Introduction
Hepatocellular carcinoma (HCC) is the second leading
cause of cancer-associated mortality worldwide and is generally
associated with hepatitis B or C viruses, which account for 80% of
HCC (1). Cirrhosis is also a risk
factor for HCC, irrespective of the etiology of the cirrhosis. The
annual risk of developing HCC among patients with cirrhosis is
between 1 and 6% (2). Despite
performed resection and locoregional therapies, recurrence occurs
often and is lethal. For this reason, in order to prevent early
recurrence, the use of antiviral therapies is essential and also
adjuvant treatment with tyrosine kinase inhibitors following
curative modality is recommended in certain previous studies
(3,4).
Using sorafenib in addition to transarterial chemoembolization
(TAKE) is currently under investigation. However, if there is
evidence of residual or recurrent tumor, sorafenib treatment
following arterially directed therapies is appropriate (5).
Renal cell cancer (RCC) is a tumor with increasing
incidence among patients with cancer. The 5 year survival rate of
kidney cancer is 98.1% for localized disease and 12.3% for advanced
disease. Following curative treatment of the localized disease,
observation remains the standard of care. Patients with stage IV
disease also may benefit from cytoreductive surgery (6). For advanced disease, targeted therapy
with tyrosine kinase and vascular endothelial growth factor (VEGF)
antibodies (e.g. sunitinib, sorafenib, pazopanib, axitinib,
temsirolimus, everolimus and bevacizumab, in combination with
interferon) are used sequentially (7–9).
In the present case, a patient presenting with liver
cirrhosis demonstrated improvement in Child-Pugh scores following
treatment with sunitinib for renal cell carcinoma. Sunitinib is an
agent exhibiting improvements in liver cirrhosis findings at
preclinical study (10). In this
context, the present study can pave the way for designing future
studies investigating the use of sunitinib in the treatment of
liver cirrhosis. Also, sunitinib can be used for patients who have
sorafenib intolerance in HCC with concomitant RCC treatment.
Case report
A 62-year-old male was diagnosed with Child-Pugh
class C (score, 10) liver cirrhosis with chronic hepatitis B
infection in 2005. Antiviral therapy with lamivudine was initiated
following the diagnosis. In February 2007, the patient presented
gross hematuria. Ultrasonography indicated a solid mass in the
right kidney. Right radical nephrectomy was performed on the
patient and postoperative pathological examination confirmed
clear-cell carcinoma (tumor >7 cm in greatest dimension, limited
to kidney with unknown lymph node status and unknown distant
metastases; pT2NxMx). In March 2007, a computed tomography (CT)
scan revealed multiple millimeter sized lung metastases. A lung
metastasectomy was performed and lung metastasis was confirmed. The
patient had an Eastern Cooperative Oncology Group performance
status of 1. Interferon treatment was administered for 6 months.
Subsequently, temsirolimus treatment was initiated as a result of
the progression of lung metastasis. This treatment was terminated
due to inadequate hepatocellular function (Child-Pugh class C;
score, 12). The patient was not examined for 2 years as a personal
choice. During this examination, decompensation of liver functions
with ascites was observed. The patient subsequently resumed
antiviral therapy. In February 2010, the patient complained of
fatigue and itching, and was evaluated as Child-Pugh class C
(score, 10; controlled ascites with diuretics). A CT scan revealed
progression of the lung metastasis, right kidney mass (size, 37×28
mm) and left surrenal mass (size, 5×4.5 cm). Sunitinib was
administered at 50 mg once daily on a standard 4 weeks on, 2 weeks
off schedule of 6 week cycles. Following treatment with sunitinib
for 6 months and no change in antiretroviral therapy, the
hepatocellular parenchyma failure finding was improved unexpectedly
to Child-Pugh class A (score, 5). During the treatment period,
grade 2 thrombocytopenia and grade 2 anemia developed. Due to this
toxicity, the dose was adjusted to 37.5 mg once daily for 2 years.
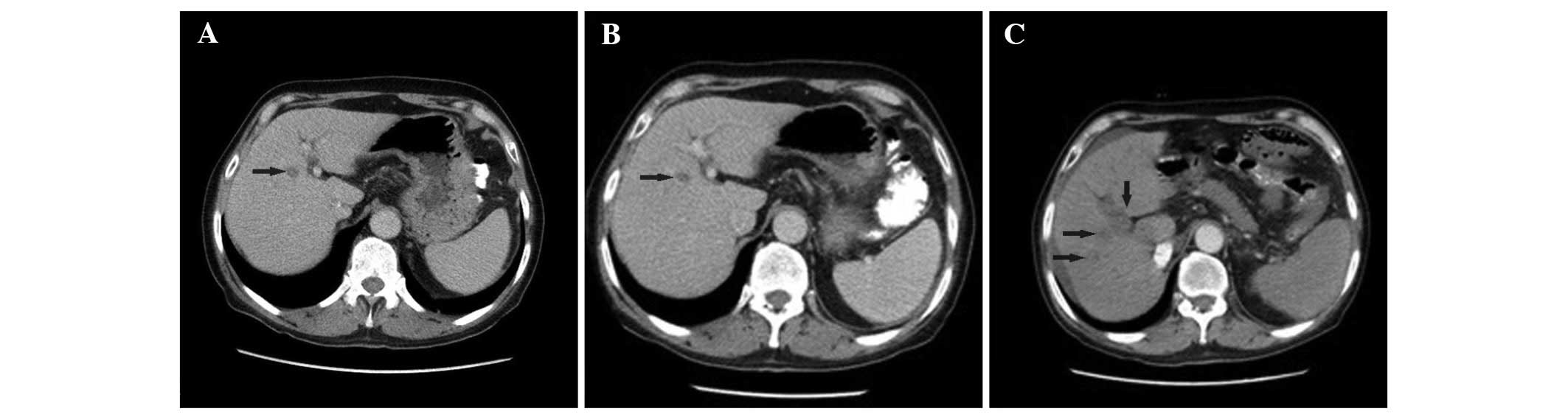
In March 2012, an abdominal CT scan revealed a mass in the segment
4b of the liver (size, 1.5×1 cm; Fig.
1A. This lesion was closely monitored. In April 2013, a lesion
biopsy was performed due to minimal progression (Fig. 1B). Pathological examination determined
well differentiated HCC. The level of α-fetoprotein (AFP) was 20
ng/ml and the patient was Child-Pugh class A. TAKE with doxorubicin
was performed three times during this period (May-July 2013). Due
to concomitant HCC and RCC, sunitinib treatment was modified to
sorafenib. However, the patient refused this modification due to
development grade III fatigue, asthenia and confusion with
sorafenib treatment. Therefore, sunitinib treatment was continued.
In April 2014, an abdominal CT scan revealed a necrotic mass in the
location at which TAKE was performed, in right portal vein and
para-aorto-cavale pathologic lymph nodes (Fig. 1C). The level of AFP was 4,548 ng/ml.
Progression of RCC was not observed. Sunitinib treatment was
discontinued as a result of HCC progression.
Discussion
The majority of patients with HCC are diagnosed at
the advanced stages. The prognosis of advanced stage HCC is poor
and the overall survival (OS) rate is <5% (10). According to two large phase 3
randomized trials, the Asia-Pacific study and the SHARP study,
sorafenib is an effective treatment in patients with advanced HCC
(11,12); however, it can be more toxic and lead
to acute hepatocellular failure in the patients with Child-Pugh
class B and C cirrhosis. Based on the National Comphrehensive
Cancer Network guideline (http://www.nccn.org/professionals/physician_gls/f_guidelines.asp),
sorafenib is recommended as a category 1 option for patients with
Child-Pugh class A liver function and as a category 2A option for
patients with Child-Pugh class B liver function.
Sorafenib, an oral multi-kinase inhibitor, inhibits
cell proliferation by targeting the Ras/mitogen-activated protein
kinase signaling pathway at the level of the Raf kinase, and other
tyrosine kinase receptors, including an antiangiogenic effect by
inhibiting VEGF receptors 1–3, platelet-derived growth factor
(PDGF) receptor β and stem cell factor receptor. In two large phase
III placebo-controlled studies, sorafenib at a dose of 800 mg/day,
significantly prolonged the OS rate in patients with advanced HCC,
particularly with Child-Pugh class A cirrhosis (11,12).
Therefore, sorafenib is currently considered as the standard
treatment for patients with advanced HCC.
However, few patients respond to sorafenib and the
majority of patients unavoidably eventuate with progression. In
this instance, a phase II study was designed with sunitinib, which
is an another tyrosine kinase inhibitor, for patients with advanced
HCC. In the present study, sunitinib was administered at 50, 37.5
and 25 mg/day, based on toxicity dose adjustment. In the present
study, the overall incidence of severe (grade, ≥3) adverse events
was 79.4%. Toxicity was observed in the majority of the patients
with Child-Pugh class B. The most common grade III or IV events
included fatigue (47%), nausea (14.7%), liver failure (14.7%) and
hepatic encephalopathy (11.7%) (10).
A previous randomized phase III study compared the safety and
efficiency of sunitinib and sorafenib. These drugs were
administered as sunitinib at 37.5 mg once daily or sorafenib at 400
mg twice daily. Sunitinib was associated with more frequent and
severe adverse events. The OS with sunitinib was inferior to
sorafenib, however, it was similar in Asian and hepatitis
B-infected patients (14).
In the present study, two findings are of great
interest. Firstly, a patient without the diagnosis of HCC exhibited
cirrhosis with Child-Pugh class C (score, 10) at the initiation of
sunitinib treatment for RCC. Following sunitinib treatment, the
Child-Pugh score regressed and decompensation of liver function
with ascites was not observed. Majumder et al (15) performed in vitro experiments
and the efficacy of Sunitinib for cirrhotic liver and its effect on
angiogenesis were assessed. The development of HCC from malignant
hepatocytes is frequently associated with intra-peritumoral
accumulation of connective tissue arising from activated hepatic
stellate cells (HSC). HSC also perform hepatic functions frequently
ascribed to fibroblast cells, including deposition of collagen and
migration in response to angiogenic stimuli. These cells have an
active role in cirrhosis and carcinogenesis. In the present study,
the receptor tyrosine kinase inhibitor, sunitinib, which is a PDGF
and VEGF inhibitor, may inhibit activated HSC functions and
angiogenesis. Therefore, it can prevent the progression of
cirrhotic liver (15). In this
circumstance, improvement of hepatic functions with sunitinib
treatment may be explained in patients who have not yet developed
HCC. Therefore, further studies are required with different doses
of sunitinib and other tyrosine kinase inhibitors in order to
evaluate the efficacy on treatment of hepatic parenchyma findings
or cirrhosis progression, as observed in the present patient.
Secondly, sunitinib treatment was well tolerated by
the patient. Although sorafenib was preferred, the patient refused
this treatment due to the development of grade III fatigue,
asthenia and confusion. The patient with concomitant stage IV RCC
and HCC experienced no grade III–IV toxicity during sunitinib
therapy. Sunitinib was demonstrated to have equal efficiency in
hepatitis B patients, as indicated in the phase III study mentioned
previously (14). Diagnosis of HCC
secondary to hepatitis B in the present patient may explain the
similar results of sorafenib treatment consistent with the
literature mentioned previously. Sorafenib is the most efficient
agent for HCC treatment. However, in the cases of sorafenib
intolerance or concomitant HCC-RCC, sunitinib may be considered as
an alternative treatment, since there is no other agent promising a
proven efficiency.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ikeda K, Saitoh S, Koida I, Arase Y,
Tsubota A, Chayama K, Kumada H and Kawanishi M: A multivariate
analysis of risk factors for hepatocellular carcinogenesis: A
prospective observation of 795 patients with viral and alcoholic
cirrhosis. Hepatology. 18:47–53. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang W, Zhao G, Wei K, Zhang Q, Ma W,
Song T, Wu Q, Zhang T, Kong D and Li Q: Adjuvant sorafenib reduced
mortality and prolonged overall survival and post-recurrence
survival in hepatocellular carcinoma patients after curative
resection: A single-center experience. Biosci Trends. 8:333–338.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang SN, Chuang SC and Lee KT: Efficacy of
sorafenib as adjuvant therapy to prevent early recurrence of
hepatocellular carcinoma after curative surgery: A pilot study.
Hepatol Res. 44:523–531. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chung YH, Han G, Yoon JH, Yang J, Wang J,
Shao GL, Kim BI, Lee TY and Chao Y: Interim analysis of START:
Study in Asia of the combination of TACE (transcatheter arterial
chemoembolization) with sorafenib in patients with hepatocellular
carcinoma trial. Int J Cancer. 132:2448–2458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Culp SH, Tannir NM, Abel EJ, Margulis V,
Tamboli P, Matin SF and Wood CG: Can we better select patients with
metastatic renal cell carcinoma for cytoreductive nephrectomy?
Cancer. 116:3378–3388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Albiges L, Kube U, Eymard JC, Schmidinger
M, Bamias A, Kelkouli N, Mraz B, Florini S, Guderian G, Cattaneo A,
et al: Everolimus for patients with metastatic renal cell carcinoma
refractory to anti-VEGF therapy: Results of a pooled analysis of
non-interventional studies. Eur J Cancer. 51:2368–2374. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oudard S and Vano Y: The role of
rechallenge with targeted therapies in metastatic renal-cell
carcinoma. Curr Opin Urol. 25:402–410. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eichelberg C, Vervenne WL, De Santis M,
von Weikersthal Fischer L, Goebell PJ, Lerchenmüller C, Zimmermann
U, Bos MM, Freier W, Schirrmacher-Memmel S, et al: SWITCH: A
Randomised, Sequential, Open-label Study to Evaluate the Efficacy
and Safety of Sorafenib-sunitinib Versus Sunitinib-sorafenib in the
Treatment of Metastatic Renal Cell Cancer. Eur Urol. 68:837–847.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wilhelm SM, Adnane L, Newell P, Villanueva
A, Llovet JM and Lynch M: Preclinical overview of sorafenib, a
multikinase inhibitor that targets both Raf and VEGF and PDGF
receptor tyrosine kinase signaling. Mol Cancer Ther. 7:3129–3140.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Luo R, Feng J, Ye S, Yang TS, et al: Efficacy and safety of
sorafenib in patients in the Asia-Pacific region with advanced
hepatocellular carcinoma: A phase III randomised, double-blind,
placebo-controlled trial. Lancet Oncol. 10:25–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Barone C, Basso M, Biolato M, Pompili M,
Rufini V, Miele L, Basso M, De Gaetano AM, Castaldi P, Iaculli A,
et al: A phase II study of sunitinib in advanced hepatocellular
carcinoma. Dig Liver Dis. 45:692–698. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cheng AL, Kang YK, Lin DY, Park JW, Kudo
M, Qin S, Chung HC, Song X, Xu J, Poggi G, et al: Sunitinib versus
sorafenib in advanced hepatocellular cancer: Results of a
randomized phase III trial. J Clin Oncol. 31:4067–4075. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Majumder S, Piguet AC, Dufour JF and
Chatterjee S: Study of the cellular mechanism of sunitinib mediated
inactivation of activated hepatic stellate cells and its
implications in angiogenesis. Eur J Pharmacol. 705:86–95. 2013.
View Article : Google Scholar : PubMed/NCBI
|