Introduction
Uterine sarcoma is a rare malignant tumor of the
female reproductive system. The incidence of uterine sarcoma among
women is only 1.23–1.70/100,000 (1,2),
comprising 1–3% of gynecological malignant tumors and 3.0–5.0% of
malignant tumors of the uterine body (3). Uterine sarcoma is a highly aggressive
malignancy; even if treated at its early stages, patients often
develop local recurrence and distant metastasis. Total hysterectomy
is the standard treatment for early-stage uterine sarcoma. There is
currently no standard therapy for advanced or recurrent uterine
sarcomas, but chemotherapy is the preferred approach. However,
chemotherapy only achieves a 30% response rate in advanced or
recurrent uterine leiomyosarcomas (3). Therefore, it is crucial to investigate
other medications and therapeutic regimens for advanced or
recurrent uterine sarcomas. This study retrospectively analyzed 4
patients with advanced or recurrent uterine sarcoma who were
treated with bevacizumab (BEV) combined with chemotherapy in our
hospital between May, 2006 and May, 2014, with the aim to determine
the efficacy of this combination in uterine sarcoma.
Patients and methods
Patient characteristics
We retrospectively analyzed the clinical data of 4
patients with advanced or recurrent refractory uterine sarcoma who
were treated with BEV combined with chemotherapy in our hospital
between May, 2006 and May, 2014. Of the 4 cases, 2 had
advanced-stage (IV) persistent uterine sarcoma (1 patient received
BEV as first-line treatment and 1 patient as second-line
treatment), whereas the remaining 2 patients had recurrent uterine
sarcoma (1 patient received BEV as second-line treatment and 1
patient as third-line treatment). The pathological types were
undifferentiated sarcoma of the uterus in 1 case, uterine
carcinosarcoma in 2 cases and uterine leiomyosarcoma in 1 case. The
mean age of the patients was 61 years (range, 44–79 years) and the
Karnofsky performance status score was ≥80 prior to treatment. The
patient characteristics are summarized in Table I.
 | Table I.Characteristics of the 4 patients. |
Table I.
Characteristics of the 4 patients.
| N | Age (years) | Initial
treatment | Pathology | Prior
chemotherapy | Usage of BEV | Combined
chemotherapy | RECIST | Toxicity
(BM/suppression GI reactions) | Follow-up PFS/OS
(months) | Outcome |
|---|
| 1 | 44 | TAH + BSO | Undifferentiated
endometrial sarcoma | IFO + Doxil | 7.5 mg/kg every 2
weeks, 12 times | DTIC + VP-16 +
DDP | CR | II/II | 96/96 | Alive |
| 2 | 57 | TAH + BSO | Carcinosarcoma | PEI, TI | 7.5 mg/kg every 3
weeks, 11 times | DTIC + VP-16 +
DDP | PR | IV/I | 13/25 | Deseased |
| 3 | 63 | TAH + BSO | Leiomyosarcoma | EI | 7.5 mg/kg every 3
weeks, 4 times | DTIC + EPI + DDP | SD | II/II | 9/24 | Deseased |
| 4 | 79 | TAH + BSO | Carcinosarcoma | – | 7.5 mg/kg every 3
weeks, 6 times | PC, IFO + EPI | PD | II/I | 3/9 | Deseased |
Therapeutic method
BEV (Avastin; Genentech, South San Francisco, USA)
7.5 mg/kg with 100 ml 0.9% NaCl, 1 h prior to or following
chemotherapy, as an intravenous drip over 60 min, repeated every 2
or 3 weeks. The chemotherapeutic drugs included dacarbazine (DTIC),
cisplatin (DDP), etoposide (VP-16), adriamycin, paclitaxel and
carboplatin. The patients received 4–12 cycles of BEV treatment,
with a mean of 8.3 cycles. The completed cycles of BEV combined
with chemotherapy were 4–8, with a mean of 6 cycles. One patient
received BEV 7.5 mg/kg as an intravenous drip every 2 weeks, for a
total of 12 times (2 BEV administrations per 4-week cycle); the
other 3 patients received BEV 7.5 mg/kg as an intravenous drip
every 3 weeks, synchronously combined with chemotherapy. However, 1
patient was unable to tolerate treatment due to grade 4 bone marrow
suppression (thrombocytopenia, platelet count 13 G/l) after 8
cycles of chemotherapy, and thus she was administered single-agent
BEV maintenance therapy for 3 cycles (once every 3 weeks).
Therapeutic evaluation
All the patients were evaluated after receiving
>2 cycles of treatment with BEV according to the World Health
Organisation Response Evaluation Criteria In Solid Tumors, version
1.1 (2009). Response to treatment was classified as complete
response (CR), partial response (PR), stable disease (SD) or
progressive disease (PD). Chemotherapy response rate (CR+PR) and
clinical benefit rate (CR+PR+SD) were also evaluated.
Toxicity evaluation
The adverse reactions to BEV combined with
chemotherapy were evaluated according to the toxicity evaluation
standards of the National Cancer Institute. Cardiovascular toxicity
was evaluated with a sphygmomanometer, electrocardiogram (ECG)
monitor and ECG examination; urinary system toxicity was evaluated
with routine urine and kidney function tests. Toxicity was graded
as 0–4.
Follow-up
The patients were followed up in an outpatient
setting or telephonically, until death or until the last follow-up
in October, 2014.
Statistical analysis
Statistical analysis was performed by SPSS 19.0
software (SPSS Inc., Chicago, IL, USA). The ×2 inspection was used
for count data and PFS was estimated with the Kaplan Meier method,
using the log-rank test. Of the 4 patients, 3 had succumbed to
disease progression at the last follow-up, with a progression-free
survival (PFS) of 13, 9 and 3 months. The PFS of the surviving
patient was 96 months. The overall survival (OS) of the deceased
patients was 25, 24 and 9 months, and of the surviving patient 96
months (Table I).
Results
Objective effect
The total chemotherapy response rate of BEV combined
with chemotherapy (CR+PR) was 50% (2/4) and the clinical benefit
rate was 75% (3/4). The clinical evaluation of the 4 patients was
CR in 1 case, PR in 1 case, SD in 1 case and PD in 1 case. The mean
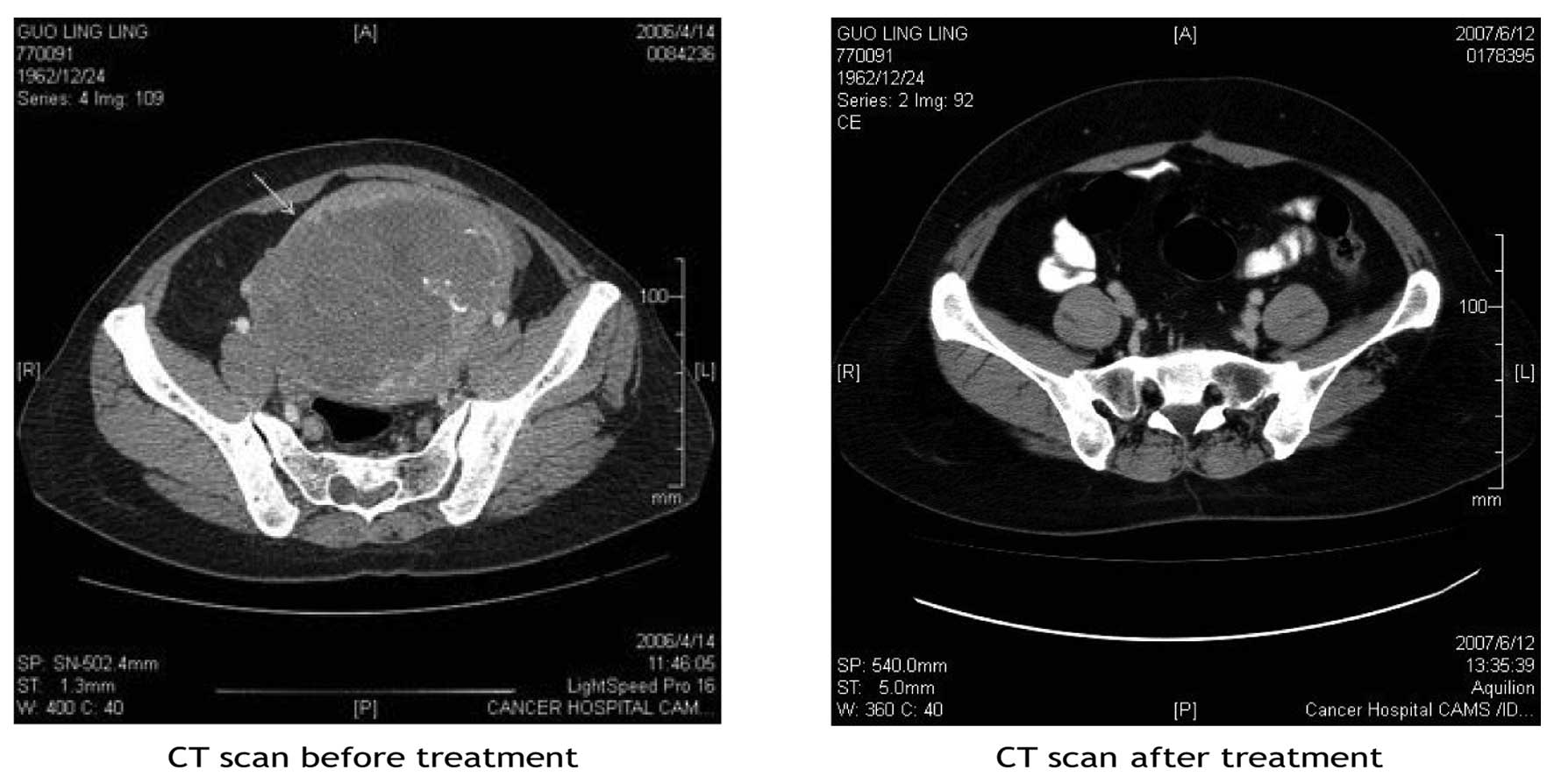
PFS was 30.25 months and the mean OS 38.5 months. The one patient
who achieved CR survived for 96 months and remained alive at the
last follow-up; her computed tomography scans prior to and
following treatment are shown in Fig.
1.
Toxicity reaction
The treatment-related adverse reactions mainly
included bone marrow suppression; 1 patient had grade 4
thrombocytopenia [platelet (PLT) count 13 G/l], while the remaining
3 patients had grade 2 bone marrow suppression (leukopenia); the
non-hematological toxicities included grade 2 gastrointestinal
reactions in 2 patients and grade 1 in the remaining 2
patients.
Discussion
Uterine sarcomas are a group of heterogeneous
malignant tumors derived from uterine mesenchymal tissue, and
include leiomyosarcoma of the uterus, endometrial stromal sarcoma,
uterine adenosarcoma and carcinosarcoma. At present, uterine
carcinosarcoma is classified into endometrial carcinoma. The
prognosis of uterine sarcoma is poor, with a 5-year survival rate
of only 30% (4). Prognosis is poor,
as this type of cancer may easily recur and metastasize following
primary treatment. Furthermore, certain patients are not diagnosed
until they are at an advanced stage (with distant metastasis), in
which case the 5-year survival rate is <15% (5). The effect of treatment on persistent
and/or recurrent uterine sarcomas is poor, particularly for
patients who have recurred after first-line chemotherapy. When
these patients receive >2 lines of chemotherapy, re-using mono-
or combination chemotherapy (including ifosfamide, doxorubicin,
cisplatin, topotecan, paclitaxel, docetaxel, gemcitabine and
gemcitabine combined with docetaxel), the effectiveness of these
regimens is only 5–27% (6–8). Several tumor treatment centers worldwide
have been actively investigating treatment methods for uterine
sarcomas, including novel chemotherapeutic drugs and
molecular-targeted agents, but the results have not been
satisfactory. Bernstein-Molho et al (9) reported that the efficiency rate was 0%
with trabectedin as second- or third-line therapy for metastatic
leiomyosarcoma, whereas the SD rate was 60%. Aghajanian et
al (10) reported that the
response rate with iniparib combined with paclitaxel and
carboplatin (as first-line chemotherapy) was 23.5% (4/17) in late
persisitent and/or recurrent uterine sarcomas, but 35.3% (6/17) of
the patients only achieved SD. Hensley et al (11) reported that, according to the
Gynecologic Oncology Group (GOG), the results of sunitinib used as
single-drug therapy in a phase II clinical study to treat
persistent and/or recurrent uterine sarcomas achieved a PR rate of
8.7% (2/23); the rate of grade 3 hematological toxicity was 17.4%
and of grade 3–4 non-hematological toxicity ≤30% (11). Gynecological oncologists must
continuously investigate effective agents for the treatment of
uterine sarcomas, as the availability of relevant data is currently
limited.
Inhibition of the vascular endothelial growth factor
(VEGF) pathway has been used in cancer treatment in recent years.
BEV is a type of humanized restructuring anti-VEGF monoclonal
antibody, and a number of researchers have already confirmed that
BEV is effective in treating a variety of solid tumors, such as
colorectal, lung, breast and ovarian cancer; however, very few
reports have been published to date on BEV as treatment for uterine
sarcomas and/or other soft tissue sarcomas. It was previously
demonstrated that VEGF is strongly expressed in the epithelium and
stroma of uterine carcinosarcomas (12), and GOG is currently conducting a phase
III randomized clinical study (12)
to assess the curative effect of gemcitabine combined with
docetaxel, with or without BEV, in patients with advanced or
recurrent uterine leiomyosarcomas (12). In the present study, 4 cases of
uterine sarcomas received BEV combined with chemotherapy; 2 cases
had advanced-stage disease (IV) and persistent uterine sarcoma (1
case received BEV as first-line and 1 case as second-line therapy),
whereas the other 2 cases presented with recurrent uterine sarcomas
post-treatment (1 case received BEV as second-line and 1 case as
third-line therapy). The pathological tumor type was
undifferentiated sarcoma of the uterus in 1 case, uterine
carcinosarcoma in 2 cases and uterine leiomyosarcoma in 1 case. The
patients received 4–12 cycles of BEV treatment, with a mean of 8.3
cycles. The completed cycles of BEV combined with chemotherapy were
4–8, with a mean of 6 cycles. One patient received BEV 7.5 mg/kg as
an intravenous drip every 2 weeks, for a total of 12 times (2 BEV
administrations per 4-week cycle); the other 3 patients received
BEV 7.5 mg/kg as an intravenous drip every 3 weeks, synchronously
combined with chemotherapy. However, 1 patient was unable to
tolerate treatment due to grade 4 bone marrow suppression
(thrombocytopenia, PLT count 13 G/l) after 8 cycles of
chemotherapy, and thus she was administered single-agent BEV
maintenance therapy for 3 cycles (once every 3 weeks). The clinical
evaluation of the 4 patients was CR in 1 case, PR in 1 case, SD in
1 case and PD in 1 case. Cases 1 and 2 achieved CR and PR,
respectively, and received >6 cycles of DTIC + VP-16 + DDP
chemotherapy combined with BEV, with a PFS of 96 and 13 months, and
an OS of 96 and 25 months, respectively. This is consistent with
the results of Olawalye et al (13), who reported that a patient with
recurrent uterine epithelioid angiosarcoma achieved CR following
treatment with BEV combined with chemotherapy for 6 cycles; the
patient exhibited a disease-free survival for 12 months until the
time the report was published.
In our study, 1 patient (case 3) was evaluated as SD
after 4 cycles of BEV combined with chemotherapy; the pelvic
recurrence completely disappeared after treatment, and the multiple
metastases in her lungs were also evaluated as SD, with a PFS of 9
months. The patient eventually succumbed to morbidities associated
with changing the therapeutic regimen and tumor progression. These
findings demonstrated that BEV combined with chemotherapy may be an
effective second- and/or third-line therapy to treat advanced
persistent (case 1) and recurrent (cases 2 and 3) uterine sarcomas,
and that patients with persistent or recurrent uterine sarcomas may
achieve CR with appropriate dosage and dosing intervals of BEV
combined with the appropriate chemotherapy regimens, with a PFS of
up to 96 months. Of the 4 patients, only 1 (case 4) was clinically
evaluated as PD after BEV combined with chemotherapy as first-line
therapy. This PD may be associated with the fact that the patient
had been irradiated radically for cervical cancer prior to the
development of uterine sarcomas; therefore, the patient's uterus
and surrounding tissues may have developed fibrosis and resulted in
a different vascular distribution and tumor formation compared with
the patients who only received chemotherapy. Furthermore, the
patient's sarcoma comprised complex components (leiomyosarcoma,
chondrosarcoma and endometrial stromal sarcoma). Thus, this patient
did not benefit from BEV treatment combined with chemotherapy.
Treatment-related adverse reactions were mainly bone
marrow suppression and gastrointestinal reactions in all 4 patients
who received a mean of 8.3 cycles of BEV therapy in the present
study. Grade 4 thrombocytopenia (PLT count 13 G/l) was only
observed in 1 patient (case 2) and may have been associated with
several factors: The patient was elderly (63-years old) and
received a total of 13 cycles of chemotherapy (3 lines), while the
remaining 3 patients exhibited grade 2 bone marrow suppression
(leukopenia). The main non-hematological toxicity was grade 2
gastrointestinal reactions in 2 patients and grade 1 in the
remaining 2 patients. There were no severe adverse reactions to
BEV, such as bowel perforation, bleeding or blood vessel embolism,
high blood pressure, or severe proteinuria (14). Thus, it may be safe to use BEV
combined with chemotherapy to treat advanced persistent and/or
recurrent uterine sarcomas.
In conclusion, BEV combined with chemotherapy
exhibits efficacy in the treatment of advanced or recurrent uterine
sarcomas, with a tolerable toxicity profile. Treatment with BEV may
achieve a CR, and potentially long-term disease-free survival, and
may be considered as a safe and effective candidate regimen for
advanced or recurrent uterine sarcomas, in addition to providing a
theoretical basis for further clinical research involving larger
samples.
Acknowledgements
This study was supported by the Gynecologic Oncology
of Cancer Institute and Hospital, Chinese Academy of Medical
Sciences.
References
|
1
|
Nordal RR and Thoresen So: Uterine
sarcomas in Norway 1956-1992: Incidence survival and mortality. Eur
J Cancer. 33:907–911. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Oláh KS, Gee H, Blunt S, Dunn JA, Kelly K
and Chan KK: Retrospective analysis of 318 cases of uterine
sarcoma. Eur J Caner. 27:1095–1099. 1991. View Article : Google Scholar
|
|
3
|
Major FJ, Blessing JA, Silverberg SG,
Morrow CP, Creasman WT, Currie JL, Yordan E and Brady MF:
Prognostic factors in early-stage uterine sarcoma. Histopathology.
Cancer. 71((Suppl 4)): S1702–S1709. 1993. View Article : Google Scholar
|
|
4
|
Tropé CG, Abeler VM and Kristensen GB:
Diagnosis and treatment of sarcoma of the uterus. Histopathology.
Acta Oncol. 51:694–705. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Powell MA, Filiac VL, Rose PG, Mannel RS,
Hanjani P, Degeest K, Miller BE, Susumu N and Ueland FR: Phase II
evaluation of paclitaxel and carboplatin in the treatment of
carcinosarcoma of the uterus, A Gynecologic Oncology Group study. J
Clin Oncol. 28:2727–2731. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miller BE, Blessing JA, Stehman FB, Shahin
MS, Yamada SD, Secord AA, Warshal DP, Abulafia O, Richards WE and
Van Le L: A phase II evaluation of weekly gemcitabine and docetaxel
for second-line treatmentof recurrent carcinosarcoma of the uterus.
A Gynecologic Oncology Group study. Gynecol Oncol. 118:139–144.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yoo HJ, Lim MC, Lim S, Park JY, Kang S,
Park SY and Seo SS: Phase II study of paclitaxel in combination
with carboplatin for patients with recurrent or persistent uterine
sarcoma. Arch Gynecol Obstet. 286:1529–1535. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gupta AA, Yao X, Verma S, Mackay H and
Hopkins L: Chemotherapy (gemcitabine, docetaxel plus gemcitabine,
doxorubicin, or trabectedin) in inoperable, locally advanced,
recurrent, or metastatic uterine leiomyosarcoma, A clinical
practice guideline. Curr Oncol. 20:e448–e454. 2012.
|
|
9
|
Bernstein-Molho R, Grisaro T, Soyfer V,
Safra T and Merimsky O: Metastatic uterine leiomyosarcomas, A
single-institution experience. Int J Gynecol Cancer. 20:255–260.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aghajanian C, Sill MW, Secord AA, Powell
MA and Steinhoff M: Iniparib plus paclitaxel and carboplatin as
initial treatment of advanced or recurrent uterine carcinosarcoma,
A Gynecologic Oncology Group study. Gynecol Oncol. 126:424–427.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hensley ML, Sill MW, Scribner DR Jr, Brown
J, Debernardo RL, Hartenbach EM, McCourt CK, Bosscher JR and Gehrig
PA: Sunitinib malate in the treatment of recurrent or persistent
uterine leiomyosarcoma: A Gynecologic Oncology Group phase II
study. Gynecol Oncol. 115:460–465. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gupta A, Yao X, Verma S, Mackay H and
Hopkins L: the Sarcoma Disease Site Group (DSG) and the Gynecology
Cancer DSG. Chemotherapy (i.e., gemcitabine, docetaxel plus
gemcitabine, doxorubicin, or trabectedin) for inoperable, locally
advanced, recurrent, or metastatic uterine leiomyosarcoma.
Evidence-based series. 11(11)2012.(Toronto, On). Cancer Care
Ontario, Program in Evidence-Based Care. 2012.
|
|
13
|
Olawaiye AB, Morgan JA, Goodman AK, Fuller
AF Jr and Penson RT: Epithelioid angiosarcoma of the uterus: A
review of management. Arch Gynecol Obstet. 278:401–404. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Homesley HD, Filiaci V, Markman M,
Bitterman P, Eaton L, Kilgore LC, Monk BJ and Ueland FR:
Gynecologic Oncology Group: Phase III trial of ifosfamide with or
without paclitaxel in advanceed uterine carcinosarcoma. A
Gynecologic Oncology Group study. Clin Oncol. 25:526–531. 2007.
View Article : Google Scholar
|