Introduction
Febrile neutropenia is a life-threatening condition
characterized by fever in addition to chemotherapy-induced
neutropenia. The longer the duration of neutropenia, the higher the
risk of developing febrile neutropenia.
Therefore, granulocyte-colony stimulating factor
(G-CSF) is often used to manage chemotherapy-induced neutropenia.
Furthermore, prophylactic administration of G-CSF is recommended
for patients at high risk of febrile neutropenia by several
guidelines (1,2).
Pegfilgrastim is a long-acting granulocyte-colony
stimulating factor (G-CSF) formulation. A phase III randomized
trial demonstrated that pegfilgrastim significantly reduced the
incidence of febrile neutropenia in breast cancer patients
(3). According to the data,
pegfilgrastim has been approved in Japan since 2014 and several
associated adverse effects, including rash, joint pain, diarrhea
and fever, have been reported. However, interstitial lung disease
(ILD) during pegfilgrastim treatment has rarely been reported. We
herein present a case of ILD following administration of
pegfilgrastim.
Case report
An asymptomatic 64-year-old man was referred to
another hospital due to abnormal nodular shadows in the left upper
lung on chest computed tomography (CT) scan. The smoking history
was 44 pack-years. Thoracoscopic pleural biopsy was performed and
the patient was diagnosed with small-cell lung cancer (SCLC) stage
IV (T4N2M1a). The patient was treated every 4 weeks with four
cycles of first-line chemotherapy, including cisplatin (60
mg/m2) and irinotecan (60 mg/m2) on day 1 and
irinotecan (60 mg/m2) alone on day 8. After the
first-line chemotherapy, complete response (CR) was observed.
However, 1 month after the fourth cycle of
chemotherapy, the infiltrative shadows progressed. SCLC progression
was hypothesized and the patient was treated with amrubicin
monotherapy (40 mg/m2 on days 1, 2 and 3) as second-line
chemotherapy every 3 weeks. Due to certain circumstances of the
patient, he was transferred to our hospital.
After the fourth cycle of chemotherapy, the SCLC
continued to progress. The patient received combination
chemotherapy with carboplatin (area under the curve=5) and
etoposide (80 mg/m2 on days 1, 2 and 3) as third-line
chemotherapy every 3 weeks. The adverse events of the first course
were grade 4 neutropenia and grade 3 thrombocytopenia. Prior to the
second cycle of chemotherapy, the performance status (PS) of the
patient was 3. However, we decided to administer a second cycle of
chemotherapy, as this combination therapy was considered to be
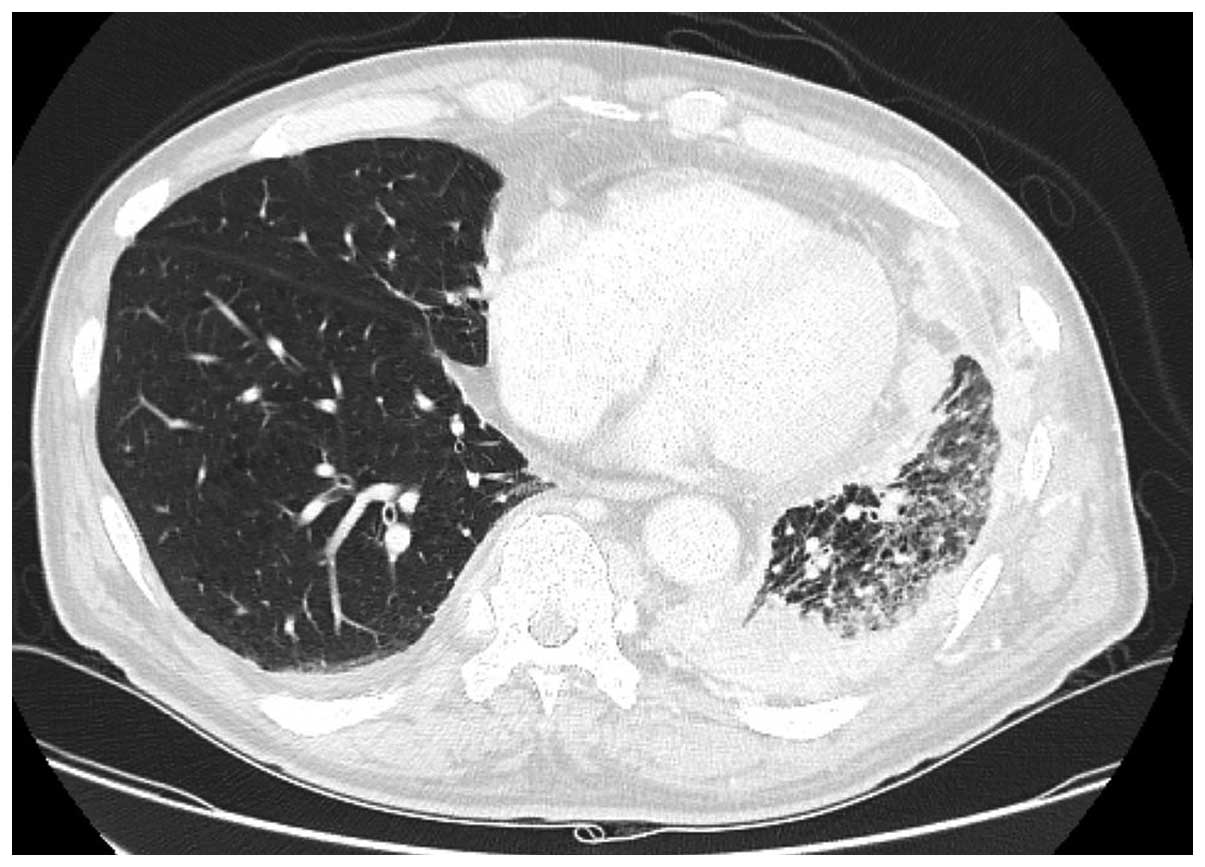
effective (Fig. 1). Therefore,
pegfilgrastim was added to prevent febrile neutropenia.
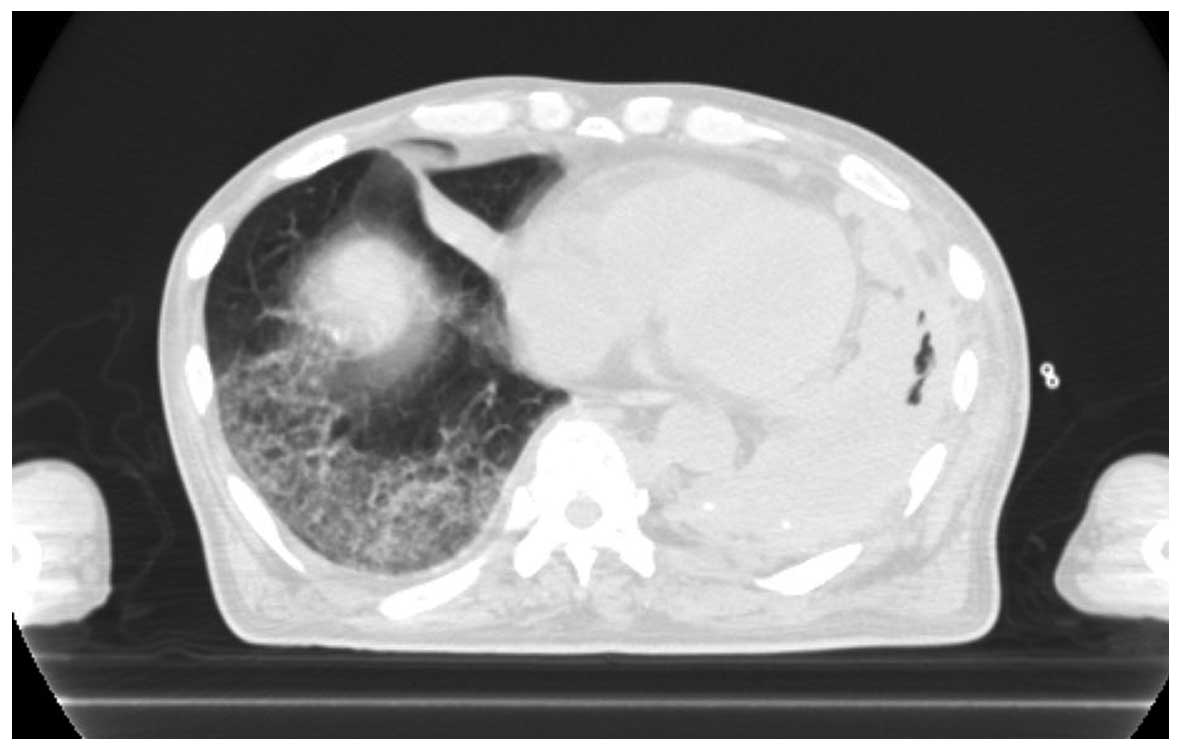
One day after pegfilgrastim administration, the
patient experienced sudden deterioration of his respiratory status.
On physical examination there were fine crackles on the right side
of the chest, and the CT scan revealed diffuse infiltrative shadows
(Fig. 2). Subsequent tests for
infectious diseases, such as sputum, blood and urine cultures, were
all normal. From these results, we considered that the patient's
clinical course was due to drug-induced lung injury. The patient
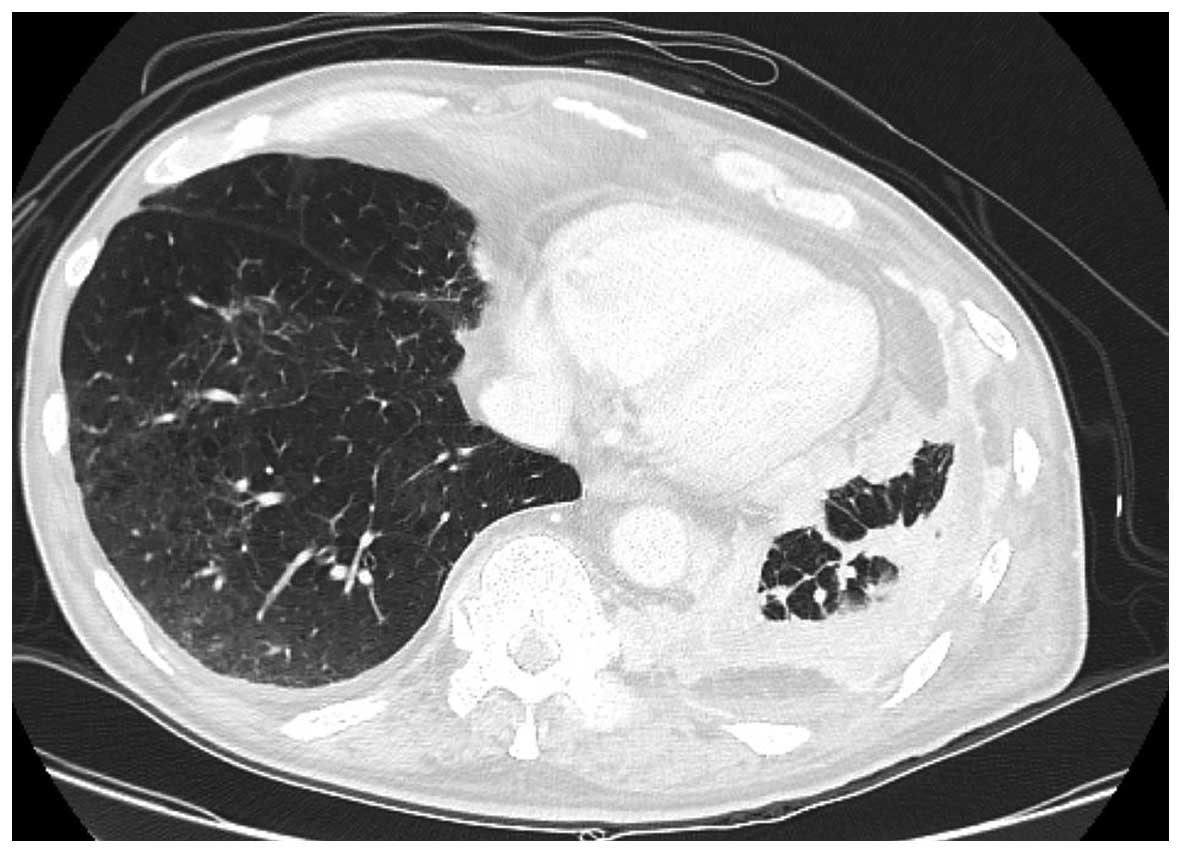
was treated with methylprednisolone (1,000 mg/day for 3 days). The
interstitial pneumonia improved after 10 days of pulse steroid
therapy (Fig. 3). However, the
patient succumbed to cancer progression 1 month after the
occurrence of interstitial pneumonia.
Discussion
Our patient was administered pegfilgrastim for the
prevention of febrile neutropenia, but ILD developed on the next
day. Apart from pegfilgrastim, carboplatin and etoposide were
suspected as the other possible offending drugs. The drug-induced
lymphocyte stimulation test for these three drugs was negative. ILD
secondary to carboplatin and etoposide is extremely rare (4). Moreover, the disease did not manifest
in the patient after the first course of combination chemotherapy
with carboplatin and etoposide. Therefore, in this case, we
considered that the drug most likely responsible for ILD was
pegfilgrastim.
To the best of our knowledge, interstitial pneumonia
has not been reported in clinical trials on pegfilgrastim (3,5–8). However, several reports indicated that
the administration of G-CSF may be associated with lung injury.
Matthews reported that pneumotoxicity occurred in 3 out of 5
patients with Hodgkin's lymphoma who received G-CSF with
doxorubicin, bleomycin, vinblastine and dacarbazin (ABVD) therapy
(9). Yokose et al reported
that pulmonary toxicity had occurred in 6 out of 52 patients with
non-Hodgkin lymphoma who received G-CSF with cyclophosphamide,
doxorubicin, vincristine and prednisolone (CHOP) therapy (10). The study demonstrated that the mean
peak leukocyte count with each therapy cycle had been associated
with development of pulmonary toxicity, and concluded that lowering
the G-CSF dose appeared to be useful in the prevention of this
toxicity. Furthermore, Ruiz-Argüelles et al reported a case
of pulmonary toxicity after the administration of G-CSF without any
chemotherapy (11).
Adachi et al reported that the mechanism of
ILD due to G-CSF was enhancement of the infiltration of the alveoli
by alkaline phosphatase-positive neutrophils (12). However, this mechanism has not been
fully elucidated.
Smoking history and poor PS were reported to be risk
factors of drug-induced lung injury (13). Furthermore, Niitsu et al
reviewed 20 cases of interstitial pneumonia secondary to treatment
with G-CSF, and reported that it occurred predominantly in patients
aged ≥60 years (14).
Our patient was 64 years old, had a history of heavy
smoking and his PS was 3 at the start of pegfilgrastim
administration, placing him at risk to develop ILD.
In conclusion, drug-induced lung injury by
pegfilgrastim is rare. However, physicians should be aware of the
possibility of this adverse effect.
References
|
1
|
Smith TJ, Khatcheressian J, Lyman GH, Ozer
H, Armitage JO, Balducci L, Bennett CL, Cantor SB, Crawford J,
Cross SJ, et al: 2006 update of recommendations for the use of
white blood cell growth factors: An evidence-based clinical
practice guideline. J Clin Oncol. 24:3187–3205. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aapro MS, Bohlius J, Cameron DA, Dal Lago
L, Donnelly JP, Kearney N, Lyman GH, Pettengell R, Tjan-Heijnen VC,
Walewski J, et al: 2010 update of EORTC guidelines for the use of
granulocyte-colony stimulating factor to reduce the incidence of
chemotherapy-induced febrile neutropenia in adult patients with
lymphoproliferative disorders and solid tumours. Eur J Cancer.
47:8–32. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kosaka Y, Rai Y, Masuda N, Takano T, Saeki
T, Nakamura S, Shimazaki R, Ito Y, Tokuda Y and Tamura K: Phase III
placebo-controlled, double-blind, randomized trial of pegfilgrastim
to reduce the risk of febrile neutropenia in breast cancer patients
receiving docetaxel/cyclophosphamide chemotherapy. Support Care
Cancer. 23:1137–1143. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Okamoto H, Watanabe K, Kunikane H,
Yokoyama A, Kudoh S, Asakawa T, Shibata T, Kunitoh H, Tamura T and
Saijo N: Randomised phase III trial of carboplatin plus etoposide
vs split doses of cisplatin plus etoposide in elderly or poor-risk
patients with extensive disease small-cell lung cancer: JCOG 9702.
Br J Cancer. 97:162–169. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamamoto N, Sekine I, Nakagawa K, Takada
M, Fukuoka M, Tanigawara Y and Saijo N: A pharmacokinetic and dose
escalation study of pegfilgrastim (KRN125) in lung cancer patients
with chemotherapy-induced neutropenia. Jpn J Clin Oncol.
39:425–430. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee KH, Kim JY, Lee MH, Han HS, Lim JH,
Park KU, Park IH, Cho EK, Yoon SY, Kim JH, et al: A randomized,
multicenter, phase II/III study to determine the optimal dose and
to evaluate the efficacy and safety of pegteograstim (GCPGC) on
chemotherapy-induced neutropenia compared to pegfilgrastim in
breast cancer patients: KCSG PC10-09. Support Care Cancer.
24:1709–1717. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Masuda N, Tokuda Y, Nakamura S, Shimazaki
R, Ito Y and Tamura K: Dose response of pegfilgrastim in Japanese
breast cancer patients receiving six cycles of docetaxel,
doxorubicin, and cyclophosphamide therapy: A randomized controlled
trial. Support Care Cancer. 23:2891–2898. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang BB, Morrow PK, Wu X, Moxness M and
Padhi D: Comparison of pharmacokinetics and safety of pegfilgrastim
administered by two delivery methods: On-body injector and manual
injection with a prefilled syringe. Cancer Chemother Pharmacol.
75:1199–1206. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matthews JH: Pulmonary toxicity of ABVD
chemotherapy and G-CSF in Hodgkin's disease: Possible synergy.
Lancet. 342:9881993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yokose N, Ogata K, Tamura H, An E,
Nakamura K, Kamikubo K, Kudoh S, Dan K and Nomura T: Pulmonary
toxicity after granulocyte colony-stimulating factor-combined
chemotherapy for non-Hodgkin's lymphoma. Br J Cancer. 77:2286–2290.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ruiz-Argüelles GJ, Arizpe-Bravo D,
Sánchez-Sosa S, Rojas-Ortega S, Moreno-Ford V and Ruiz-Argüelles A:
Fatal G-CSF-induced pulmonary toxicity. Am J Hematol. 60:82–83.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Adachi K, Suzuki M, Sugimoto T, Yorozu K,
Takai H, Uetsuka K, Nakayama H and Doi K: Effects of granulocyte
colony-stimulating factor (G-CSF) on bleomycin-induced lung injury
of varying severity. Toxicol Pathol. 31:665–673. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kudoh S, Kato H, Nishiwaki Y, Fukuoka M,
Nakata K, Ichinose Y, Tsuboi M, Yokota S, Nakagawa K, Suga M, et
al: Interstitial lung disease in Japanese patients with lung
cancer: A cohort and nested case-control study. Am J Respir Crit
Care Med. 177:1348–1357. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Niitsu N, Iki S, Muroi K, Motomura S,
Murakami M, Takeyama H, Ohsaka A and Urabe A: Interstitial
pneumonia in patients receiving granulocyte colony-stimulating
factor during chemotherapy: Survey in Japan 1991–96. Br J Cancer.
76:1661–1666. 1997. View Article : Google Scholar : PubMed/NCBI
|