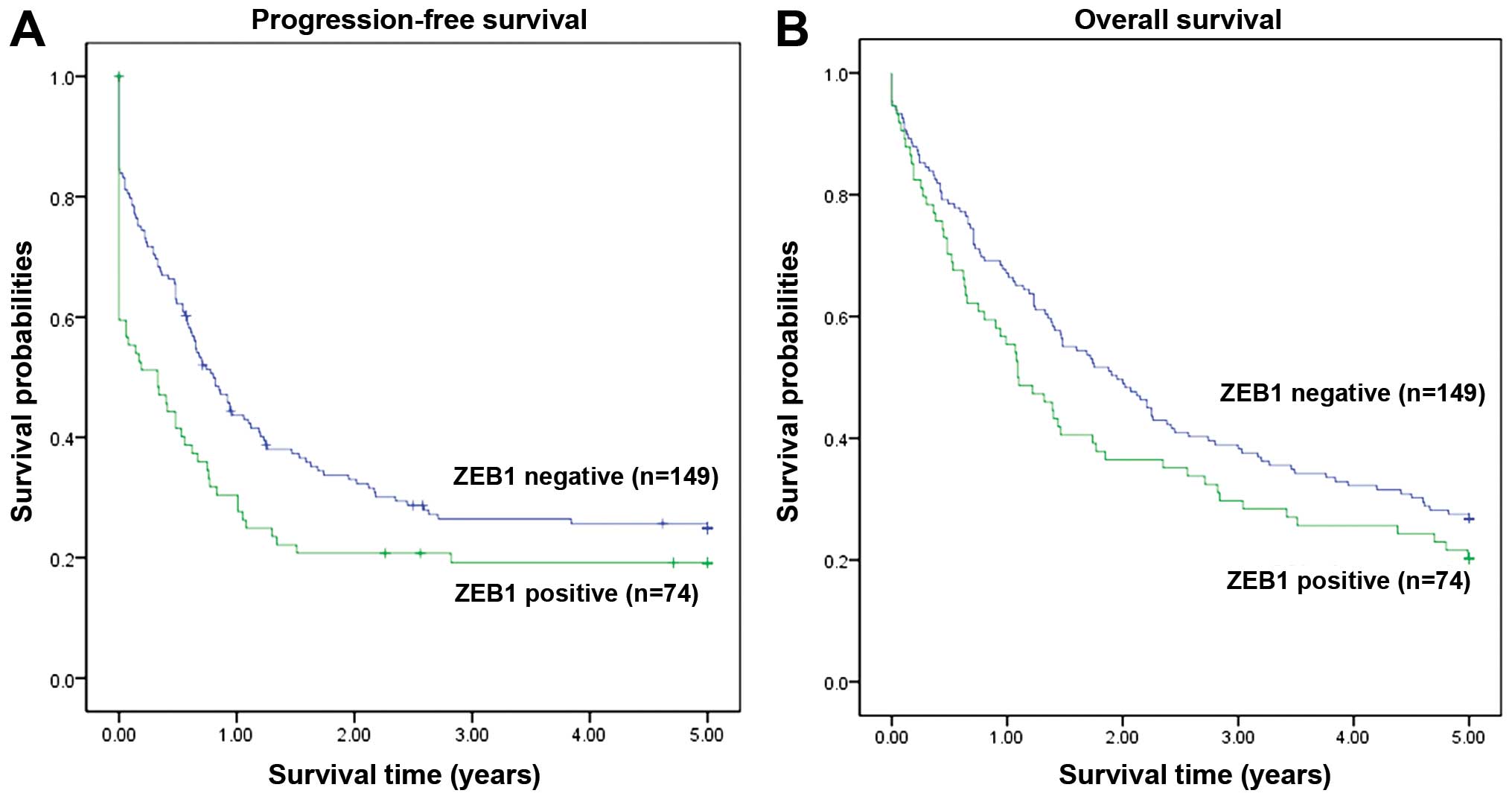
The survival analysis indicated that high expression
of ZEB1 was associated with poor progression-free survival (PFS)
(P=0.021, Fig. 2A). A similar
tendency was also observed for the association of ZEB1 expression
with 5-year overall survival (OS), although it did not reach
statistical significance (P=0.118, Fig.
2B).
Multivariate analysis of 5-year
PFS
The multivariate analysis revealed that the status
of ZEB1 expression in cancer cells (P=0.021, Table II), tumor differentiation (P=0.020,
Table II) and FIGO stage
(P<0.001, Table II) were
independent predictors of 5-year PFS in EOC.
 | Table II.Multivariate analysis of 5-year
progession-free survival in 238 confirmed ovarian carcinoma
patients. |
Table II.
Multivariate analysis of 5-year
progession-free survival in 238 confirmed ovarian carcinoma
patients.
| Factors | HR | 95% CI | P-value |
|---|
| ZEB1
expressiona | 1.491 | 1.063–2.091 |
0.021 |
| Ageb | 1.104 | 0.961–1.269 |
0.163 |
|
Differentiationc | 1.379 | 1.052–1.808 |
0.020 |
| FIGO
staged | 1.689 | 1.334–2.140 | <0.001 |
| Histological
subtypee | 0.905 | 0.811–1.267 |
0.905 |
Discussion
Ovarian cancer has the highest mortality rate among
all gynecological malignancies and EOC accounts for 90% of all
ovarian cancers (2). Despite advances
in surgery and chemotherapy, the 5-year survival rate remains ~30%,
which is mainly attributed to these cancers being diagnosed at an
advanced stage (2). The leading cause
of relapse and death in patients with ovarian cancer is metastasis.
Metastasis to the omentum, peritoneum, diaphragm and small bowel
mesentery, have been confirmed as poor prognostic factors for EOC
patients (17). Therefore, it is
crucial to elucidate the mechanism underlying ovarian cancer
metastasis.
The ZEB family of zinc finger transcription factors
is necessary for embryonic development (12). Over the last few years, ZEB1 has
emerged as an important regulator of EMT, required for cancer
development and metastasis. A growing body of evidence indicates
that ZEB1 overexpression may promote tumor progression (18–21). ZEB1
promotes EMT by repressing the genes contributing to the epithelial
phenotype, while activating those associated with the mesenchymal
phenotype (4,5). ZEB1 is expressed in estrogen-responsive
tissues, such as breast, bone, uterus, endometrium, ovary, and the
cardiovascular system, and high expression of this gene in normal
ovarian and endometrial tissue is correlated with high estrogen
levels. Measurements of ZEB1 mRNA in reproductive carcinomas have
revealed high levels, yet independent of estrogen, in poorly
differentiated endometrial and ovarian carcinomas (12). ZEB1 contributes to cell proliferation
and migration and suppresses cell differentiation (4,5). Our study
aimed to investigate the expression pattern of ZEB1 at the protein
level in EOC tissues, evaluate its associations with tumor
progression and patient prognosis, and evaluate ZEB1 as a
prognostic marker and potential therapeutic target.
In this study, we observed that immunoreactive ZEB1
was variably detected in ovarian carcinoma cells and stromal cells.
ZEB1 expression in the stromal cells was generally common and it
was rather strong, which is seldom mentioned in other studies.
Strong ZEB1 expression in stromal cells may be attributed to the
fact that ZEB1 is able to activate genes required for the
mesenchymal phenotype. However, the association of ZEB1 expression
in the stromal cells with the clinicopathological parameters was
not included in our present study.
IHC revealed that ZEB1 expression in ovarian
carcinoma cells was significantly higher in FIGO stage IV cases
(46.1%) (P=0.027, Table I). FIGO is
the most popular clinically used staging system, and it is an
important prognostic predictor in ovarian cancer. This supports the
conclusion that ZEB1 may be associated with the development, as
well as the invasion and metastasis of EOC. A study conducted by
Yang et al (21) reported that
overexpression of ZEB1 in esophageal squamous cell carcinoma was
associated with tumor stage, lymph node metastasis, histological
grade and depth of invasion. Furthermore, a study on gastric cancer
conducted by Jia et al (19)
demonstrated that overexpression of ZEB1 was associated with tumor
differentiation, stage and depth of invasion. Theoretically, tumor
differentiation is associated with the invasive and metastatic
ability of tumors. Unlike the abovementioned studies on other
carcinomas, the present investigation found no significant
correlation between the expression of ZEB1 and tumor
differentiation in EOC (P=0.249). Additionally, the expression of
ZEB1 in gastric cancer tissue was independent of the patients' age
(P>0.05); however, ZEB1 expression in tumor cells tended to be
negative in mucinous and endometrioid carcinoma, although no
significant difference was found in the present study.
The survival analysis indicated that high expression
of ZEB1 was associated with poor 5-year PFS. A similar tendency was
also observed for the association between high expression of ZEB1
and 5-year OS, although it did not reach statistical significance.
In light of these findings, we suggest that the expression of ZEB1
in EOC is associated with an unfavorable prognosis. In addition to
its effect on EMT, ZEB1 expression was also reported to be
significantly associated with poor response to chemotherapy at
diagnosis (22). Moreover, the
multivariate analysis in our study demonstrated that the status of
ZEB1 expression was an independent predictor of 5-year PFS in EOC,
together with histological grade and FIGO stage.
Taken together, our data support the evidence
suggesting that ZEB1 may play a crucial role in promoting
aggressive EOC behavior and progression. Therefore, ZEB1 may serve
as a predictive marker and a potential target for therapeutic
intervention in EOC.
Acknowledgements
We would like to thank the Inger and John Fredriksen
Foundation, the Radium Hospital Research Foundation and The
Norwegian Cancer Society for the financial support. We would also
like to thank Ellen Hellesylt, Mette Synnøve Førsund, Mai Nguyen
and Don Trinh for technical support with IHC.
References
|
1
|
Dutta DK and Dutta I: Origin of ovarian
cancer: Molecular profiling. J Obstet Gynaecol India. 63:152–157.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Spaderna S, Schmalhofer O, Wahlbuhl M,
Dimmler A, Bauer K, Sultan A, Hlubek F, Jung A, Strand D, Eger A,
et al: The transcriptional repressor ZEB1 promotes metastasis and
loss of cell polarity in cancer. Cancer Res. 68:537–544. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aigner K, Dampier B, Descovich L, Mikula
M, Sultan A, Schreiber M, Mikulits W, Brabletz T, Strand D, Obrist
P, et al: The transcription factor ZEB1 (deltaEF1) promotes tumour
cell dedifferentiation by repressing master regulators of
epithelial polarity. Oncogene. 26:6979–6988. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: An alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang P, Sun Y and Ma L: ZEB1: At the
crossroads of epithelial-mesenchymal transition, metastasis and
therapy resistance. Cell Cycle. 14:481–487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Feng G, Wang X, Cao X, Shen L and Zhu J:
ZEB1 expression in endometrial biopsy predicts lymph node
metastases in patient with endometrial cancer. Dis Markers.
2014:6803612014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li P, Wang J, Chu M, Zhang K, Yang R and
Gao WQ: Zeb1 promotes androgen independence of prostate cancer via
induction of stem cell-like properties. Exp Biol Med (Maywood).
239:813–822. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Quan Y, Jin R, Huang A, Zhao H, Feng B,
Zang L and Zheng M: Downregulation of GRHL2 inhibits the
proliferation of colorectal cancer cells by targeting ZEB1. Cancer
Biol Ther. 15:878–887. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sayan AE: Tumour-promoting role of
EMT-inducing transcription factor ZEB1 in mantle cell lymphoma.
Cell Death Differ. 21:194–195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wellner U, Brabletz T and Keck T: ZEB1 in
pancreatic cancer. Cancers (Basel). 2:1617–1628. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hurt EM, Saykally JN, Anose BM, Kalli KR
and Sanders MM: Expression of the ZEB1 (deltaEF1) transcription
factor in human: Additional insights. Mol Cell Biochem. 318:89–99.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cho KR and Shih IeM: Ovarian cancer. Annu
Rev Pathol. 4:287–313. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zivanovic O, Sima CS, Iasonos A, Hoskins
WJ, Pingle PR, Leitao MM Jr, Sonoda Y, Abu-Rustum NR, Barakat RR
and Chi DS: The effect of primary cytoreduction on outcomes of
patients with FIGO stage IIIC ovarian cancer stratified by the
initial tumor burden in the upper abdomen cephalad to the greater
omentum. Gynecol Oncol. 116:351–357. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang R, Ma Y, Holm R, Trope CG, Nesland
JM and Suo Z: Sex hormone-binding globulin (SHBG) expression in
ovarian carcinomas and its clinicopathological associations. PloS
one. 8:e832382013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang R, Wu D, Yuan Y, Li X, Holm R, Trope
CG, Nesland JM and Suo Z: CD117 expression in fibroblasts-like
stromal cells indicates unfavorable clinical outcomes in ovarian
carcinoma patients. PloS One. 9:e1122092014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sehouli J, Senyuva F, Fotopoulou C,
Neumann U, Denkert C, Werner L and Gülten OO: Intra-abdominal tumor
dissemination pattern and surgical outcome in 214 patients with
primary ovarian cancer. J Surg Oncol. 99:424–427. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Harada K, Miyake H, Kusuda Y and Fujisawa
M: Expression of epithelial-mesenchymal transition markers in renal
cell carcinoma: Impact on prognostic outcomes in patients
undergoing radical nephrectomy. BJU Int. 110:E1131–E1137. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jia B, Liu H, Kong Q and Li B:
Overexpression of ZEB1 associated with metastasis and invasion in
patients with gastric carcinoma. Mol Cell Biochem. 366:223–229.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Miyahara S, Hamasaki M, Hamatake D,
Yamashita S, Shiraishi T, Iwasaki A and Nabeshima K:
Clinicopathological analysis of pleomorphic carcinoma of the lung:
Diffuse ZEB1 expression predicts poor survival. Lung Cancer.
87:39–44. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang X, Wang Q, Dai W, Zhang J and Chen X:
Overexpression of zinc finger E-box binding homeobox factor 1
promotes tumor invasiveness and confers unfavorable prognosis in
esophageal squamous cell carcinoma. Tumour Biol. 35:11977–11984.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Davidson B, Holth A, Hellesylt E, Tan TZ,
Huang RY, Tropé C, Nesland JM and Thiery JP: The clinical role of
epithelial-mesenchymal transition and stem cell markers in
advanced-stage ovarian serous carcinoma effusions. Hum Pathol.
46:1–8. 2015. View Article : Google Scholar : PubMed/NCBI
|