Introduction
Considering patients with acute myeloid leukemia
(AML), >20% fail to achieve complete remission (CR) following a
standard first line induction treatment (1). The majority of patients with CR relapsed
within 1–2 years and the chance of long-term disease-free survival
for relapsed patients is <10% (2).
For the majority of relapsed patients, regimens involving
fludarabine have been performed in previous studies for treatment,
with a CR rate ranging between 30 and 60% (3–8). Liso
et al (9) and Kern et
al (10) have also reported a
high-dose of cytarabine, combined with mitoxantrone and etoposide,
as a salvage regimen. In these cases, the CR rates were 47.4 and
47%, and the median overall survival (OS) rates were 8.1 months and
4.2 months, respectively.
An optimum strategy for patients with primary
induction failure or relapse remains to be elucidated. A pilot
study was performed in order to assess the effect of the MAC
regimen for re-induction therapy in patients who suffered primary
induction failure or relapsed in the Institute of Hematology and
Blood Diseases Hospital (Tianjin, China). Cyclophosphamide, which
is seldom used in the treatment of AML, was combined in this
regimen. In the present report, the efficacy and toxicity of the
MAC salvage regimen were assessed. The MAC salvage regimen was
selected in the present study to reduce the leukemic burden and
induce remission. It was hypothesized that there would be a longer
survival rate as a result of this combination chemotherapy.
Patients and methods
Patients
Between September 2001 and October 2011, 91 newly
diagnosed patients with de novo AML in the Institute of
Hematology and Blood Diseases Hospital (Tianjin, China) were
enrolled in the present study. Patients with acute promyelocytic
leukemia were excluded. Absence of heart, lung, liver, or kidney
damage, as well as no active infection, was required for enrollment
in the study. Written informed consent was obtained from each
patient. The diagnosis was based on bone marrow cell morphology,
cytochemistry, immunophenotyping and chromosomal analysis. The
final follow-up was March 2014. For the entire cohort, the median
follow-up time was 46 months.
Disease evaluation
The diagnosis and classification of AML were based
on the criteria of the French-American-British (FAB) Cooperative
Study Group (11) and the more recent
World Health Organization classification (12). The disease status was assessed by
bone-marrow cytology and flow cytometric analysis (FACSCalibur;
Becton Dickinson, San Jose, CA, USA). Bone marrow or peripheral
samples were harvested following 1–3 days of culture, containing no
stimulating factors. Chromosomal analysis was performed using the
R-banding method and karyotyped, according to the International
System for Human Cytogenetic Nomenclature (13). Mutations in several genes, including
NPM1, FLT3/ITD, FLT3/TKD, AML1, CEBPA, C-KIT and JAK2, were
investigated. For cytogenetic analysis, the chromosome karyotype
and/or fusion gene (e.g. AML1/ETO or CBFβ/MYH11) mutation was
analyzed successfully in 85/87 patients. A total of 85 patients
were divided into three cytogenetic risk groups, according to the
Southwest Oncology Group (SWOG) criteria: Favorable group with 28
cases, including t(8;21) and inv(16); intermediate group with 45 cases,
including normal karyotype, 2Y, 18, 16, del(12p); adverse group
with 12 cases, including 25/27, abnormal 3q, 9q, 11q, 21q, 17p,
t(6;9) and complex karyotype. Since the prognosis of the unknown
group was similar to that of the intermediate group, these patients
were incorporated into the intermediate group.
Treatment protocol
The initial induction regimen used in patients with
primary induction failure (n=44) included cytarabine (100
mg/m2 infusion) for 7 consecutive days and daunorubicin
(45 mg/m2) for 3 consecutive days, cytarabine (100
mg/m2 infusion) for 7 consecutive days and Idamycin (12
mg/m2) for 3 consecutive days, and homoharringtonine
(2–2.5 mg/m2 infusion) for 7 consecutive days, a
standard dose (sd) of cytarabine (100 mg/m2) for 7
consecutive days and daunorubicin (45 mg/m2) for 3
consecutive days. Daunorubicin or idarubicin, combined with 2–2.5
mg m−2 ·d−1 homoharringtonine on days 1–7 and
a sd or intermediate dose of cytarabine (1 g m−2 q12 h
on days 5–7) was also performed as a first induction regimen.
The MAC regimen consisted of 8 mg m−2
·d−1 mitoxantrone on days 1–3, 100 mg m−2
·d−1 cytarabine on days 1–7 and 400 mg m−2
·d−1 cyclophosphamide on days 2 and 5.
Efficacy and safety evaluations
The efficacy was established, according to the
criteria of Cheson et al (14): i) CR, ≤5% blasts infiltration in bone
marrow without Auer's rodes, with ANC >1×109/l and
PLT >100×109/l; ii) Partial remission, 5–25% of blast
infiltration and blast reduction by 50% in bone marrow or <5% of
blasts in the bone marrow, however, with Auer's rods, and with a CR
criteria for peripheral blood; iii) Non-remission, the patients who
succumbed to mortality prior to the assessment of remission or
those who failed to achieve CR or PR; iv) Early mortality was
defined as mortality from any cause during the first 30 days from
the beginning of the induction treatment. Patients were considered
relapsed when they had >5% blasts in the bone marrow
examination.
Statistics
Disease-free survival (DFS) was calculated from the
date of CR to the date of relapse, mortality, undergoing stem cells
transplantation (SCT) or the final follow-up. The OS was calculated
from the first day of MAC administration until mortality,
undergoing SCT or the final follow-up.
Survival distributions were estimated by the
Kaplan-Meier method. Statistical differences in survival
distributions were evaluated using the log-rank test. Data analysis
was performed using R 2.10.1 software (R Foundation for Statistical
Computing, Vienna, Austria). P<0.05 was considered to indicate a
statistically significant difference.
Results
Patients
Of the 91 patients, 3 patients had a history of
myelodysplastic syndrome. No patient had other hematological
disorders at diagnosis. The median hemoglobin was 78.0 g/l
(36.0–136.0 g/l), the median white blood cell and platelet count
was 29.5×109/l (1.1–417.8×109/l) and
36.0×109/l (4.0–305.0×109/l), respectively. A
total of 15 patients exhibited hyperleucocytosis (white blood
cells>100×109/l). The blast count in bone marrow
aspiration at diagnosis ranged between 28.5 and 97.0%, the median
blast count was 68.5%. The clinical and hematological
characteristics of these patients are listed in Table I. It was observed that 10/73 (13.7%)
patients exhibited FLT3/ITD, and FLT3/TKD was positive in another 2
patients. Karyotypic studies revealed unfavorable chromosomal
abnormalities in 18 patients, including −7, 3q, 11q and complex
karyotype. A total of 11 and 53 patients were in favorable and
intermediate groups, respectively. The other 9 patients exhibited
no assessed cytogenetic factors. The risk categories were defined
according to the SWOG criteria. A total of 44 patients experienced
primary induction failure and 47 cases experienced relapse,
including 20 cases of early relapse (42.6%) and 27 cases of late
relapse (57.4%).
 | Table I.Clinical and laboratory patient
characteristics. |
Table I.
Clinical and laboratory patient
characteristics.
| Characteristic | No. patients
(n=91) | % |
|---|
| Median age,
years | 37 (14–72) |
|
| Gender |
|
|
| Male | 47 | 51.6 |
|
Female | 44 | 48.4 |
| FAB criteria |
|
|
| M0 | 2 | 2.2 |
| M1 | 4 | 4.4 |
| M2 | 31 | 34.1 |
| M4
(M4Eo) | 12 (7) | 13.2 (7.7) |
| M5 | 38 | 41.7 |
| M6 | 4 | 4.4 |
| Cytogenetic
subgroups |
|
|
| Good | 11 | 12.1 |
|
Intermediate | 53 | 58.2 |
| Poor | 18 | 19.8 |
| Not
available | 9 | 9.9 |
| NCCN categories |
|
|
|
Favorable | 9 | 9.9 |
|
Intermediate | 39 | 42.9 |
|
Unfavorable | 17 | 18.6 |
| Not
available | 26 | 28.6 |
| Primary
induction failure | 44 | 48.4 |
| Early
relapse | 20 | 21.9 |
| Late
relapse | 27 | 29.7 |
Response to MAC regimen
All 91 patients were assessed until the final
follow-up and 68/91 (74.7%) patients achieved CR (CR + CR with
incomplete platelet recovery) following the MAC regimen. The CR
rate was 72.7 (32/44) and 76.6% (36/47) in patients with primary
induction failure and those who experienced relapse, respectively.
For the relapsed cases, it was 65.0 (13/20) and 85.2% (23/27) in
cases experienced early relapse and late relapse, respectively. No
difference in the CR rate was observed between these groups. A
total of 4/10 patients with FLT3/ITD positive achieved CR following
the MAC regimen, PR and non-remission (NR) was observed in three
cases, respectively. The overall CR rate was 81.8 (9/11) and 79.2%
(42/53) for patients with favorable and intermediate cytogenetic
groups, while it was 66.7% (12/18) for the unfavorable cytogenetic
group. No difference in CR rate was observed for patients in
different cytogenetic risk groups. The patients with unavailable
cytogenetic factors had a CR rate of 55.5% (5/9). Early mortality
was observed in only one case during the agranulocytosis period
following chemotherapy, as a result of a severe lung infection and
gastrointestinal hemorrhage.
A total of 27 patients underwent at least two
high-dose cytarabine (Ara-c; 3 gm−2 q12 h on days 1–3)
or intermediate dose Ara-c plus anthracyclines prior to the MAC
regimen. A total of 7/27 patients underwent SCT following the MAC
salvage regimen.
Post-re-induction treatment
Among the 68 patients who achieved CR following the
MAC regimen, five underwent allogenic-SCT, one succumbed to relapse
following SCT, and four remain in CR. The other 63 patients entered
consolidation chemotherapy. Among them, 26 patients were referred
to 1–3 courses high-dose Ara-c (3 gm−2 q12 h on days
1–3) or intermediate dose Ara-c plus anthracyclines consolidation
chemotherapy, other patients received consolidation chemotherapy
with daunorubicin, homoharringtonine, mitozantrone or amsacrine
combined with a sd of cytarabine. A total of 32 patients remain
alive in CR, 2/31 patients with relapse succumbed to subsequent
progressive disease, and 2 patients with partial remission
following the MAC regimen achieved CR with a sd of mitoxantrone and
cytarabine. Each patient underwent allogenic-SCT and remain in CR.
Among the 21 patients with NR, 18 failed to achieve CR even though
they underwent G-CSF (300 µg) administered 12 hours prior to
fludarabine (30 mg⁄m2) in 0.5 h infusions for 5
consecutive days and cytarabine (2 g⁄m2) in 4 h
infusions for 5 consecutive days (FLAG), aclacinomycin (5–7
mg⁄m2) for 8 consecutive days, cytarabine (10
mg⁄m2) for 14 consecutive days and G-CSF (300 µg)
regimen re-induction and the final three were lost at
follow-up.
OS and DFS
The median follow-up duration was 46 months (range,
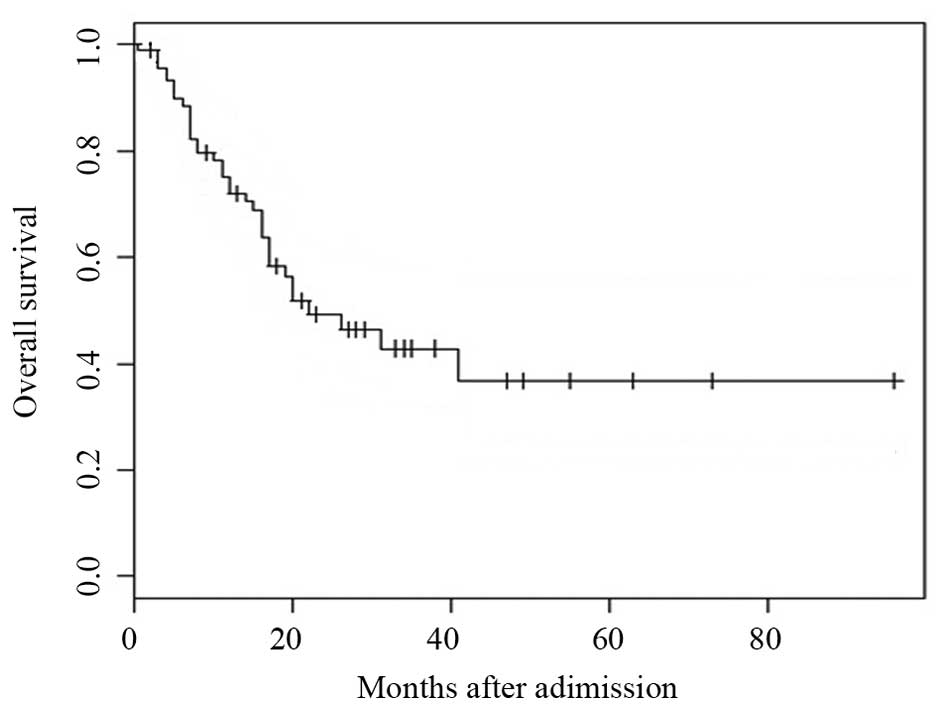
2–69 months). The median OS of the 91 patients was 22 months
(range, 0.5–73 months; Fig. 1). The
OS at 1, 3 and 5-years for the entire study population was 72.1,
42.9 and 36.7%, respectively (Fig.
1).
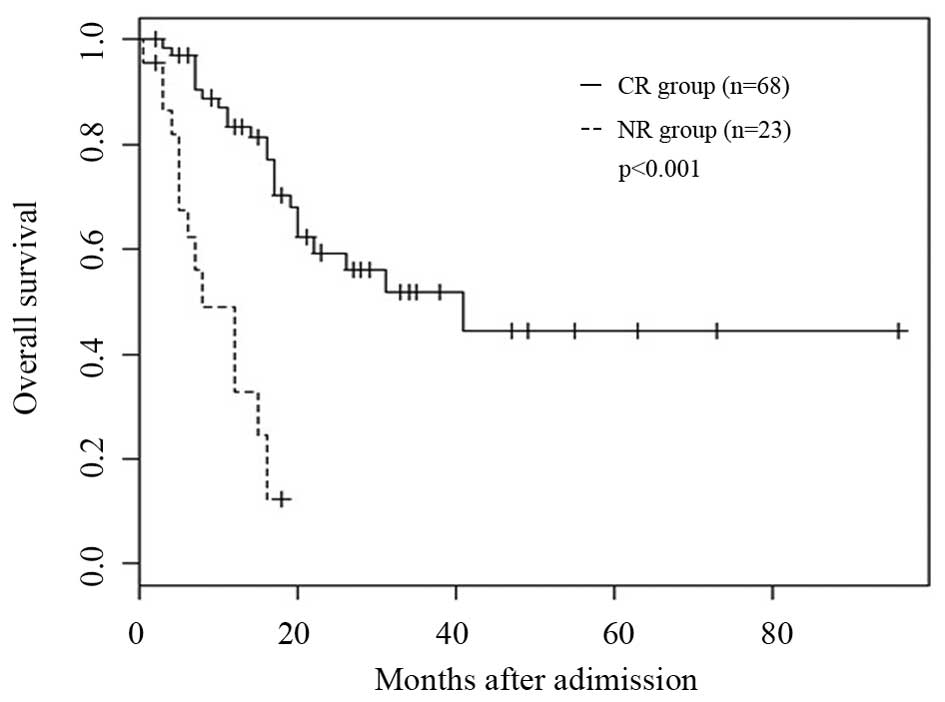
For patients who attained CR (n=68) or failed to
(n=23), 1 and 3-year OS rate were 83.3, vs. 32.7% (P<0.001) and
57.3, vs. 10.3% (P<0.001), respectively (Fig. 2). The median survival was 12 (4–73)
and 5 (0.5–20) months in patients with a white blood cell count
<100×109/l and ≥100×109/l (P=0.0001), and
it was 7.5 and 4 months in FLT3 positive and negative patients,
respectively (P=0.0001). In a multivariate analysis, the white
blood cell counts at diagnosis and FLT3 mutation were independent
factors for OS (P=0.035 and P=0.008).
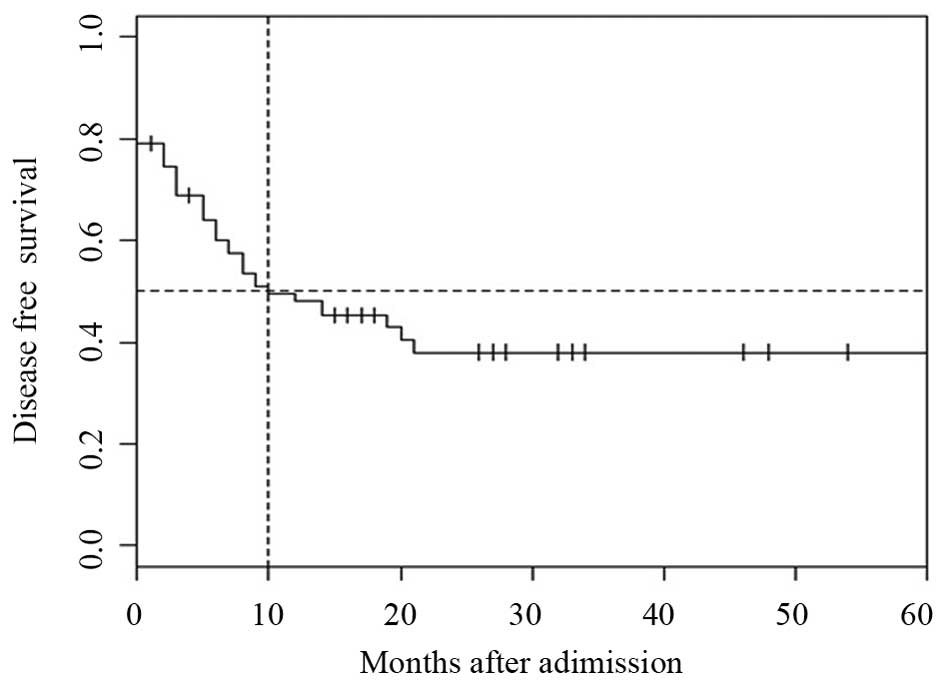
For the entire cohort, the 1 and 3-year DFS rates
were 48.1 and 38.0%, respectively. The median DFS was 10 months
(Fig. 3). For patients who attained
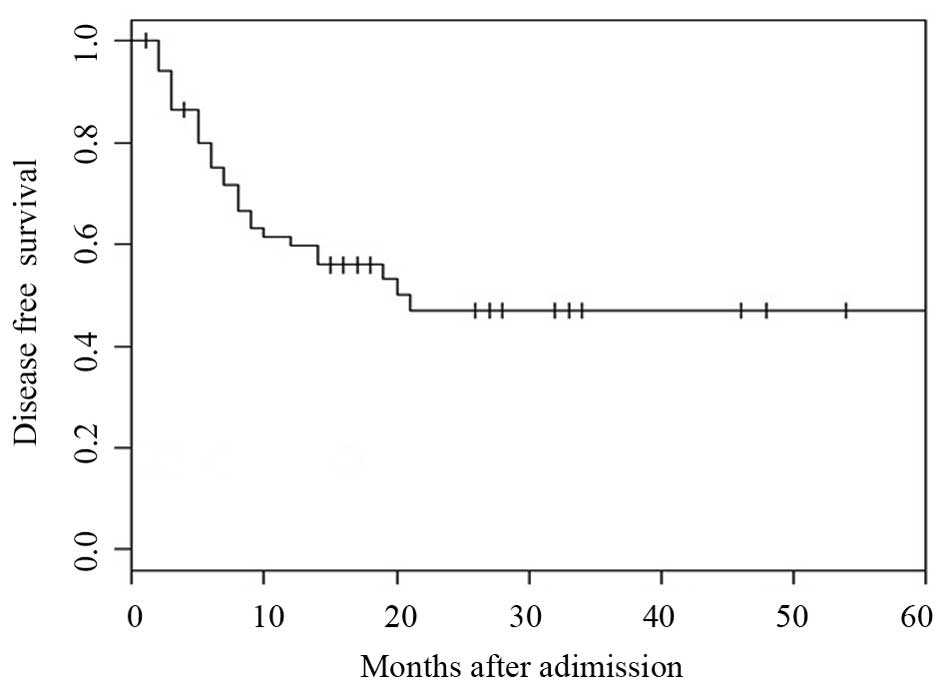
CR following the MAC regimen, the DFS rate at 1, 3 and 5-years was
59.7, 47.1 and 43%, respectively (Fig.
4). For the OS rate (P=0.32), as well as the DFS rate (P=0.19),
no difference was observed in different cytogenetic risk
groups.
The median OS and DFS in seven transplanted patients
was not significantly different compared with the group treated
with chemotherapy alone, and they were 31 and 17, and 23 and 12
months, respectively (P=0.32 and P=0.18).
Toxicity associated with the MAC
regimen
Hematological toxicity and infections were the most
prominent toxicities of the MAC treatment. All patients developed
grade IV neutropenia, according to the NCI CTC4.0 criteria
following the MAC therapy. The median duration of neutrophils
<0.5×109/l and platelets <20×109/l was
11 (range, 6–22) days and 9 (range, 5–25) days, respectively. The
patients received a median of 10 units of packed red cells (range,
3–29) and 6 units (range, 2–12) of platelets. The median duration
of hospitalization was 26 (range, 19–56) days. Severe infections
(grade III or IV) were observed in 29% (n=26) of the cases, among
which fungal was observed in 8% (n=7). The median duration of
antibiotic therapy was 11 days. A total of 5 (5.5%) patients had
lightly elevated levels of aminotransferase and nausea, which
resolved shortly following the completion of chemotherapy. Liver,
kidney, circulatory failure or neuropathy following the MAC regimen
was not observed.
Discussion
The outcomes of patients who experienced relapse are
very poor despite age, cytogenetics and refractory free survival
following CR1 (period from first CR until refractory or mortality)
(15). For the majority of relapsed
patients, fludarabine is used, as it acts synergistically with
cytarabine. Certain regimens, including FLAG, Flag-Ida and
fludarabine (30 mg⁄m2; 0.5 h; 3 consecutive days),
cytarabine (1 g⁄m2; 4 h infusions; 3 consecutive days),
mitoxantrone (10 mg⁄m2; 3 consecutive days) and G-CSF
(300 µg) administered 12 hours prior to fludarabine, have been
involved in studies for treating relapsed AML and in those with a
poor-risk disease, with a CR rate ranging between 30 and 60%. As an
extensively used second-line regimen, purine nucleoside analogues,
were combined with Ara-C for AML as salvage regimens in the last
decade. The novel agent, cladribine, has been extensively employed
in refractory/relapsed (RR)-AML. According to Pricea et al
(16), the CR rate was 37.9% for
cladribine (5 mg⁄m2; 2 h infusions; 5 consecutive days),
cytarabine (2 g⁄m2; 4 h infusions; 5 consecutive days)
and G-CSF (300 µg; 6 days; CLAG) and the median OS was 7.3 months.
In the initial relapse, the CR rate was 36.8% and median OS was 6.7
months. The Poland adult leukemia group (17) also reported that in poor risk
refractory/relapsed patients with AML, 58% achieved CR following
one or two courses of CLAG with mitoxantrone (10 mg⁄m2;
3 days), and the probability of OS at 4 years was 14%. The
probability of 4 year DFS was 30% for all 66 patients in CR.
Therefore, a modified therapeutic regimen and combined use of
chemotherapeutic agents for experienced primary induction failure
or relapsed patients with AML is urgently required.
As a conventional cytotoxic agent, mitozantrone has
no cross-resistance with other anthracycline agents and has
significant potentially synergistic effects with cytarabine. With
regards to the mechanism of action, mitozantrone is known to be
active against leukemia cell lines resistant to daunorubicin
(18). Liso et al and Kern
et al (9,10) reported a high-dose of cytarabine,
combined with mitozantrone and etoposide, as a salvage regimen, and
the CR rates were 47.4 and 47%, the median OS rates were 8.1 and
4.2 months, respectively. The present study attempted to use
cyclophosphamide instead of etoposide and comprise a novel regimen,
MAC, as the salvage treatment. Cyclophosphamide, which was seldom
used in the salvage regimens for AML, combined with high-dose
cytarabine has represented a promising therapeutic approach to
induce a second CR in younger patients (19). In the present study, cytarabine was
performed with an sd in the MAC regimen to avoid severe marrow
suppression. At the same time, the overall treatment costs were
reduced. The CR rate (74.7%) was encouraging. FLT3 (ITD/TKD) was
the only statistically significant factor affecting CR, while
different cytogenetic risk groups revealed no influence on the
treatment results. The result was similar to the observation made
by others (20–21). The prognosis of adult patients with
AML resistant to first-line or relapsed following CR1 within <6
months duration is poor. A striking a balance between the
treatment-associated mortality, response rates of salvage regimens,
and the likelihood of long-term overall survival are critical in
planning a treatment approach for the patients with relapsed or
experienced primary induction failure AML. High efficacy and a low
toxicity profile are preferable properties of this regimen. Only
once case of early mortality was observed. Severe infections (grade
III or IV) were observed in 29% of the cases and they were all
under control. The observed CR rate (74.7%) can be considered
excellent in this subset of patients, which included those with
primary induction failure disease following standard or
intermediate-dose Ara-c induction (72.7% CR), even if the best
response rate was obtained in the group of relapsed patients. As
for relapsed patients, the response rate to salvage therapy
correlated with the duration of the initial CR. Indeed, when the
patients were stratified according to the initial CR duration into
two groups (<6 and >6 months), an improved outcome was
observed for patients with initial CR duration >6 months, and
the CR rate was 65.0 and 85.2%, respectively. The median duration
of OS and DFS was 22 and 10 months for the entire cohort. For
patients who achieved CR following the MAC regimen, the DFS rate
was 47.1 and 43%, and the OS rate was 42.9 and 36.7% at 3 and 5
years, respectively. For patients who achieved CR or not, 1 and 3
year OS rate were 83.3, vs. 32.7% (P<0.0001) and 57.3, vs. 10.3%
(P<0.0001), respectively.
In conclusion, the MAC regimen may be a
highly-effective salvage regimen in the treatment of AML and is
worthy of further studies. The MAC regimen proved to be highly
effective, as indicated by an overall CR rate of 74.7% and 5 years,
and an OS and DFS of 36.7 and 43%, respectively.
Acknowledgements
The authors would like to thank Professor Jacob M.
Rowe (Rambam Medical Center, Haifa, Israel) for his constructive
suggestions for preparing the manuscript.
References
|
1
|
Litzow MR: Progress and strategies for
patients with relapsed and refractory acute myeloid leukemia. Curr
Opin Hematol. 14:130–137. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cassileth PA, Harrington DP, Appelbaum FR,
Lazarus HM, Rowe JM, Paietta E, Willman C, Hurd DD, Bennett JM,
Blume KG, et al: Chemotherapy compared with autologous or
allogeneic bone marrow transplantation in the management of acute
myeloid leukemia in first remission. N Engl J Med. 339:1649–1656.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Clavio M, Carrara P, Miglino M, Pierri I,
Canepa L, Balleari E, Gatti AM, Cerri R, Celesti L, Vallebella E,
et al: High efficacy of fludarabine-containing therapy (FLAG-FLANG)
in poor risk acute myeloid leukemia. Haematologica. 81:513–520.
1996.PubMed/NCBI
|
|
4
|
Estey EH: Treatment of relapsed and
refractory acute myelogenous leukemia. Leukemia. 14:476–479. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Montillo M, Mirto S, Petti MC, Latagliata
R, Magrin S, Pinto A, Zagonel V, Mele G, Tedeschi A and Ferrara F:
Fludarabine, cytarabine and G-CSF (FLAG) for the treatment of poor
risk acute myeloid leukemia. Am J Hematol. 58:105–109. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Byrne JL, Dasgupta E, Pallis M, Turzanski
J, Forman K, Mitchell D, Haynes AP and Russell NH: Early allogeneic
transplantation for refractory or relapsed acute leukaemia
following remission induction with FLAG. Leukemia. 13:786–791.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carella AM, Cascavilla N, Greco MM,
Melillo L, Sajeva MR, Ladogana S, D'Arena G, Perla G and Carotenuto
M: Treatment of ‘poor risk’ acute myeloid leukemia with
fludarabine, cytarabine and G-CSF (FLAG regimen): A single center
study. Leuk Lymphoma. 40:295–303. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jackson G, Taylor P, Smith GM, Marcus R,
Smith A, Chu P, Littlewood TJ, Duncombe A, Hutchinson M, Mehta AB,
et al: A multicentre, open, non-comparative phase II study of a
combination of fludarabine phosphate, cytarabine and granulocyte
colony-stimulating factor in relapsed and refractory acute myeloid
leukaemia and de novo refractory anaemia with excess of blasts in
transformation. Br J Haematol. 112:127–137. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liso V, Lacopino P, Avvisati G, Petti MC,
Broccia G, Carotenuto M, Falda M, Fazi P, Lazzarino M, Leoni P, et
al: Outcome of patients with acute myeloid leukemia who failed to
respond to a single course of first-line induction therapy: A
GIMEMA study of 218 unselected consecutive patients. Gruppo
italiano malattie ematologiche maligne dell'adulto. Leukemia.
10:1443–1452. 1996.PubMed/NCBI
|
|
10
|
Kern W, Aul C, Maschmeyer G,
Schönrock-Nabulsi R, Ludwig WD, Bartholomäus A, Bettelheim P,
Wormann B, Büchner T and Hiddemann W: Superiority of high-dose over
intermediate-dose cytosine arabinoside in the treatment of patients
with high-risk acute myeloid leukemia: Results of a age-ajusted
prospective randomized comparison. Leukemia. 12:1049–1055. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bennet JM, Catovsky D, Daniel MT, Flandrin
G, Galton DA, Gralnick HR and Sultan C: Criteria for the diagnosis
of acute leukemia of megakaryocytic lineage (M7): A report of the
French-American-British (FAB) cooperative group. Ann Intern Med.
103:460–462. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vardiman JW, Harris NL and Brunning RD:
The world health organization (WHO) classification of the myeloid
neoplasms. Blood. 100:2292–2302. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Slovak ML, Kopecky KJ, Cassileth PA,
Harrington DH, Theil KS, Mohamed A, Paietta E, Willman CL, Head DR,
Rowe JM, et al: Karyotypic analysis predicts outcome of
pre-remission and post-remission therapy in adult acute myeloid
leukemia: A southwest oncology group/eastern cooperative oncology
group study. Blood. 96:4075–4083. 2000.PubMed/NCBI
|
|
14
|
Cheson BD, Bennett JM, Kopecky KJ, Büchner
T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister
TA, et al: Revised recommendations of the international working
group for diagnosis, standardization of response criteria,
treatment outcomes and reporting standards for therapeutic trials
in acute myeloid leukemia. J Clin Oncol. 21:4642–4649. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Burnett AK, Wheatley K, Goldstone AH,
Stevens RF, Hann IM, Rees JH and Harrison G: Medical Research
Council Adult and Paediatric Working Parties: The value of
allogeneic bone marrow transplant in patients with acute myeloid
leukaemia at differing risk of relapse: Results of the UK MRC AML
10 trial. Br J Haematol. 118:385–400. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pricea SL, Lancetb JE, Georgea TJ,
Wetzstein GA, List AF, Ho VQ, Fernandez HF, Pinilla-Ibarz J,
Kharfan-Dabaja MA and Komrokji RS: Salvage chemotherapy regimens
for acute myeloid leukemia: Is one better? Efficacy comparison
between CLAG and MEC regimens. Leuk Res. 35:301–304. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wierzbowska1 A, Robak1 T, Pluta1 A,
Wawrzyniak E, Cebula B, Hołowiecki J, Kyrcz-Krzemień S, Grosicki S,
Giebel S, Skotnicki AB, et al: Cladribine combined with high doses
of arabinoside cytosine, mitoxantrone and G-CSF (CLAG-M) is a
highly effective salvage regimen in patients with refractory and
relapsed acute myeloid leukemia of the poor risk: A final report of
the polish adult leukemia group. Euro J Haematol. 80:115–126. 2008.
View Article : Google Scholar
|
|
18
|
Fujimoto S and Ogawa M: Antitumor activity
of mitozantrone against murine experimental tumors: Comparative
analysis against various antitumor antibiotics. Cancer Chemother
Pharmacol. 8:157–162. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schnetzke U, Fix P, Spies-Weisshart B,
Schrenk K, Glaser A, Fricke HJ, LaRosée P, Hochhaus A and Scholl S:
Efficacy and feasibility of cyclophosphamide combined with
intermediate-dose or high-dose cytarabine for relapsed and
refractory acute myeloid leukemia (AML). J Cancer Res Clin Oncol.
140:1391–1397. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
De la Rubia J, Regadera AA, Martín G,
Cervera J, Sanz G, Martínez J, Jarque I, García I, Andreu R,
Moscardó F, et al: FLAG-IDA regimen (fludarabine, cytarabine,
idarubicin and G-CSF) in the treatment of patients with high-risk
myeloid malignancies. Leuk Res. 26:725–730. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Steinmetz HT, Schulz A, Staib P, Scheid C,
Glasmacher A, Neufang A, Franklin J, Tesch H, Diehl V and Dias
Wickramanayake P: Phase-II trial of idarubicin, fludarabine,
cytosine arabinoside and filgrastim (Ida-FLAG) for treatment of
refractory, relapsed and secondary AML. Ann Hematol. 78:418–425.
1999. View Article : Google Scholar : PubMed/NCBI
|