Introduction
Combined chemotherapy regimens for colon cancer,
including the combination of 5-fluorouracil (FU), leucovorin (LV)
and oxaliplatin (FOLFOX), and the combination of capecitabine and
oxaliplatin ± bevacizumab, the combination of FU/LV and irinotecan
(FOLFIRI) ± cetuximab and FOLFOX ± cetuximab, have assisted in
prolonging survival (1–3). However, the high cost of these
treatments has often been discussed (4).
The use of bevacizumab, cetuximab or panitumumab as
second-line therapy for colon cancer after first-line chemotherapy
is expensive. Therefore, it is important to apply the concept of
drug economics for reducing medical expenses. The consideration of
drug economics is more popular in western countries compared with
in Japan. The effectiveness and economic efficiency of these
treatments for advanced and recurrent colorectal cancer have been
evaluated by numerous previous studies (5–15). For
instance, for metastatic colorectal cancer, the incremental
cost-effectiveness ratio (ICER) of XELOX as a first-line therapy
was £105,000 per quality-adjusted life year (QALY) and that of
first-line FOLFOX was £108,000 per QALY. The ICER of cetuximab for
treating wild-type KRAS colorectal cancer was ¥16×106
per QALY (11). Therefore, none of
these therapies is adequately cost-effective.
In general, the cost-effectiveness of anticancer
agents for treating conditions with a short life expectancy (last
line) is worsening. The American Society of Clinical Oncology and
the National Comprehensive Cancer Centre Network recommend
regorafenib or the trifluridine/tipiracil combination tablet as a
third-line or later treatment for advanced and recurrent colorectal
cancer. In Japan, this treatment is also recommended and is
generally used (16). The
trifluridine/tipiracil combination tablet is used based on the
results of the RECOURSE study (17).
In Europe, the Committee for Medicinal Products for Human Use of
the European Medicines Agency has also approved this
recommendation. For third-line or later treatment of advanced and
recurrent colorectal cancer, no previous studies have, to the best
of our knowledge, directly compared regorafenib and the
trifluridine/tipiracil combination tablet.
The present study evaluated the cost-effectiveness
of regorafenib vs. the trifluridine/tipiracil combination tablet as
treatments for advanced and recurrent colorectal cancer in order to
facilitate decision making during treatment selection.
Patients and methods
Treatment regimens
Regorafenib was administered at a dose of 160 mg/day
during 3-week courses with 1-week intervals between the courses.
The trifluridine/tipiracil combination tablet (with each dose
consisting of 35 mg/m2) was administered twice daily
after morning and evening meals for 5 days, followed by 2 days of
rest and then again for 2 weeks, followed by a 14-day resting
period, thus completing one treatment cycle. These are the patient
data from the previous CORRECT and RECOURSE trials. The patients
were administered two courses or more of either the regorafenib
regimen (n=10) or the trifluridine/tipiracil combination tablet
regimen (n=34) for treating advanced and recurrent colorectal
cancer.
Calculation of cost
Cost data included direct costs occurring at the
time of drug therapy. Fees for medication (including supportive
care), inspection and medical examination of outpatients were
calculated. Information on drug prices from the Insurance Drug
Encyclopaedia (18) and medical fees
from the Medical Fee Points Table (19) was retrieved to calculate total
medical expenses. The cost of diagnostic imaging (chest computed
tomography scan) and the labour of medical staff was included for
each drug treatment and therefore did not require adding. The
running and depreciation costs of facilities per patient were
excluded as they were difficult to determine.
Determination of therapeutic
efficacy
To obtain the therapeutic efficacy of the
regorafenib and trifluridine/tipiracil combination tablet regimens,
the CORRECT study (20) and the
RECOURSE study (17) were used as
data sources of the median survival time (MST).
Calculation of cost-effectiveness
The cost-effectiveness analysis was performed using
the cost and effectiveness data of each drug regimen obtained as
stated. The cost-effectiveness ratio of each drug regimen was
calculated by dividing the expected cost by the MST. In addition,
the ICER was determined to compare the cost-effectiveness of the
trifluridine/tipiracil combination tablet regimen vs. that of the
regorafenib regimen using the following equation: ICER (¥/MST) =
(expected cost of trifluridine/tipiracil combination tablet regimen
– expected cost of regorafenib regimen) / (MST of
trifluridine/tipiracil combination tablet regimen – MST of
regorafenib regimen). Since the data available were for a
time-period of no more than 1 year, the timing of the discount was
not adjusted.
Analysis of adverse events (AEs)
AEs were investigated for each patient
retrospectively. The date of the occurrence of each AE was
identified using electronic charts and pharmacy service records for
the patients treated at Ogaki Municipal Hospital. The severity of
AEs was classified according to the Common Terminology Criteria for
Adverse Events (21).
Statistical analysis
Welch's t-test was used to analyse the variables and
Student's t-test was used to analyse the number of outpatient
visits and patient characteristics. In all significance tests,
P<0.05 was considered to indicate a statistically significant
difference. All statistical analyses were performed using JMP 8
software (SAS Institute Inc., Cary, NC, USA) and the data are
presented as the mean ± standard deviation.
Ethical considerations
The present study was approved by the Institutional
Review Board of Ogaki Municipal Hospital (Ogaki, Japan).
Results
Patient characteristics
The patient characteristics are summarised in
Table I. The median age of the
patients who received regorafenib and trifluridine/tipiracil
combination tablet regimens was 67.5 years (range, 53–71 years) and
69 years (range, 37–77 years), respectively, and the median number
of previous treatments was 3.5 courses (range, 3–5 courses) and 3
courses (range, 3–5 courses), respectively.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | Regorafenib |
Trifluridine/tipiracil combination
tablet | P-value |
|---|
| Number | 10 | 34 |
|
| Median age, years
(range) | 67.5 (53–71) | 69 (37–77) | 0.9491 |
| No.
males/females | 8/2 | 18/16 | 0.1211 |
| ECOG performance
status |
|
| 0.1211 |
| 0 | 4 | 17 |
|
| 1 | 5 | 14 |
|
| 2 | 1 | 3 |
|
| Median number of
previous treatment lines (range) | 3.5 (3–5) | 3 (3–5) | 0.1024 |
| Median body surface
area, m2, (range) | 1.69 (1.34–1.82) | 1.53 (1.09–1.96) | 0.2042 |
| Disease status |
|
| 0.2175 |
|
Unresectable | 2 | 2 |
|
|
Recurrent | 8 | 32 |
|
| Metastatic site |
|
| 0.4829 |
|
Liver | 6 | 19 |
|
| Lung | 3 | 15 |
|
|
Peritoneum | 0 | 11 |
|
| Lymph
node | 3 | 11 |
|
| Bone | 1 | 4 |
|
| Skin | 1 | 1 |
|
Cost data
For the regorafenib regimen, the calculated direct
medical cost included medication fee (anti-cancer drugs,
¥658,424.0; supportive care drugs, ¥14,780.3), an inspection fee of
¥10,062.0 and an outpatient medical examination fee of ¥6,716.0.
For the trifluridine/tipiracil combination tablet regimen, the
calculated direct medical cost included medication fee (anti-cancer
drugs, ¥349,685.0; supportive care drugs, ¥261.0), an inspection
fee of ¥5,535.0 and an outpatient fee of ¥3,453.5. The regorafenib
regimen was found to be more expensive compared with the
trifluridine/tipiracil combination tablet regimen in total
(¥705,330.3 vs. ¥371,198.7; P<0.0001), as well as for each
medical expense (Table II).
 | Table II.Treatment costs. |
Table II.
Treatment costs.
| Source of cost | Regorafenib |
Trifluridine/tipiracil combination
tablet | P-value |
|---|
| Medication |
|
|
|
|
| Anticancer drugs | ¥658,424.0 | ¥349,685.0 | <0.0001 |
| Supportive care
drugs | ¥14,780.3 | ¥261.0 | 0.0184 |
| Inspection | ¥10,062.0 | ¥5,535.0 | <0.0001 |
| Outpatient medical
examination | ¥6,716.0 | ¥3,453.5 | <0.0001 |
| Management of
malignant tumour-specific substances and therapeutic
management | ¥9,000.0 | ¥9,000.0 | 1 |
| Othersa | ¥6,348.0 | ¥3,264.2 | <0.0001 |
| Total | ¥705,330.3 | ¥371,198.7 | <0.0001 |
Cost-effectiveness analysis
The cost-effectiveness ratio (¥/month) was
¥110,207.9/MST for the regorafenib regimen and ¥52,281.5/MST for
the trifluridine/tipiracil combination tablet regimen. A
significant difference between the two groups was identified
(P<0.0001; Table III). The
incremental cost-effectiveness ratio of the regorafenib regimen vs.
the trifluridine/tipiracil combination tablet regimen was
¥477,330.9/MST. The trifluridine/tipiracil combination tablet
regimen was therefore found to be more cost-effective compared with
the regorafenib regimen.
 | Table III.Cost-effectiveness ratio. |
Table III.
Cost-effectiveness ratio.
| Treatment | Expected cost per
person | Cost-effectiveness
ratioa | MST (months) |
|---|
| Regorafenib | ¥705,330.3 | ¥110,207.9 | 6.4 |
|
Trifluridine/tipiracil combination
tablet | ¥371,198.7 | ¥52,281.5 | 7.1 |
| P-valueb | <0.0001 | <0.0001 | NA |
Number of outpatient visits
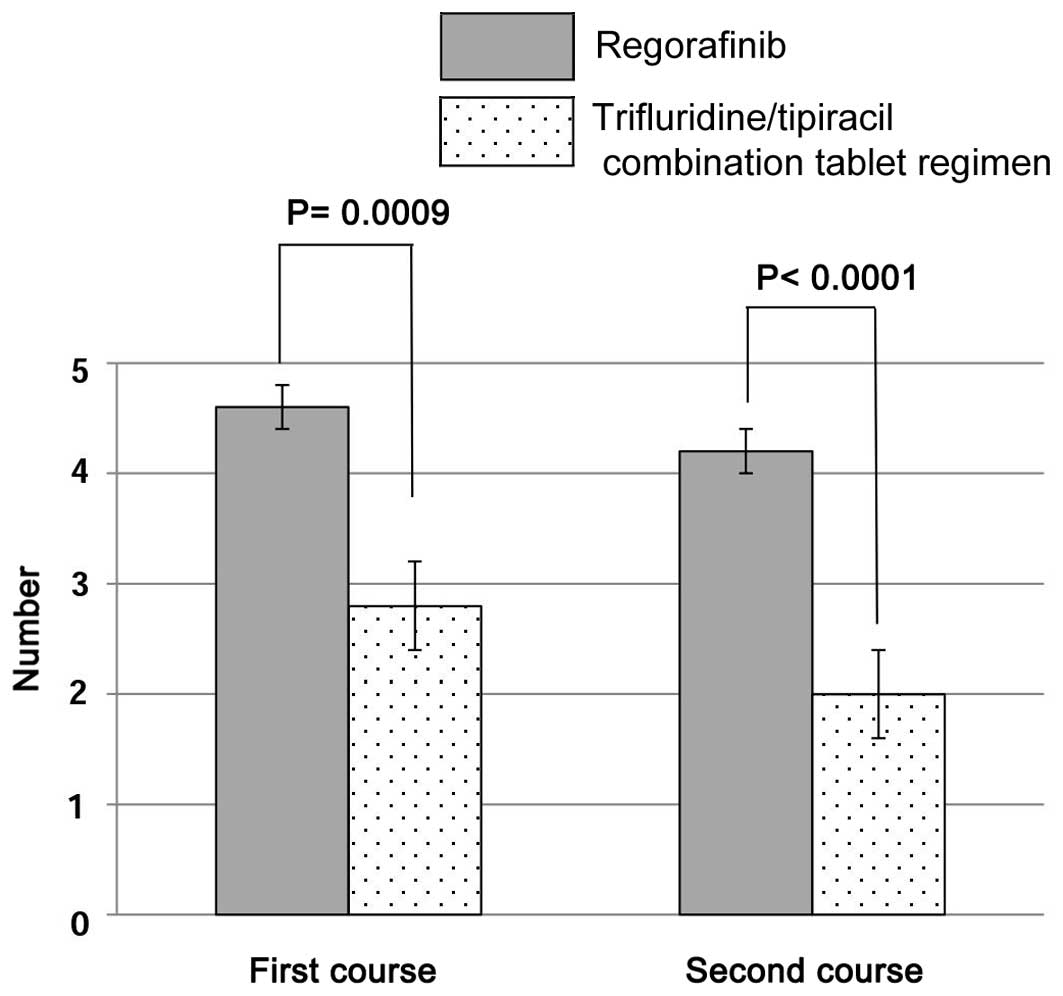
The number of outpatient visits in the first and
second course was 4.6±0.5 and 4.0±0.5, respectively, for the
regorafenib regimen, and 2.8±1.1 and 2.0±0.7, respectively, for the
trifluridine/tipiracil combination tablet regimen. Significant
differences were observed between the two groups (Fig. 1).
AEs
The major AEs are summarised in Table IV. For the regorafenib regimen,
these comprised hand-foot syndrome (70.0%), malaise (70.0%),
hypertension (60.0%), hoarseness (60.0%) and an increase in
aspartate transaminase and alanine transaminase (50.0%). Among
these AEs, 90.0% were grade 3 or higher; rhabdomyolysis was also
observed. For the trifluridine/tipiracil combination tablet regime,
major AEs comprised neutropenia (61.8%), anaemia (58.8%), nausea
(44.1%) and anorexia (20.6%). Among these, 37.5% were grade 3 or
higher.
 | Table IV.Adverse events. |
Table IV.
Adverse events.
| A, Patients treated
with regorafenib (n=10) |
|---|
|
|---|
|
| Grade |
|
|---|
|
|
|
|
|---|
| Adverse event | 1 | 2 | 3 | 4 | All grades, n
(%) |
|---|
| Thrombocytopenia | 1 | 0 | 1 | 0 | 2 (20.0) |
| Anaemia | 1 | 0 | 0 | 0 | 1 (10.0) |
| T-Bil increase | 0 | 1 | 2 | 0 | 3 (30.0) |
| AST/ALT
increase | 0 | 2 | 2 | 1 | 5 (50.0) |
| CPK | 0 | 0 | 0 | 1 | 1 (10.0) |
| Malaise | 4 | 2 | 1 | 0 | 7 (70.0) |
| Anorexia | 2 | 0 | 1 | 0 | 3 (30.0) |
| Nausea | 2 | 0 | 0 | 0 | 2 (20.0) |
| Stomatitis | 2 | 0 | 0 | 0 | 2 (20.0) |
| HFS | 2 | 1 | 4 | 0 | 7 (70.0) |
| Rash (face) | 1 | 0 | 0 | 0 | 1 (10.0) |
| Hypertension | 2 | 1 | 3 | 0 | 6 (60.0) |
| Proteinuria | 0 | 1 | 0 | NA | 1 (10.0) |
| Hoarseness | 6 | 0 | 0 | 0 | 6 (60.0) |
| Fever | 2 | 0 | 0 | 0 | 2 (20.0) |
|
| B, Patients treated
with trifluridine/tipiracil combination tablet (n=34) |
|
|
|
|
|
|
| Grade |
|
|
|
|
|
| Adverse event | 1 | 2 | 3 | 4 | All grades, n
(%) |
|
| Neutropenia | 2 | 8 | 8 | 3 | 21 (61.8) |
|
Thrombocytopenia | 5 | 1 | 0 | 0 | 6 (17.6) |
| Anaemia | 7 | 8 | 5 | 0 | 20 (58.8) |
| T-Bil increase | 0 | 0 | 1 | 0 | 1 (2.9) |
| AST/ALT
increase | 0 | 1 | 0 | 0 | 1 (2.9) |
| Creatinine
increase | 2 | 0 | 0 | 0 | 2 (5.9) |
| Malaise | 5 | 3 | 1 | 0 | 9 (26.5) |
| Anorexia | 3 | 4 | 0 | 0 | 7 (20.6) |
| Nausea | 12 | 0 | 3 | 0 | 15 (44.1) |
| Vomiting | 3 | 0 | 0 | 0 | 3 (8.8) |
| Stomatitis | 1 | 0 | 0 | 0 | 1 (2.9) |
| Diarrhoea | 2 | 1 | 0 | 0 | 3 (8.8) |
| Alopecia | 1 | 0 | NA | NA | 1 (2.9) |
| Dysgeusia | 2 | 0 | NA | NA | 2 (5.9) |
| Headache | 1 | 0 | 0 | 0 | 1 (2.9) |
| Eye disorders
(conjunctivitis) | 1 | 0 | 0 | 0 | 1 (2.9) |
| Hyperkalaemia | 0 | 1 | 0 | 0 | 1 (2.9) |
Discussion
In the present study, a drug-economics analysis was
performed to compare regorafenib and trifluridine/tipiracil
combination tablet regimens as third-line or later treatments of
advanced and recurrent colorectal cancer. It was revealed that the
MST (months) was almost equal for the two regimens, and that the
trifluridine/tipiracil combination tablet regimen has a superior
cost-effectiveness compared with that of the regorafenib
regimen.
The outcome (survival) is included in the
cost-effectiveness value. Suppression of recurrence/progression of
cancer, maintenance of quality of life (QOL) and extension of
survival time are desired effects of anticancer agent treatments.
However, advanced and recurrent cancer cannot be cured by these
treatments. In certain cases, progression-free survival (PFS) and
overall survival were extended from a few weeks to several months.
The median PFS of patients with advanced and recurrent colorectal
cancer treated with regorafenib and trifluridine/tipiracil
combination tablet is only 1.9 and 2.0 months, respectively
(17,20).
Regarding cost-effectiveness, Goldstein et al
(22) reported that regorafenib
compared with the best supportive care, provides a minimal
incremental benefit at high incremental cost per QALY in the
third-line management of metastatic colorectal cancer. The present
study found that the ICER for the trifluridine/tipiracil
combination tablet regimen vs. regorafenib was ¥477,330.9/MST. This
figure objectively showed that patients can save almost
¥500,000/MST by opting for the trifluridine/tipiracil combination
tablet regimen. However, the data on the cost determined by the
present study are based on the patient population of a single
facility. In the future, if data on cost are collected from several
facilities, the results can be applied more widely.
Since the present study did not take QOL into
account, it was not possible to accurately determine the
cost-effectiveness in the common units ¥/QALI. However, upon
examining AEs, it was speculated that the high incidence of
hand-foot syndrome (HFS) in patients subjected to the regorafenib
regimen reduced their QOL. Go et al (23) reported that HFS and skin damage by
anticancer agents decreased patient's QOL. In addition, patients
receiving regorafenib more frequently presented with AEs of grade 3
or higher, including HFS, fatigue, high blood pressure and liver
dysfunction. Thus, more frequent outpatient visits are required,
which may represent a burden for patients. In the present study,
the cost of supportive care agents for HFS and hypertension was
added to the total cost of regorafenib.
As the cost of supportive care and outpatient visits
was lower for patients receiving the trifluridine/tipiracil
combination tablet regimen, it is considered an improved and more
cost-effective treatment compared with regorafenib. The findings of
the present study will assist medical practitioners and patients
decide whether to use regorafenib or the trifluridine/tipiracil
combination tablet for treating advanced and recurrent colorectal
cancer.
In conclusion, the present study was the first to
analyse the cost-effectiveness of two types of anticancer drug
regimens for third-line or later treatment of advanced and
recurrent colorectal cancer. The findings clearly suggested that
the trifluridine/tipiracil combination tablet regimen is more
cost-effective compared with regorafenib treatment.
References
|
1
|
Saltz LB, Clarke S, Díaz-Rubio E,
Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS,
Rivera F, et al: Bevacizumab in combination with oxaliplatin-based
chemotherapy as first-line therapy in metastatic colorectal cancer:
A randomized phase III study. J Clin Oncol. 26:2013–2019. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Van Cutsem E, Köhne CH, Láng I, Folprecht
G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D,
Tejpar S, et al: Cetuximab plus irinotecan, fluorouracil, and
leucovorin as first-line treatment for metastatic colorectal
cancer: Updated analysis of overall survival according to tumor
KRAS and BRAF mutation status. J Clin Oncol. 29:2011–2019. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bokemeyer C, Bondarenko I, Hartmann JT, de
Braud F, Schuch G, Zubel A, Celik I, Schlichting M and Koralewski
P: Efficacy according to biomarker status of cetuximab plus
FOLFOX-4 as first-line treatment for metastatic colorectal cancer:
The OPUS study. Ann Oncol. 22:1535–1546. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Meropol NJ, Schrag D, Smith TJ, Mulvey TM,
Langdon RM Jr, Blum D, Ubel PA and Schnipper LE: American Society
of Clinical Oncology: American Society of Clinical Oncology
guidance statement: The cost of cancer care. J Clin Oncol.
27:3868–3874. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goldstein DA, Chen Q, Ayer T, Howard DH,
Lipscomb J, El-Rayes BF and Flowers CR: First- and second-line
bevacizumab in addition to chemotherapy for metastatic colorectal
cancer: A United States-based cost-effectiveness analysis. J Clin
Oncol. 33:1112–1118. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carter HE, Zannino D, Simes R John,
Schofield DJ, Howard K, Zalcberg JR, Price TJ and Tebbutt NC: The
cost effectiveness of bevacizumab when added to capecitabine, with
or without mitomycin-C, in first line treatment of metastatic
colorectal cancer: Results from the Australasian phase III MAX
study. Eur J Cancer. 50:535–543. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ruiz-Millo O, Albert-Mari A, Sendra-Garcia
A and Jimenez-Torres NV: Comparative cost-effectiveness of
bevacizumab-irinotecan-fluorouracil versus irinotecan-fluorouracil
in first-line metastatic colorectal cancer. J Oncol Pharm Pract.
20:341–350. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Díaz-Rubio E, Pietrantonio F and de Braud
F: Continuing single-agent bevacizumab as maintenance therapy after
induction XELOX (or FOLFOX) plus bevacizumab in first-line
treatment of metastatic colorectal cancer. Oncologist.
17:1426–1428. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rinaldi F, George E and Adler AI: NICE
guidance on cetuximab, bevacizumab, and panitumumab for treatment
of metastatic colorectal cancer after first-line chemotherapy.
Lancet Oncol. 13:233–234. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tappenden P, Jones R, Paisley S and
Carroll C: The cost-effectiveness of bevacizumab in the first-line
treatment of metastatic colorectal cancer in England and Wales. Eur
J Cancer. 43:2487–2494. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shiroiwa T, Motoo Y and Tsutani K:
Cost-effectiveness analysis of KRAS testing and cetuximab as
last-line therapy for colorectal cancer. Mol Diagn Ther.
14:375–384. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schwartzberg LS, Rivera F, Karthaus M,
Fasola G, Canon JL, Hecht JR, Yu H, Oliner KS and Go WY: PEAK: A
randomized, multicenter phase II study of panitumumab plus modified
fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab
plus mFOLFOX6 in patients with previously untreated, unresectable,
wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol.
32:2240–2247. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nomura H, Nagai S, Shinohara T, Iwashita
M, Yajima M and Takatoya S: Compared medical costs of treating
ovarian cancer patients with weekly paclitaxel, carboplatin (TC)
chemotherapy. Gan To Kagaku Ryoho. 34:1091–1094. 2007.(In
Japanese). PubMed/NCBI
|
|
14
|
Inage S, Ise Y, Obayashi M, Katayama S and
Gemma A: Cost-effectiveness analysis comparing carboplatin and
weekly paclitaxel with cisplatin and docetaxel in the treatment of
advanced non-small cell lung carcinoma. Gan To Kagaku Ryoho.
37:2093–2100. 2010.(In Japanese). PubMed/NCBI
|
|
15
|
Kurihara T, Kobayashi M, Kogo M, Yoneyama
K, Ito N, Sunaga T, Konishi K, Imawari M, Tobe T and Kiuchi Y:
Cost-effectiveness analysis of chemotherapy with GEM or S-1 for
patients with non-resectable pancreatic cancer. Gan To Kagaku
Ryoho. 37:659–664. 2010.(In Japanese). PubMed/NCBI
|
|
16
|
Watanabe T, Itabashi M, Shimada Y, Tanaka
S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H, et
al: Japanese Society for Cancer of the Colon and Rectum (JSCCR)
guidelines 2014 for treatment of colorectal cancer. Int J Clin
Oncol. 20:207–239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mayer RJ, Van Cutsem E, Falcone A, Yoshino
T, Garcia-Carbonero R, Mizunuma N, Yamazaki K, Shimada Y, Tabernero
J, Komatsu Y, et al: Randomized trial of TAS-102 for refractory
metastatic colorectal cancer. N Engl J Med. 372:1909–1919. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pharmaceutical Society, . Insurance drug
encyclopedia. Jiho, Tokyo: Pharmaceutical Society; 2012
|
|
19
|
Kawakami Y: Medical fee points table.
35th. Social Insurance Institute; Tokyo: 2008
|
|
20
|
Grothey A, Van Cutsem E, Sobrero A, Siena
S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, et
al: Regorafenib monotherapy for previously treated metastatic
colorectal cancer (CORRECT): An international, multicentre,
randomised, placebo-controlled, phase 3 trial. Lancet. 381:303–312.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
US Department Of Health And Human
Services, . Common terminology criteria for adverse events (CTCAE)
version 4.0. United States: National Cancer Institute; 2009,
http://www.
acrin.org/Portals/0/Administration/Regulatory/CTCAE_4.02_2009-09-15_QuickReference_5X7.pdf
|
|
22
|
Goldstein DA, Ahmad BB, Chen Q, Ayer T,
Howard DH, Lipscomb J, El-Rayes BF and Flowers CR:
Cost-effectiveness analysis of regorafenib for metastatic
colorectal cancer. J Clin Oncol. 33:3727–3732. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Go M, Ikagawa M, Kimura M, Iwai M, Usami
E, Yoshimura T and Yasuda K: Awareness of adverse events and
health-related quality of life in outpatients receiving molecular
targeted drugs for lung cancer patients. J Jpn Soc Hosp Pharm.
51:1462–1466. 2015.(In Japanese).
|